
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adam M. Zawada | -- | 1944 | 2022-11-04 09:17:57 | | | |
| 2 | Beatrix Zheng | + 2 word(s) | 1946 | 2022-11-07 04:07:10 | | |
Video Upload Options
The dialyzer is the core element in the hemodialysis treatment of patients with end-stage kidney disease (ESKD). During hemodialysis treatment, the dialyzer replaces the function of the kidney by removing small and middle-molecular weight uremic toxins, while retaining essential proteins. Meanwhile, a dialyzer should have the best possible hemocompatibility profile as the perpetuated contact of blood with artificial surfaces triggers complement activation, coagulation and immune cell activation, and even low-level activation repeated chronically over years may lead to undesired effects. During hemodialysis, the adsorption of plasma proteins to the dialyzer membrane leads to a formation of a secondary membrane, which can compromise both the uremic toxin removal and hemocompatibility of the dialyzer. Hydrophilic modifications of novel dialysis membranes have been shown to reduce protein adsorption, leading to better hemocompatibility profile and performance stability during dialysis treatments.
1. Introduction
During hemodialysis treatment, the adsorption of plasma proteins to the blood-side surface of the dialyzer membrane can strongly impact both performance as well as the hemocompatibility profile of the dialyzer. Importantly, hydrophilic membrane modifications reduce protein adsorption and improve the performance and hemocompatibility profile of a dialyzer.
2. Reduction in Membrane Fouling by Hydrophilic Modifications
Over the course of the last several decades, dialyzer membrane research has focused on improving both performance and hemocompatibility. As protein adsorption to the membrane impacts both–performance and hemocompatibility–membrane modifications with the aim to reduce secondary membrane formation during dialysis treatment were in the focus of latest dialyzer development. For synthetic membranes, such as polysulfone or polyethersulfone-based membranes, polyvinylpyrrolidone (PVP) is commonly used as a hydrophilic agent. PVP has good physiological inertness and reduces protein adsorption via repulsive hydration force of the formed water layer [18][19][20][21][22][23]. Wang et al. fabricated polyethersulfone-based membranes with increased PVP content and found that those membranes with higher PVP content showed stronger water adsorption and were associated with reduced albumin adsorption as well as increased blood coagulation time [74]. In line, Zhu and colleagues prepared and characterized polysulfone membranes with different amounts of PVP and found that membranes with higher PVP content showed lower protein adsorption, reduced platelet adhesion and deformation as well as improved blood clotting characteristics [19]. Differences in PVP content in polysulfone-based membranes also affect the roughness of the membrane in dry or wet condition and are strong determinants for the swelling of the membrane after contact with water [18][22]. These findings are schematically summarized in Figure 1.
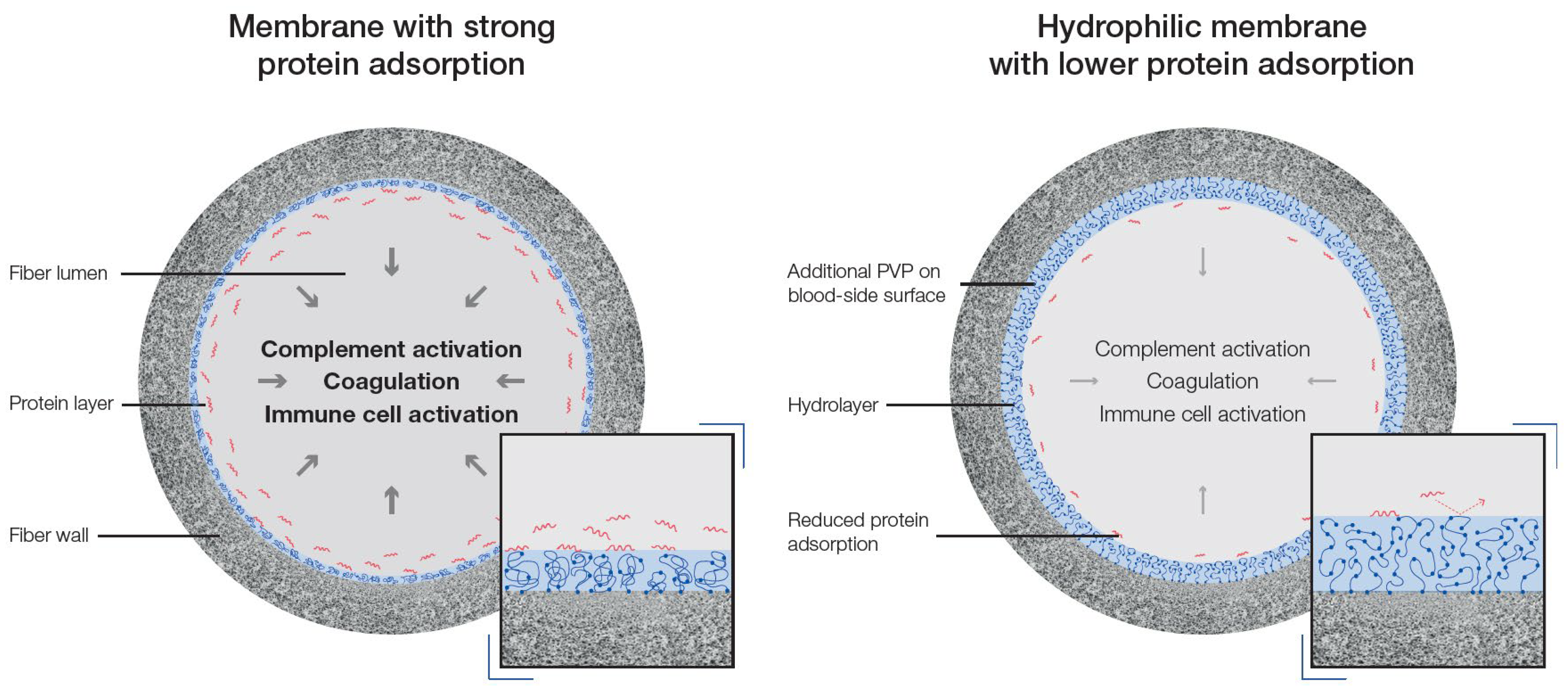
3. Maintaining Hydrophilic Modification of Dialysis Membranes
3.1. Undesirable Effects of Elutable PVP
3.2. Factors Influencing PVP Elution
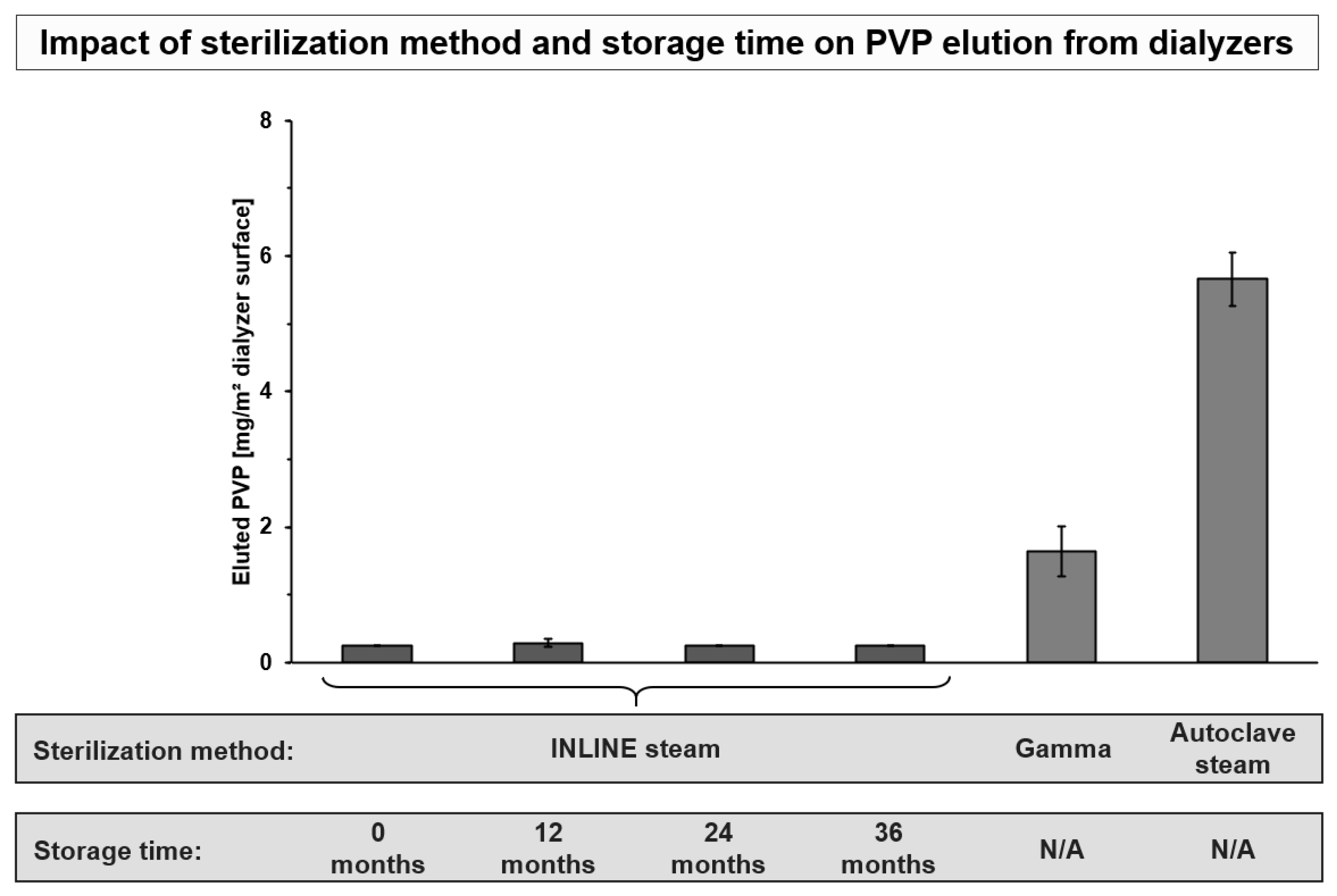
References
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107.
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75, A6–A7.
- Benabed, A.; Henri, P.; Lobbedez, T.; Goffin, E.; Baluta, S.; Benziane, A.; Rachi, A.; van der Pijl, J.W.; Bechade, C.; Ficheux, M. Hémodialyse quotidienne à bas débit de dialysat à domicile: Résultats cliniques et biologiques des 62 premiers patients traités en France et en Belgique. Nephrol. Ther. 2017, 13, 18–25.
- Pattharanitima, P.; El Shamy, O.; Chauhan, K.; Saha, A.; Wen, H.H.; Sharma, S.; Uribarri, J.; Chan, L. The Association between Prevalence of Peritoneal Dialysis versus Hemodialysis and Patients’ Distance to Dialysis-Providing Facilities. Kidney360 2021, 2, 1908–1916.
- Bello, A.K.; Okpechi, I.G.; Osman, M.A.; Cho, Y.; Htay, H.; Jha, V.; Wainstein, M.; Johnson, D.W. Epidemiology of Haemodialysis Outcomes. Nat. Rev. Nephrol. 2022, 18, 378–395.
- Said, N.; Lau, W.J.; Ho, Y.-C.; Lim, S.K.; Zainol Abidin, M.N.; Ismail, A.F. A Review of Commercial Developments and Recent Laboratory Research of Dialyzers and Membranes for Hemodialysis Application. Membranes 2021, 11, 767.
- Bowry, S.K.; Kircelli, F.; Nandakumar, M.; Vachharajani, T.J. Clinical Relevance of Abstruse Transport Phenomena in Haemodialysis. Clin. Kidney J. 2021, 14, i85–i97.
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Mironova, M.V.; Anokhina, T.S.; Arkharova, N.A. Morphology and Transport Properties of Membranes Obtained by Coagulation of Cellulose Solutions in Isobutanol. Carbohydr. Polym. 2021, 254, 117472.
- Yamashita, A.C.; Sakurai, K. Dialysis Membranes—Physicochemical Structures and Features. In Updates in Hemodialysis; Suzuki, H., Ed.; IntechOpen: London, UK, 2015.
- Bowry, S.K. Dialysis Membranes Today. Int. J. Artif. Organs 2002, 25, 447–460.
- Bowry, S.K.; Chazot, C. The Scientific Principles and Technological Determinants of Haemodialysis Membranes. Clin. Kidney J. 2021, 14, i5–i16.
- Ronco, C.; Clark, W.R. Haemodialysis Membranes. Nat. Rev. Nephrol. 2018, 14, 394–410.
- Canaud, B. Recent Advances in Dialysis Membranes. Curr. Opin. Nephrol. Hypertens. 2021, 30, 613–622.
- Poppelaars, F.; Faria, B.; Gaya da Costa, M.; Franssen, C.F.M.; van Son, W.J.; Berger, S.P.; Daha, M.R.; Seelen, M.A. The Complement System in Dialysis: A Forgotten Story? Front. Immunol. 2018, 9, 71.
- Losappio, V.; Franzin, R.; Infante, B.; Godeas, G.; Gesualdo, L.; Fersini, A.; Castellano, G.; Stallone, G. Molecular Mechanisms of Premature Aging in Hemodialysis: The Complex Interplay between Innate and Adaptive Immune Dysfunction. Int. J. Mol. Sci. 2020, 21, 3422.
- Ekdahl, K.N.; Soveri, I.; Hilborn, J.; Fellström, B.; Nilsson, B. Cardiovascular Disease in Haemodialysis: Role of the Intravascular Innate Immune System. Nat. Rev. Nephrol. 2017, 13, 285–296.
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759.
- Hayama, M.; Yamamoto, K.; Kohori, F.; Uesaka, T.; Ueno, Y.; Sugaya, H.; Itagaki, I.; Sakai, K. Nanoscopic Behavior of Polyvinylpyrrolidone Particles on Polysulfone/Polyvinylpyrrolidone Film. Biomaterials 2004, 25, 1019–1028.
- Zhu, L.; Song, H.; Wang, J.; Xue, L. Polysulfone Hemodiafiltration Membranes with Enhanced Anti-Fouling and Hemocompatibility Modified by Poly(Vinyl Pyrrolidone) via in Situ Cross-Linked Polymerization. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 159–166.
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and Antimicrobial Polymer Membranes Based on Bioinspired Polydopamine and Strong Hydrogen-Bonded Poly( N -Vinyl Pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904.
- Ran, F.; Nie, S.; Zhao, W.; Li, J.; Su, B.; Sun, S.; Zhao, C. Biocompatibility of Modified Polyethersulfone Membranes by Blending an Amphiphilic Triblock Co-Polymer of Poly(Vinyl Pyrrolidone)-b-Poly(Methyl Methacrylate)-b-Poly(Vinyl Pyrrolidone). Acta Biomater. 2011, 7, 3370–3381.
- Hayama, M.; Yamamoto, K.; Kohori, F.; Sakai, K. How Polysulfone Dialysis Membranes Containing Polyvinylpyrrolidone Achieve Excellent Biocompatibility? J. Membr. Sci. 2004, 234, 41–49.
- Wang, H.; Yu, T.; Zhao, C.; Du, Q. Improvement of Hydrophilicity and Blood Compatibility on Polyethersulfone Membrane by Adding Polyvinylpyrrolidone. Fibers Polym. 2009, 10, 1–5.
- Matsuda, M.; Sato, M.; Sakata, H.; Ogawa, T.; Yamamoto, K.; Yakushiji, T.; Fukuda, M.; Miyasaka, T.; Sakai, K. Effects of Fluid Flow on Elution of Hydrophilic Modifier from Dialysis Membrane Surfaces. J. Artif. Organs 2008, 11, 148–155.
- Namekawa, K.; Matsuda, M.; Fukuda, M.; Kaneko, A.; Sakai, K. Poly(N-Vinyl-2-Pyrrolidone) Elution from Polysulfone Dialysis Membranes by Varying Solvent and Wall Shear Stress. J. Artif. Organs 2012, 15, 185–192.
- Hulme, B.; Dykes, P.W.; Appleyard, I.; Arkell, D.W. Retention and Storage Sites of Radioactive Polyvinylpyrrolidone. J. Nucl. Med. 1968, 9, 389–393.
- Takahashi, K.; Eto, K.; Takeya, M.; Naito, M.; Yaginuma, Y.; Ichihara, A. Long-Term Polyvinylpyrrolidone Storage. Pathol. Int. 1983, 33, 985–997.
- Shu, K.-H.; Kao, T.-W.; Chiang, W.-C.; Wu, V.-C. A Case of Anaphylactic Shock Induced by FX60 Polysulfone Hemodialyzer but Not F6-HPS Polysulfone Hemodialyzer: Polysulfone Hemodialyzer Anaphylaxis. Hemodial. Int. 2014, 18, 841–845.
- Bacelar Marques, I.D.; Pinheiro, K.F.; de Freitas do Carmo, L.P.; Costa, M.C.; Abensur, H. Anaphylactic Reaction Induced by a Polysulfone/Polyvinylpyrrolidone Membrane in the 10th Session of Hemodialysis with the Same Dialyzer. Hemodial. Int. 2011, 15, 399–403.
- Martin-Navarro, J.; Esteras, R.; Castillo, E.; Carriazo, S.; Fernández-Prado, R.; Gracia-Iguacel, C.; Mas Fontao, S.; Ortíz, A.; González-Parra, E. Reactions to Synthetic Membranes Dialyzers: Is There an Increase in Incidence? Kidney Blood Press. Res. 2019, 44, 907–914.
- Alvarez-de Lara, M.A.; Martín-Malo, A. Hypersensitivity Reactions to Synthetic Haemodialysis Membranes’ an Emerging Issue? Nefrologia 2014, 34, 698–702.
- Ohashi, N.; Yonemura, K.; Goto, T.; Suzuki, H.; Fujigaki, Y.; Yamamoto, T.; Hishida, A. A Case of Anaphylactoid Shock Induced by the BS Polysulfone Hemodialyzer but Not by the F8-HPS Polysulfone Hemodialyzer. Clin. Nephrol. 2003, 60, 214–217.
- Konishi, S.; Fukunaga, A.; Yamashita, H.; Miyata, M.; Usami, M. Eluted Substances from Hemodialysis Membranes Elicit Positive Skin Prick Tests in Bioincompatible Patients. Artif. Organs 2015, 39, 343–351.
- Zawada, A.M.; Melchior, P.; Erlenkötter, A.; Delinski, D.; Stauss-Grabo, M.; Kennedy, J.P. Polyvinylpyrrolidone in Hemodialysis Membranes: Impact on Platelet Loss during Hemodialysis. Hemodial. Int. 2021, 25, 498–506.
- Miyata, M.; Konishi, S.; Shimamoto, Y.; Kamada, A.; Umimoto, K. Influence of Sterilization and Storage Period on Elution of Polyvinylpyrrolidone from Wet-Type Polysulfone Membrane Dialyzers. ASAIO J. 2015, 61, 468–473.
- Allard, B.; Begri, R.; Potier, J.; Coupel, S. Dialyzers Biocompatibility and Efficiency Determinants of Sterilization Method Choice. Pharm. Hosp. Clin. 2013, 48, e15–e21.
- Namekawa, K.; Kaneko, A.; Sakai, K.; Kunikata, S.; Matsuda, M. Longer Storage of Dialyzers Increases Elution of Poly(N-Vinyl-2-Pyrrolidone) from Polysulfone-Group Dialysis Membranes. J. Artif. Organs 2011, 14, 52–57.
- Ehlerding, G.; Ries, W.; Kempkes-Koch, M.; Ziegler, E.; Erlenkoetter, A.; Zawada, A.M.; Kennedy, J.; Ottillinger, B.; Stauss-Grabo, M.; Lang, T. Randomized Comparison of Three High-Flux Dialyzers during High Volume Online Hemodiafiltration—The ComPERFORM Study. Clin. Kidney J. 2021, 15, 672–680.
- Ehlerding, G.; Erlenkötter, A.; Gauly, A.; Griesshaber, B.; Kennedy, J.; Rauber, L.; Ries, W.; Schmidt-Gürtler, H.; Stauss-Grabo, M.; Wagner, S.; et al. Performance and Hemocompatibility of a Novel Polysulfone Dialyzer: A Randomized Controlled Trial. Kidney360 2021, 2, 937–947.
- Melchior, P.; Erlenkötter, A.; Zawada, A.M.; Delinski, D.; Schall, C.; Stauss-Grabo, M.; Kennedy, J.P. Complement Activation by Dialysis Membranes and Its Association with Secondary Membrane Formation and Surface Charge. Artif. Organs 2021, 45, 770–778.
- Zawada, A.M.; Melchior, P.; Schall, C.; Erlenkötter, A.; Lang, T.; Keller, T.; Stauss-Grabo, M.; Kennedy, J.P. Time-resolving Characterization of Molecular Weight Retention Changes among Three Synthetic High-flux Dialyzers. Artif. Organs 2022, 46, 1318–1327.
- Kiaii, M.; Aritomi, M.; Nagase, M.; Farah, M.; Jung, B. Clinical Evaluation of Performance, Biocompatibility, and Safety of Vitamin E-Bonded Polysulfone Membrane Hemodialyzer Compared to Non-Vitamin E-Bonded Hemodialyzer. J. Artif. Organs 2019, 22, 307–315.
- Calò, L.A.; Naso, A.; D’Angelo, A.; Pagnin, E.; Zanardo, M.; Puato, M.; Rebeschini, M.; Landini, S.; Feriani, M.; Perego, A.; et al. Molecular Biology-Based Assessment of Vitamin E-Coated Dialyzer Effects on Oxidative Stress, Inflammation, and Vascular Remodeling: THOUGHTS AND PROGRESS. Artif. Organs 2011, 35, E33–E39.




