Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Przemysław Kowal | -- | 1274 | 2022-10-31 11:26:41 | | | |
| 2 | Conner Chen | Meta information modification | 1274 | 2022-11-01 03:11:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Otieno, J.; Kowal, P.; Mąkinia, J. Historical Perspective of Microorganisms Involved in EBPR. Encyclopedia. Available online: https://encyclopedia.pub/entry/32092 (accessed on 08 February 2026).
Otieno J, Kowal P, Mąkinia J. Historical Perspective of Microorganisms Involved in EBPR. Encyclopedia. Available at: https://encyclopedia.pub/entry/32092. Accessed February 08, 2026.
Otieno, Jeremiah, Przemysław Kowal, Jacek Mąkinia. "Historical Perspective of Microorganisms Involved in EBPR" Encyclopedia, https://encyclopedia.pub/entry/32092 (accessed February 08, 2026).
Otieno, J., Kowal, P., & Mąkinia, J. (2022, October 31). Historical Perspective of Microorganisms Involved in EBPR. In Encyclopedia. https://encyclopedia.pub/entry/32092
Otieno, Jeremiah, et al. "Historical Perspective of Microorganisms Involved in EBPR." Encyclopedia. Web. 31 October, 2022.
Copy Citation
The application of enhanced biological phosphorus removal (EBPR) in wastewater treatment plants (WWTPs) has commonly been utilized worldwide. The EBPR in biological nutrient removal (BNR) systems is mainly carried out by a group of microorganisms known as polyphosphate-accumulating organisms (PAOs).
phosphorus removal
Tetrasphaera
denitrification
enhanced biological phosphorus removal
1. Introduction
Enhanced biological phosphorus removal (EBPR) has emerged as the most powerful phosphorus (P) removal process during municipal and industrial wastewater treatment. For a long time, the EBPR has been considered as one of the most complex processes involved in the metabolic activity of activated sludge systems and has shown promise in terms of the cost, reliability and sustainability [1]. Recent years have brought many research contributions to expand knowledge and improve the process efficiency based on the recognition of the pathways and microorganisms involved.
The EBPR in biological nutrient removal (BNR) systems is mainly carried out by a group of microorganisms known as polyphosphate-accumulating organisms (PAOs). Conventionally, P removal via PAO activity is achieved by triggering anaerobic–aerobic conditions, which considerably increase operational costs related to energy consumption by aerators. The focus has recently been on P removal by denitrifying PAOs (DPAOs) under anaerobic–anoxic conditions to reduce the costs. The DPAOs are capable of using alternative electron sources (nitrate or nitrite) to metabolize intracellular organic compounds under anoxic conditions, and P uptake and denitrification is performed simultaneously [2][3][4][5].
Due to the growing interest in the implementation of P removal under anaerobic-anoxic conditions, a special attention has been paid to the microorganisms responsible for that process. Representatives of the genus Tetrasphaera are among the recently confirmed putative denitrifying PAO attracting attention of the scientific community. Members of Tetrasphaera are able to perform either denitrification or aerobic respiration, depending on the local environmental conditions [6]. All currently characterized Tetrasphaera isolates have proven the capability of reducing nitrate only to nitrite, whereas some members revealed the ability to reduce nitric acid to nitrous oxide. Moreover, the Tetrasphaera group is capable of carrying out the complete physiological EBPR process compared to other known PAOs, whose activity is more dependent on interspecies relationships [7].
The key aspects of Tetrasphaera have been studied intensively, including their classification and taxonomy, development of methods for detection [8], abundance in wastewater treatment plants (WWTPs) [9][10], functions in EBPR and biochemistry [6][11][12]. The ubiquitous occurrence of Tetrasphaera in diverse ecological niches, the use of various carbon sources and the ability to produce volatile fatty acids (VFAs) show their extraordinary metabolic potential [4][13].
Interactions and competition between DPAOs and other functional microbial groups in the anaerobic-aerobic cycle enable P removal optimization in activated sludge systems [14][15][16]. In addition, the interest in DPAO ecophysiology, in particular in the context of Tetrasphaera activity related to nitrous oxide emissions, was significant [3]. An emerging approach to enhance the full-scale EBPR is optimization by the application of mathematical modelling. The conventional models are thought to favor Ca. Accumulibacter over other PAOs, such as Ca. Halomonas phosphatis, Tessaracoccus, as well Tetrasphaera [8]. To ensure the highest prediction accuracy of the model, it is strongly recommended to extend and develop currently available models for multiple PAO groups, differentiated in terms of the growth rate and physiology.
2. Historical Perspective of Microorganisms Involved in EBPR
The first observations of EBPR date back to the mid-20th century and the findings obtained in laboratory scale experiments [17] and to a minor extent at full scale plants [18]. The principles of EBPR were formulated by Barnard [19][20], whose experiments clarified the need for anaerobic contact between activated sludge and influent wastewater prior to aerobic treatment to accomplish P removal. Subsequently, Barnard [21] used the term Phoredox to represent any process with an anaerobic/aerobic sequence to promote the EBPR technology concept (Figure 1a).
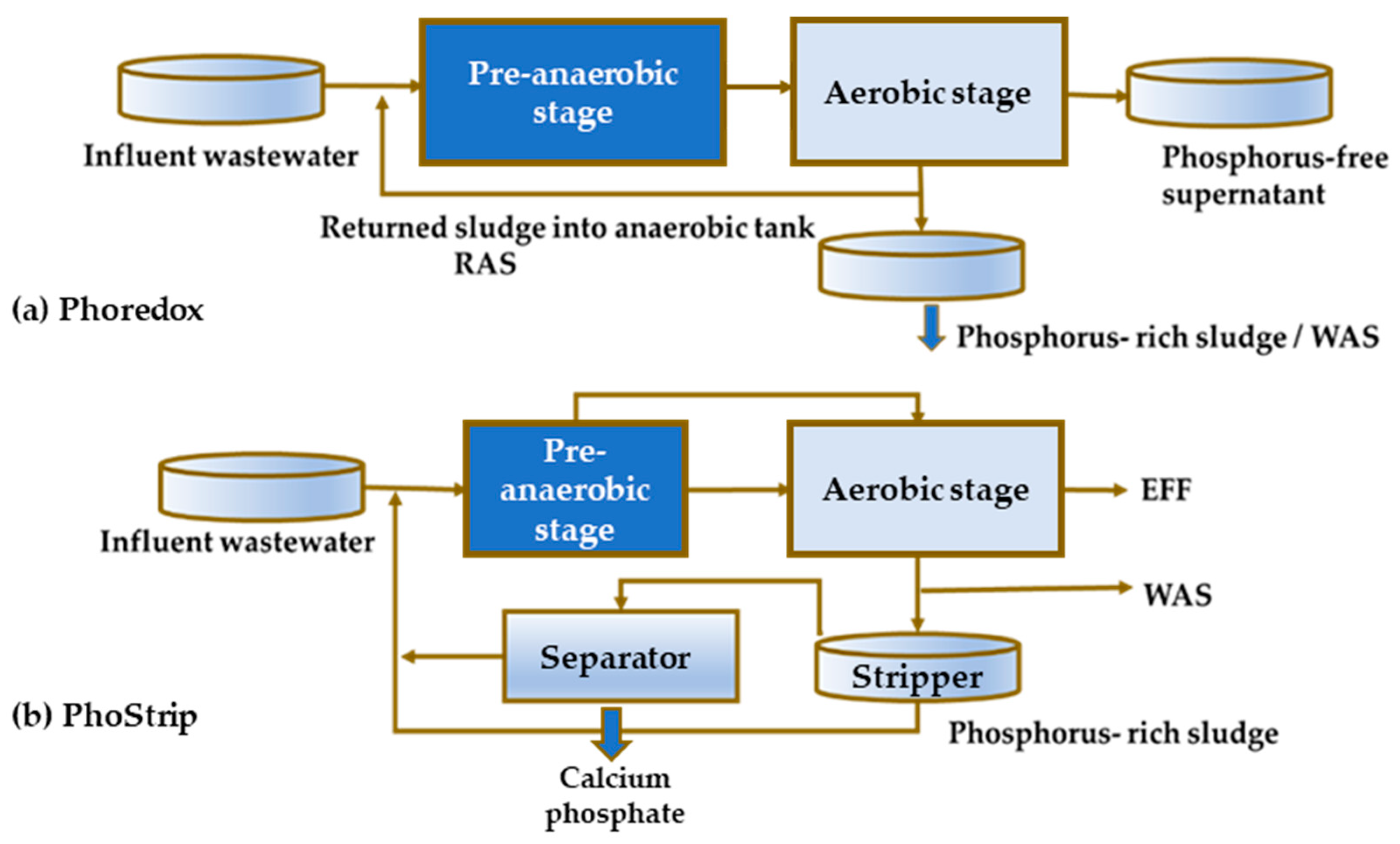
Figure 1. A typical configuration for P removal in the mainstream the mainstream EBPR (Phoredox process) (a), sidestream EBPR (PhoStrip process) (b) (abbreviations: EFF—effluent, RAS—recirculated activated sludge, WAS—waste activated sludge).
The performance and start-up process of the first full scale Phoredox system, which was launched in 1973, was briefly reported by Levin et al. [22]. In parallel, an alternative side-stream P removal technology, called PhoStrip, [1]. was developed based on the separation of the enriched side stream liquor treated with lime (Figure 1b).
While the nature of P removal was initially considered as chemical, Fuhs and Chen [23] found Acinetobacter as the primary microorganisms responsible for EBPR. These organisms responded to VFA in the influent wastewater under anaerobic conditions by releasing stored phosphate. Bacteria affiliated to Acinetobacter were considered as the key PAO responsible for the EBPR, mainly due to the limitations of the cultivation techniques applied for the microbial characterization at that time [11]. Significant advances in the microbial research have been achieved over the years and novel bacterial groups involved in the P metabolism were identified. Those microorganisms were able to store P in their cells in the form of energy-rich polyphosphates, resulting in the P content as high as 20 to 30 percent by dry weight [1]. The anaerobic zone free of nitrate and DO was found to favour the PAOs activity over other heterotrophs. In the following years, Betaproteobacteria were determined as the dominant bacterial group in the P metabolism [24], as well as the presence of Rhodocyclus-related bacteria was observed and linked with EBPR [25][26]. Subsequently, modern microbial tools without the cultivation step, established Ca. Accumulibacter as the most important member of PAOs, with the share ranging from 0.6 to 33.1% [27][28]. Insights into the biochemical characteristics of the Ca. Accumulibacter were applied to propose mathematical models of the EBPR within Activated Sludge Model [29]. Basic metabolic models are based on the assumption that PAOs exhibit intracellular phosphorus and energy storage in the form of poly-P and polyhydroxyalkanoates (PHA), respectively [30]. The stored PHA provide energy for the growth of PAOs when exposed to anoxic condition due to the capability of simultaneous denitrification and P uptake.
The novel microbial tools comprise deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) polymorphism analyses (e.g., 16S rRNA high-throughput gene sequencing, metagenomics, fluorescence in situ hybridization (FISH) and their modification) as well as flow cytometry and Raman spectroscopy. Along with the development of those tool, the contribution of particular bacterial groups in EBPR could be revised greatly [4][28][31].
The next advance in understanding the microbiology of EBPR was the discovery of new PAO and capability of simultaneous P and nitrogen (N) removal under anoxic conditions. Genus Tetrasphaera is the most recently confirmed putative PAO. From the first characterization of the isolated Tetrasphaera strain [32][33], it was found that representatives of this genera show a large and often predominant number in full-scale WWTPs [7][34]. Moreover, versatile metabolic capabilities of Tetrasphaera have been recognised, including the capability of fermenting glucose and amino acids to produce VFA in the anaerobic zone, thereby enhancing a pool of the available substrates for EBPR. The main difference with respect to the typical PAO is that Tetrasphaera are capable of storing other (than PHA) intracellular compounds and use nitrate, but not nitrite, in addition to DO as an electron acceptor [1]. The characterization of Tetrasphaera and their assignment to PAO drew attention to this group, especially in terms of the competition and interaction with Ca. Accumulibacter [3][35].
In the recent years, several new genera have been proposed as potential PAOs, including Dechloromonas or Candidatus Microthrix, but only members of the betaproteobacterial genera Ca. Accumulibacter [7] and the actinobacterial genus Tetrasphaera were consistently found in high abundances in full-scale EBPR plants [34]. For instance, it was proven that approximately 24–70% of total P removed in Danish WWTPs was directly attributed to Ca. Accumulibacter and Tetrasphaera [7]. The relative abundances of other PAO within activated sludge are usually significantly lower. In the study by Seviour and McIlroy [36], the relative abundance of Acinetobacter reached 1.2%, whereas the relative abundances below 1% were reported for Dechloromonas, another newly characterized PAO group [37].
References
- Metcalf and Eddy Inc. Wastewater Engineering Treatment and Reuse, 5th ed.; McGraw-Hill: New York, NY, USA, 2014.
- Barnard, J.L.; Dunlap, P.; Steichen, M. Rethinking the Mechanisms of Biological Phosphorus Removal. Water Environ. Res. 2017, 89, 2043–2054.
- Nielsen, P.H.; Saunders, A.M.; Hansen, A.A.; Larsen, P.; Nielsen, J.L. Microbial communities involved in enhanced biological phosphorus removal from wastewater—A model system in environmental biotechnology. Curr. Opin. Biotechnol. 2012, 23, 452–459.
- Nielsen, P.H.; McIlroy, S.J.; Albertsen, M.; Nierychlo, M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr. Opin. Biotechnol. 2019, 57, 111–118.
- Rubio-Rincón, F.; Lopez-Vazquez, C.; Welles, L.; van Loosdrecht, M.; Brdjanovic, D. Cooperation between Candidatus Competibacter and Candidatus Accumulibacter clade I, in denitrification and phosphate removal processes. Water Res. 2017, 120, 156–164.
- Marques, R.; Ribera-Guardia, A.; Santos, J.; Carvalho, G.; Reis, M.A.; Pijuan, M.; Oehmen, A. Denitrifying capabilities of Tetrasphaera and their contribution towards nitrous oxide production in enhanced biological phosphorus removal processes. Water Res. 2018, 137, 262–272.
- Fernando, E.Y.; McIlroy, S.J.; Nierychlo, M.; Herbst, F.-A.; Petriglieri, F.; Schmid, M.C.; Wagner, M.; Nielsen, J.L.; Nielsen, P.H. Resolving the individual contribution of key microbial populations to enhanced biological phosphorus removal with Raman–FISH. ISME J. 2019, 13, 1933–1946.
- Zhang, Y.; Kinyua, M.N. Identification and classification of the Tetrasphaera genus in enhanced biological phosphorus removal process: A review. Rev. Environ. Sci. Technol. 2020, 19, 699–715.
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66.
- Izadi, P.; Eldyasti, A. Understanding microbial shift of Enhanced Biological Phosphorus Removal process (EBPR) under different Dissolved Oxygen (DO) concentrations and Hydraulic Retention Time (HRTs). Biochem. Eng. J. 2021, 166, 107833.
- Kristiansen, R.; Nguyen, H.T.T.; Saunders, A.M.; Nielsen, J.L.; Wimmer, R.; Le, V.Q.; McIlroy, S.J.; Petrovski, S.; Seviour, R.J.; Calteau, A.; et al. A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal. ISME J. 2013, 7, 543–554.
- Nguyen, H.T.T.; Kristiansen, R.; Vestergaard, M.; Wimmer, R.; Nielsen, P.H. Intracellular Accumulation of Glycine in Polyphosphate-Accumulating Organisms in Activated Sludge, a Novel Storage Mechanism under Dynamic Anaerobic-Aerobic Conditions. Appl. Environ. Microbiol. 2015, 81, 4809–4818.
- Roy, S.; Guanglei, Q.; Zuniga-Montanez, R.; Williams, R.B.; Wuertz, S. Recent advances in understanding the ecophysiology of enhanced biological phosphorus removal. Curr. Opin. Biotechnol. 2021, 67, 166–174.
- Liu, R.; Hao, X.; Chen, Q.; Li, J. Research advances of Tetrasphaera in enhanced biological phosphorus removal: A review. Water Res. 2019, 166, 115003.
- Welles, L.; Tian, W.; Saad, S.; Abbas, B.; Lopez-Vazquez, C.; Hooijmans, C.; van Loosdrecht, M.; Brdjanovic, D. Accumulibacter clades Type I and II performing kinetically different glycogen-accumulating organisms metabolisms for anaerobic substrate uptake. Water Res. 2015, 83, 354–366.
- Wisniewski, K.; Kowalski, M.; Makinia, J. Modeling nitrous oxide production by a denitrifying-enhanced biologically phosphorus removing (EBPR) activated sludge in the presence of different carbon sources and electron acceptors. Water Res. 2018, 142, 55–64.
- Levin, G.V.; Shapiro, J. Metabolic Uptake of Phosphorus by Wastewater Organisms. Water Pollut. Control. Fed. 1965, 37, 800–821.
- Srinath, E.G.; Sastry, C.A.; Pillai, S.C. Rapid removal of phosphorus from sewage by activated sludge. Experientia 1959, 15, 339–340.
- Barnard, J.L. Cut P and N without chemicals. Water Wastes Eng. Part 1 1974, 11, 33–36.
- Barnard, J.L. Cut P and N without chemicals. Water Wastes Eng. Part 2 1974, 11, 41–43.
- Barnard, J.L. Nutrient removal in biological systems. Water Pollut. Control 1975, 74, 143–154.
- Levin, G.V.; Topol, G.J.; Tarnay, A.G. Operation of full-scale biological phosphorus removal plant. Water Pollut. Control Fed. 1975, 47, 577–590.
- Fuhs, G.W.; Chen, M. Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microb. Ecol. 1975, 2, 119–138.
- Wagner, M.; Loy, A.; Nogueira, R.; Purkhold, U.; Valeeva, A.V.; Daims, H. Microbial community composition and function in wastewater treatment plants. Antonie Leeuwenhoek 2002, 81, 665–680.
- Bond, P.L.; Hugenholtz, P.; Keller, J.; Blackall, L.L. Bacterial community structures of phosphate-removing and non-phosphate-removing activated sludges from sequencing batch reactors. Appl. Environ. Microbiol. 1995, 61, 1910–1916.
- Bond, P.L.; Keller, J.; Blackall, L.L. Characterisation of enhanced biological phosphorus removal activated sludges with dissimilar phosphorus removal performances. Water Sci. Technol. 1998, 37, 567–571.
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300.
- McIlroy, S.J.; Saunders, A.; Albertsen, M.; Nierychlo, M.; McIlroy, B.; Hansen, A.A.; Karst, S.M.; Nielsen, J.L.; Nielsen, P.H. MiDAS: The field guide to the microbes of activated sludge. Database 2015, 2015, bav062.
- Henze, M.; Gujer, W.; Mino, T.; van Loosdrecht, M. Activated Sludge Models, ASM1, ASM2, ASM2d and ASM3. Scientific and Technical Report (Volume 5); IWA Publishing: London, UK, 2000.
- Jenkins, D.; Wanner, J. Activated Sludge—100 Years and Counting; IWA Publishing: Glasgow, UK, 2014.
- Bertanza, G.; Menoni, L.; Capoferri, G.U.; Pedrazzani, R. Promoting biological phosphorus removal in a full scale pre-denitrification wastewater treatment plant. J. Environ. Manag. 2020, 254, 109803.
- Hanada, S.; Liu, W.-T.; Shintani, T.; Kamagata, Y.; Nakamura, K. Tetrasphaera elongata sp. nov., a polyphosphate-accumulating bacterium isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 2002, 52, 883–887.
- Maszenan, A.; Seviour, R.; Patel, B.; Schumann, P.; Burghardt, J.; Tokiwa, Y.; Stratton, H. Three isolates of novel polyphosphate-accumulating gram-positive cocci, obtained from activated sludge, belong to a new genus, Tetrasphaera gen. nov., and description of two new species, Tetrasphaera japonica sp. nov. and Tetrasphaera australiensis sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 593–603.
- Stokholm-Bjerregaard, M.; McIlroy, S.J.; Nierychlo, M.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. A Critical Assessment of the Microorganisms Proposed to be Important to Enhanced Biological Phosphorus Removal in Full-Scale Wastewater Treatment Systems. Front. Microbiol. 2017, 8, 718.
- Seviour, R.J.; Mino, T.; Onuki, M. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 2003, 27, 99–127.
- Seviour, R.J.; McIlroy, S.J. The microbiology of phosphorus removal in activated sludge processes-the current state of play. J. Microbiol. 2008, 46, 115–124.
- Petriglieri, F.; Singleton, C.; Peces, M.; Petersen, J.F.; Nierychlo, M.; Nielsen, P.H. “Candidatus Dechloromonas phosphatis” and “Candidatus Dechloromonas phosphovora”, two novel polyphosphate accumulating organisms abundant in wastewater treatment systems. BioRxiv 2020.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
01 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




