Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xianyuan Wei | -- | 2622 | 2022-10-31 09:09:05 | | | |
| 2 | Rita Xu | Meta information modification | 2622 | 2022-10-31 09:47:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wei, X.; Du, M.; Chen, Z.; Yuan, Z. Bacteria-Based Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/32028 (accessed on 07 February 2026).
Wei X, Du M, Chen Z, Yuan Z. Bacteria-Based Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/32028. Accessed February 07, 2026.
Wei, Xianyuan, Meng Du, Zhiyi Chen, Zhen Yuan. "Bacteria-Based Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/32028 (accessed February 07, 2026).
Wei, X., Du, M., Chen, Z., & Yuan, Z. (2022, October 31). Bacteria-Based Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/32028
Wei, Xianyuan, et al. "Bacteria-Based Cancer Treatment." Encyclopedia. Web. 31 October, 2022.
Copy Citation
Cancer refers to a disease involving abnormal cells that proliferate uncontrollably and can invade normal body tissue. It was estimated that at least 9 million patients are killed by cancer annually. Recent studies have demonstrated that bacteria play a significant role in cancer treatment and prevention. Owing to its unique mechanism of abundant pathogen-associated molecular patterns in antitumor immune responses and preferentially accumulating and proliferating within tumors, bacteria-based cancer immunotherapy has recently attracted wide attention.
tumor therapy
engineered bacteria
bacteria-based cancer treatment
1. Introduction
Cancer refers to a disease involving abnormal cells that proliferate uncontrollably and can invade normal body tissue. According to the World Health Organization (WHO), cancer is the second leading cause of death worldwide [1]. It was estimated that at least 9 million patients are killed by cancer annually. Conventional therapies for cancer include surgery, chemotherapy, and radiotherapy. However, the downside of these traditional cancer treatment methods is that patients often suffer from various side effects during treatment. In particular, conventional treatment exhibits low specificity, leading to drug resistance in cancer cells.
Meanwhile, bacteria also have played an important role in maintaining good health and preventing diseases from healthier environments for millions of years. It is estimated that the human body contains trillions of bacteria [2]. The human gastrointestinal tract is the largest reservoir of commensal bacteria [3]. Intestinal bacteria such as Firmicutes, Bacteroides, Actinomycetes, and Enterobacteriaceae promote human health by synthesizing vitamin K, preventing colonization of pathogens, and maintaining the homeostasis of intestines [4]. There are more than 500 strains of bacteria such as Streptococcus and Actinomycetes in the mouth, which forms a protective biofilm on the surface of the teeth [5][6][7]. Additionally, Lactobacillus is dominant in the human vagina, maintaining the pH homeostasis of the environment by secreting lactic acid and inhibiting the interaction of other bacteria with epithelial cells [8]. By contrast, Lactobacillus, Staphylococcus, Streptococcus, etc., exist in the skin and nasal cavity, protecting the human body from other pathogens [7][9]. More importantly, recent studies have demonstrated that bacteria play a significant role in cancer treatment and prevention.
2. Bacterial Components of Antitumor Treatment
To date, bacterial toxins produced by bacterial cells, such as the Coley toxin, diphtheria toxin, Clostridium perfringens enterotoxin, bacterial enzymes L-asparaginase and arginine deaminase, and biosurfactant, such as surface and prodigiosin-like pixels, is able to effectively inhibit tumor growth through cell-cycle arrest, tumor-cell signal-pathway interruption, and other mechanisms. In addition, the components of bacteria, bacterial outer surface, the bacterial membrane, bacterial wall, and biofilm can also specifically activate the immune response to kill tumor cells.
2.1. Bacterial Toxins
In 1891, Dr. Coley successfully cured cancer patients with a mixture of live bacteria and “Coley toxin” heat-inactivated bacteria Streptococcus pyogenes and Bacillus mirabilis, opening the door to bacterial treatment of cancer [10][11]. Subsequent studies illustrated that Coley toxins include exotoxins produced by Streptococcus pyogenes and Serratia marcescens. In addition, S. pyogenes can produce pyrogenic exotoxins SpeA, SpeB, and SpeC, which have the ability to nonspecifically stimulate CD4+ lymphocytes, resulting in stronger secretion of different cytokines [12]. Similarly, prodigiosin, produced by S. marcescens, is a low molecular weight, red pigment, and heterocyclic tripyrrole toxin with antitumor activity, causing fever and potential antitumor immune response when combined with other components in the preparation [13].
Diphtheria toxin is a toxic protein produced by Corynebacterium diphtheria, while DTAT is its modified form, which targets the vascular endothelium of the tumor, results in the regression of cancer tissues in mice [14]. Clostridium difficile toxin includes two subtypes of cytotoxin (TcdB) and enterotoxin (TadA), which can kill cancer cells by recruiting proinflammatory factors to activate immune response [15]. Clostridium perfringens enterotoxin produced by C. perfringens also has anticancer activity, which leads to dose-dependent acute toxicity by binding to the overexpressed claudin-4 receptor on pancreatic cancer cells [16]. In addition, Verotoxin 1 (vt-1) is produced by pathogenic Escherichia coli and its function is to arrest the cancer cell cycle. Exotoxin A (PE) synthesized by Pseudomonas aeruginosa inhibits protein synthesis through ADP ribosylation, leading to cancer cell apoptosis [17][18]. The recombinant protein has better anticancer activity by modifying the cell structure recognition domain of the protein and preserving the membrane translocation domain and ADP ribosylation domain [15]. Hemolysin produced by bacteria, such as hemolysin A produced by E. coli clyA gene and hemolysin O produced by Listeria monocytogenes, is toxic to cancer cells. As a bacterial virulence factor, Listeria monocytogenes is released from phagocytes by perforating the phagocyte membrane. This phagosome escape mechanism enables Listeria monocytogenes to finally induce the immune response through MHC class I molecules in the cytoplasm with the protein expressed by the vector as an endogenous antigen [19].
2.2. Bacterial Enzymes
Bacterial enzyme L-asparaginase from Escherichia coli is an effective cancer therapeutic agent, which can inhibit the progression of malignant cells by activating asparagine hydrolysis and reducing its blood concentration, thereby causing toxicity to the MCF-7, HepG2, and SK-LU-1 cell lines. Bacterial-derived asparaginase has been approved for the treatment of acute lymphoblastic leukemia and non-Hodgkin’s lymphoma [20]. In addition, Fiedler et al. demonstrated that Streptococcus pyogenes produces arginine deaminase, which can consume arginine in tumor cells, resulting in decreased proliferation of arginine-deficient tumor glioblastoma multiforme [21].
2.3. Biosurfactant
Cyclic lipopeptide is an example of a biosurfactant with extensive antibacterial and antitumor activities that is produced by Bacillus subtilis natto TK-1. Xiaohong Cao et al. demonstrated that cyclic lipopeptide inhibited proliferation of human breast cancer MCF-7 cells by inducing apoptosis and increasing ion calcium concentration in the cytoplasm. Flow cytometric analysis revealed that cyclic lipopeptide caused dose- and time-dependent apoptosis through cell arrest at G(2)/M phase [22]. Another lipopeptide such as surfactin, have also been demonstrated their potential antitumor activity against several cancer cell lines. [23]. Surfactin induces the increase in calcium ions in human breast cancer MCF-7 cells and the accumulation of tumor suppressor p53 and cyclin kinase inhibitor p21, leading to cell-cycle arrest and apoptosis [22]. The same genus Marine Bacillus subtilis sp., can also produce an L-lysine biopolymer Epsilon-poly-L-lysine with antibacterial and anticancer activity. Studies have shown that Epsilon-poly-L-lysinet has obvious cytotoxicity on the cervical adenocarcinoma cell HeLaS3 and liver cancer cell HepG2 [24]. Pseudomonas libanensis m9-3 produces a cyclic lipopeptide named viscosin with extensive antibacterial and antitumor activities. The MTT results indicated that viscosin inhibited the proliferation of MDA-MB-231 in breast cancer at 15 uM concentration. Moreover, viscosin also inhibited the migration of the prostate cancer cell line PC-3M [25][26]. The cyclic peptide AT514 (serratamolide) from Serratia marcescens is cytotoxic to B-cell chronic lymphocytic leukemia and induces endogenous apoptosis by activating the release of caspase-3, the antitumor function of which was confirmed in mouse experiments [27]. A variety of secondary metabolites, prodigiosin-like fragments, BE18591 and roseophilin with antitumor activity were isolated from Streptomyces sp. BE18591 inhibited the growth of the human Thomas cancer cell MKN-45 [28]. Roseophilin binds to the intracellular antiapoptotic receptor Mcl-1 and induces apoptosis of cancer cells [29][30]. Prodigiosin-like fragments showed significant cytotoxic activity against the colon cancer cell line HCT-116, liver cancer cell line HepG-2, and breast cancer cell line MCF-7. Table 1 lists biosurfactants with cancer cell proliferation, which are known as antitumor agents and inhibit some cancer progression processes. Biosurfactants show promising application in microemulsion-based drug formulations. Microemulsion comprises an aqueous phase, an oil phase, and a surfactant, which can encapsulate or solubilize a hydrophobic or hydrophilic drug for antitumor therapy. The combination of biosurfactant and liposome also demonstrates specific targeting to cancer cell. Shim, Ga Yong et al. revealed that surfactin enhanced cellular delivery of liposome siRNA in Hela cells. In this way, it was possible to improve the antitumor effectiveness of those nanoparticles [31]. Biosurfactants have application in broad-spectrum antitumor treatments and are viewed as safe vehicles or ingredients in drug-delivery systems.
Table 1. Biosurfactants with antitumor activity against cancer cells.
| Biosurfactant | Cancer Type | References | |
|---|---|---|---|
| Cyclic lipopeptide | Bacillus subtilis natto TK-1 | Breast cancer | [22] |
| Surfactin | Bacillus subtilis natto T-2 | Breast cancer | [23] |
| L-lysine biopolymer Epsilon-poly-L-lysine | Marine Bacillus subtilis sp. | Liver carcinoma Cervix adenocarcinoma |
[24] |
| viscosin | Pseudomonas libanensis m9-3 | Breast cancer | [25] |
| AT514 | Serratia marcescens | B-cell chronic lymphocytic leukemia | [27] |
| BE18591 | Streptomyces sp. | Gastric cancer | [28] |
| Roseophilin | Streptomyces sp. | Hematologic cancer Colon cancer |
[29] |
2.4. Extracellular Surface
Exopolysaccharides (EPS) are carbohydrate compounds secreted by Gram-positive Lactobacillus bacteria outside the cell wall and usually infiltrated into the culture medium in the process of growth and metabolism. Some adhere to the microbial cell wall to form a capsule, which are called capsular polysaccharides. They have a dose-dependent and time-dependent antitumor effect of antiproliferation, and they promote apoptosis in anticancer activity [32]. The S-layer that is composed of protein and glycoprotein on the outermost cell surface of Gram-positive bacteria also has antitumor activity. Studies have shown that the S-layer protein of Lactobacillus acidophilus CICC 6074 can be regarded as a potential antitumor drug [33].
2.5. Bacterial Cell Membrane
The bacterial membrane components used in anticancer treatment include the cytoplasmic membrane vesicles of Gram-positive bacteria and the outer membrane vesicles of negative bacteria, as well as membrane fragments. Because of its rich pathogen-associated molecular patterns (PAMPs), the bacterial membrane is recognized by antigen-presenting cells (APCs) and activates the immune activity of T cells. It can also bind and activate the toll-like receptor (TLR), which regulates the production of proinflammatory cytokines, such as IL-12, and other constituent molecules, such as CD40. Subsequently, these mediators produce interferon (IFN)-γ and start the Th1-dependent immune response, mainly mediated by CD8+ effector cells, which induces a strong immune response against cancer cells in the tumor microenvironment [34][35].
The results of Min Li et al. showed that the PAMP on E. coli outer membrane vesicles (OMVs) was effectively recognized and internalized by neutrophils in revascularization. Neutrophils then crossed the blood vessels and guided OMVs to target inflammatory tumors (Figure 1) [36].
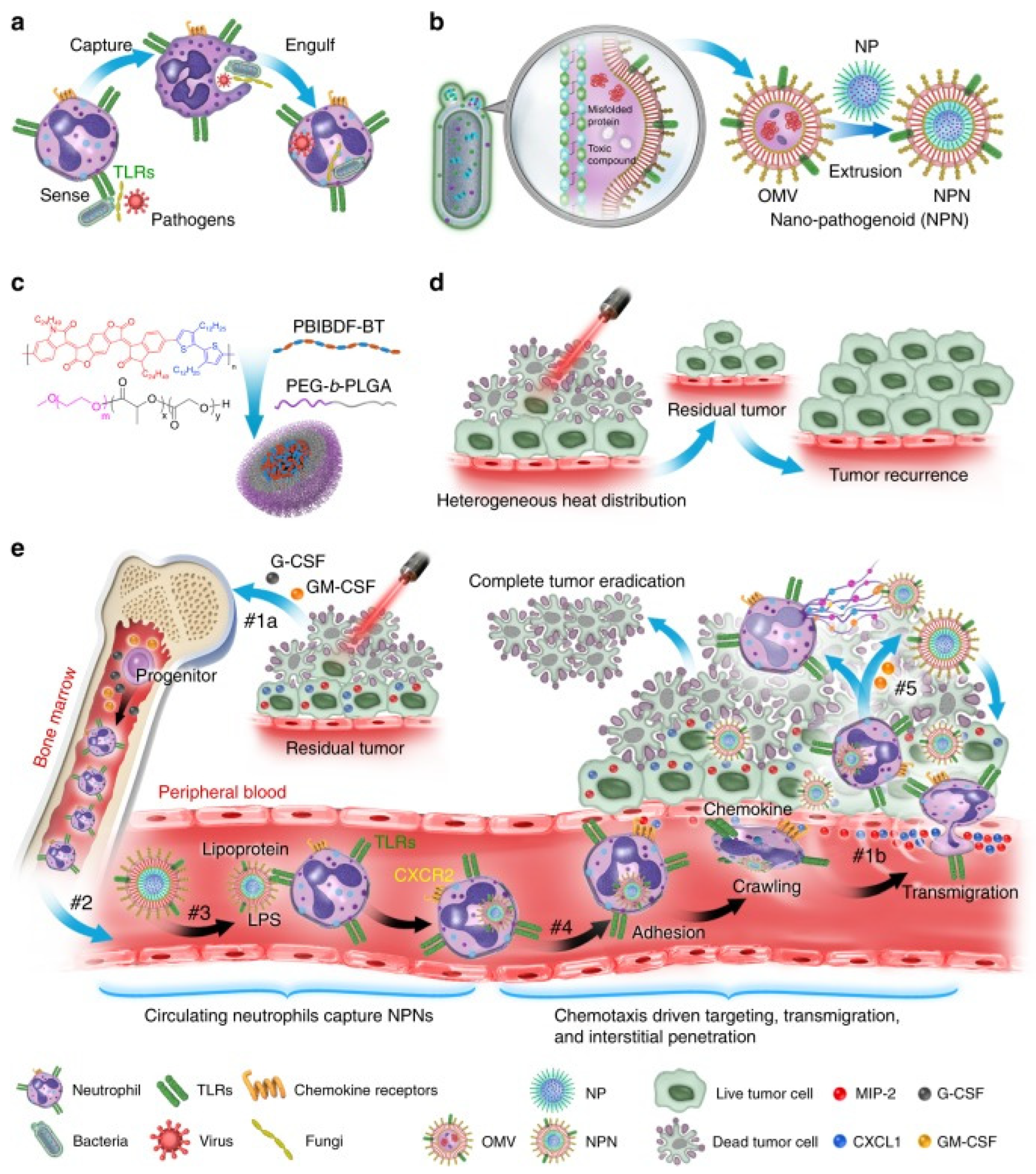
Figure 1. Schematic illustration showing the chemotaxis-driven delivery of NPNs for complete eradication of tumors post-phototherapy. (a) Neutrophils sense, capture, and engulf pathogens by recognizing the PAMPs with toll-like receptors (TLRs). (b) Preparation of NPNs by coating the OMVs on NPs, which inherit PAMPs from the OMVs. (c) Preparation of PEG-b-PLGA NPs encapsulating PBIBDF-BT (PBT) as a photothermal transducer. (d) The limited penetration of laser light used in PTT causes heterogeneous heat distribution within the tumor tissue and incomplete eradication of tumors, thus leading to tumor recurrence. (e) Treatment-induced cell death creates an inflammatory environment of the residual tumor and induces the production of granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), and chemokines CXCL1 and MIP-2. #1a The released G-CSF and GM-CSF increase neutrophil production from bone marrow. #1b The released CXCL1 and MIP-2 broadcast the location of the inflamed tumor. #2 Neutrophils enter the blood circulation and encounter the injected NPNs. #3 Neutrophils sense NPNs with the recognition of LPS and lipoprotein by TLRs and subsequently engulf them. #4 Neutrophils laden with NPNs are recruited into the tumor site in response to the chemokine gradient through the following cascade: adhesion, crawling, and transmigration. #5 NPNs are released from neutrophils to kill tumor cells along with the formation of NETs in the inflamed tumor (Adapted from reference [36] with permission).
The OMVs of Gram-negative bacteria are mostly used in anticancer research and are composed of a lipopolysaccharide (LPS), outer membrane protein (OMP), and PG similar to the outer membrane. β-Barrel assembly machine (BamA) protein guides and inserts the outer membrane protein of the OMV into the outer membrane of newly produced PG to induce the outer membrane maturation of cells [37]. Knocking out the phospholipid transporter vacj/yrb increases the production of OMVs in two Gram-negative bacteria: Haemophilus influenzae and Vibrio cholerae [38]. RNA binding protein L7ae and lysosomal escape protein listeriolysin O are modified on the surface of bacterial OMVs. L7ae specifically binds to the mRNA vaccine to deliver antigens to dendritic cells (DCs). Listeriolysin O mediates the phagosome escape mechanism. This OMV-based mRNA tumor vaccine delivery platform can significantly inhibit the growth of melanoma and colon cancer cells [39]. OMVs of Gram-negative bacteria hybridize with tumor-derived cell membranes (MTs) to form new functional vesicles. In vivo experiments showed that MT-OMVs can induce adaptive immune response and then inhibit lung metastasis of the tumor [40].
As a tool for the delivery of nanomaterial and vaccines, the bacterial membrane plays not only the role of antitumor cells, but also a role in antivirus and antibacterial infection. Hydrophobic drugs can be loaded through the incubation method with convenient operation, and common drugs can be loaded through electroporation, ultrasonic method, extrusion method, freezing cycle, and saponin treatment method. The bacterial membrane can be loaded with anticancer compounds, functional small RNA molecules, cancer cell antibodies, and cytokines, and can jointly eliminate cancer cells through chemotherapy, gene silencing or mutation, immunity, photothermal therapy, photodynamic therapy, and other methods. Bacterial extracellular vesicles (BEVs) existing in the tumor microenvironment can be engulfed by cells through antigen–antibody-specific binding or membrane fusion. The BEV then releases the cargo in the cytoplasmic space, allowing its nanomaterials to play a role leading to apoptosis, necrosis, or autophagy of cancer cells. For example, RNA drugs and antisense oligonucleotides were loaded into extracellular vesicles by ultrasound, which played a role in the mouse breast cancer model [41].
Different pathogens will prefer colonization of specific tissues, such as Klebsiella pneumonia infecting the lungs, Neisseria meningitidis and Listeria monocytogenes infecting brain nerves, which means specific bacterial membranes can be used to target the corresponding cancer sites. After incubating the OMV of Klebsiella pneumonia with doxorubicin, a broad-spectrum chemotherapy drug, and then mixing in PBS and removing the free doxorubicin, it quickly reached the vicinity of the lung tumor in A549 BALB/c nude mice, and TUNEL results indicated that it significantly induced tumor cell apoptosis (Figure 2) [42].
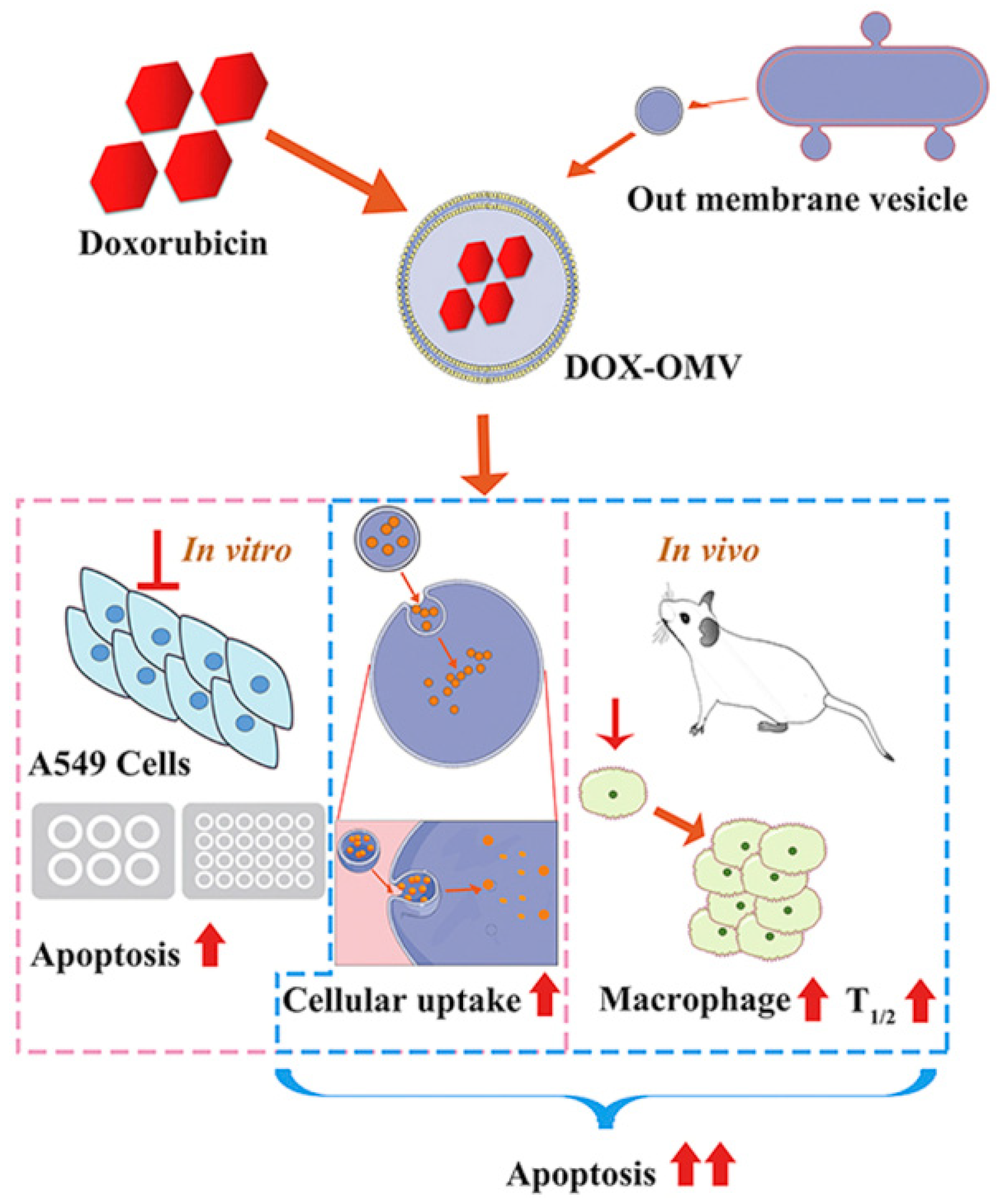
Figure 2. The attenuated Klebsiella pneumonia derived outer-membrane vesicles (OMVs), as a kind of biological drug-delivery carriers, are highly effective in transporting the chemotherapy drug doxorubicin (DOX) into nonsmall-cell lung cancer (NSCLC) A549 cells. Moreover, they can elicit appropriate immune responses, thereby enhancing the anti-NSCLC effect of DOX with no obvious toxicity in vivo (Adapted from reference [42] with permission).
2.6. Cell Wall
The bacterial cell wall is mainly composed of peptidoglycans. In addition to maintaining the shape of bacterial cells, peptide aggregation was also found to be related to regulating immune response, stimulating the production of tumor necrosis factor, interferon, and interleukin (IL-1, IL-6, IL-8, IL-12) [43].
2.7. Biofilm
Biofilm formed by bacteria also plays a role in tumor treatment. Biofilm is a glycoprotein lipid layer spontaneously formed by bacteria in the process of growth and attached to abiotic or biological surfaces including protein, DNA, metabolites, and so on. Biofilm forms in the tumor microenvironment and inhibits the growth, metastasis, and diffusion of tumor cells. The anticancer metabolites secreted by different bacteria are released in the biofilm, which can be accumulated and retained, so that they can be transferred to play a role in tumor cells [44][45].
2.8. Dormant Spores
In a harsh environment, bacteria will produce dormant spores. The spores of Clostridium can resist the harsh external environment and only revive when targeting the hypoxic tumor microenvironment. These advantages make anaerobic spores one way to target cancer cells. Studies have shown that Clostridium novyi NT spores do not contain lethal toxins, will not cause any systemic side effects in the injected host, and are an effective therapeutic agent for experimental tumors in mice [46]. The spores of Clostridium spp. are transformed into the active state in the tumor microenvironment only because of their strict anaerobic nature and are used in cancer treatment [47].
2.9. Magnetosomes
Magnetosomes are unique prokaryotic organelles containing 35 and 120 nm sized magnetite (Fe3O4) or cinerite (Fe3S4) magnetic iron mineral crystals surrounded by phospholipid bilayers. Mature magnetosomes are arranged in chains in the bacterial cytoplasm to form magnetosome chains, which cause magnetotactic bacteria to swim in the direction of the Earth’s magnetic field line [48][49]. Magnetotropic bacteria (MTB) are natural biomineralized bacteria that synthesize multiple magnetic nanoparticle chains in their own cytoplasm and can sense external magnetic fields. In order to detect and treat cancer, the bacteria can be combined with chemotherapy or radiotherapy drugs to target their delivery through magnetic force [50].
References
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, E180–E190.
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533.
- van Schaik, W. The human gut resistome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140087.
- Dulal, S.; Keku, T.O. Gut Microbiome and Colorectal Adenomas. Cancer J. 2014, 20, 225–231.
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60.
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Burgad, D.; Landay, A.; Weber, K.M.; Cohen, M.; Ravel, J.; Spear, G.T. Free Glycogen in Vaginal Fluids Is Associated with Lactobacillus Colonization and Low Vaginal pH. PLoS ONE 2014, 9, e102467.
- De Boeck, I.; Broek, M.F.V.D.; Allonsius, C.N.; Spacova, I.; Wittouck, S.; Martens, K.; Wuyts, S.; Cauwenberghs, E.; Jokicevic, K.; Vandenheuvel, D.; et al. Lactobacilli Have a Niche in the Human Nose. Cell Rep. 2020, 31, 107674.
- Nauts, H.C.; E Swift, W.; Coley, B.L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 1946, 6, 205–216.
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158.
- Dmitrieva, N.F.; Trofimov, D.I.; Eshchina, A.S.; A Riapis, L.; Pavlova, O.G.; Petrova, T.V.; A Skorkina, I.; Gerasimov, A.N.; Alekseev, L.P.; Zhuravlev, M.V.; et al. Frequency of genes speA, speB, and speC in Streptococcus pyogenes strains and the identification of the infective agent by polymerase chain reaction. J. Microbiol. Epidemiol. Immunobiol. 2002, 3–6.
- Elahian, F.; Moghimi, B.; Dinmohammadi, F.; Ghamghami, M.; Hamidi, M.; Mirzaei, S.A. The Anticancer Agent Prodigiosin Is Not a Multidrug Resistance Protein Substrate. DNA Cell Biol. 2013, 32, 90–97.
- Lewis, D.J.; Dao, H.; Nagarajan, P.; Duvic, M. Primary cutaneous anaplastic large-cell lymphoma: Complete remission for 13 years after denileukin diftitox. JAAD Case Rep. 2017, 3, 501–504.
- Siegall, C.B.; Chaudhary, V.K.; Fitzgerald, D.J.; Pastan, I. Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. J. Biol. Chem. 1989, 264, 14256–14261.
- Michl, P.; Buchholz, M.; Rolke, M.; Kunsch, S.; Löhr, M.; McClane, B.; Tsukita, S.; Leder, G.; Adler, G.; Gress, T.M. Claudin-4: A new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 2001, 121, 678–684.
- Rommasi, F. Bacterial-Based Methods for Cancer Treatment: What We Know and Where We Are. Oncol. Ther. 2022, 10, 23–54.
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015, 6, 963.
- Singh, R.; Dominiecki, M.E.; Jaffee, E.M.; Paterson, Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 2005, 175, 3663–3673.
- Mayakrishnan, V.; Kannappan, P.; Tharmalingam, N.; Bose, R.J.C.; Madheswaran, T.; Ramasamy, M. Bacterial cancer therapy: A turning point for new paradigms. Drug Discov. Today 2022, 27, 2043–2050.
- Fiedler, T.; Strauss, M.; Hering, S.; Redanz, U.; William, D.; Rosche, Y.; Classen, C.F.; Kreikemeyer, B.; Linnebacher, M.; Maletzki, C. Arginine deprivation by arginine deiminase of Streptococcus pyogenes controls primary glioblastoma growth in vitro and in vivo. Cancer Biol. Ther. 2015, 16, 1047–1055.
- Cao, X.; Wang, A.H.; Jiao, R.Z.; Wang, C.L.; Mao, D.Z.; Yan, L.; Zeng, B. Surfactin induces apoptosis and G(2)/M arrest in human breast cancer MCF-7 cells through cell cycle factor regulation. Cell Biochem. Biophys. 2009, 55, 163–171.
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675.
- El-Sersy, N.A.; Abdelwahab, A.E.; Abouelkhiir, S.S.; Abou-Zeid, D.M.; Sabry, S.A. Antibacterial and anticancer activity of epsilon-poly-L-lysine (epsilon-PL) produced by a marine Bacillus subtilis sp. J. Basic Microbiol. 2012, 52, 513–522.
- Saini, H.S.; Barragán-Huerta, B.E.; Lebrón-Paler, A.; Pemberton, J.E.; Vázquez, R.R.; Burns, A.M.; Marron, M.T.; Seliga, C.J.; Gunatilaka, A.A.L.; Maier, R.M. Efficient Purification of the Biosurfactant Viscosin from Pseudomonas libanensis Strain M9-3 and Its Physicochemical and Biological Properties. J. Nat. Prod. 2008, 71, 1011–1015.
- De Vleeschouwer, M.; Van Kersavond, T.; Verleysen, Y.; Sinnaeve, D.; Coenye, T.; Martins, J.C.; Madder, A. Identification of the Molecular Determinants Involved in Antimicrobial Activity of Pseudodesmin A, a Cyclic Lipopeptide From the Viscosin Group. Front. Microbiol. 2020, 11, 646.
- Escobar-Diaz, E.; Lopez-Martin, E.M.; Hernandez del Cerro, M.; Puig-Kroger, A.; Soto-Cerrato, V.; Montaner, B.; Giralt, E.; Garcia-Marco, J.A.; Perez-Tomas, R.; Garcia-Pardo, A. AT514, a cyclic depsipeptide from Serratia marcescens, induces apoptosis of B-chronic lymphocytic leukemia cells: Interference with the Akt/NF-kappaB survival pathway. Leukemia 2005, 19, 572–579.
- Kojiri, K.; Nakajima, S.; Suzuki, H.; Okura, A.; Suda, H. A new antitumor substance, BE-18591, produced by a streptomycete. I. Fermentation, isolation, physico-chemical and biological properties. J. Antibiot. 1993, 46, 1799–1803.
- Bracken, J.D.; Carlson, A.D.; Frederich, J.; Nguyen, M.; Shore, G.C.; Harran, P.G. Tailored fragments of roseophilin selectively antagonize Mcl-1 in vitro. Tetrahedron Lett. 2015, 56, 3612–3616.
- Kawasaki, T.; Sakurai, F.; Hayakawa, Y. A Prodigiosin from the Roseophilin Producer Streptomyces griseoviridis. J. Nat. Prod. 2008, 71, 1265–1267.
- Shim, G.Y.; Kim, S.H.; Han, S.-E.; Kim, Y.; Oh, Y.J.A.J.P.S. Cationic surfactin liposomes for enhanced cellular delivery of siRNA. Asian J. Pharm. Sci. 2009, 4, 207–214.
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022; in press.
- Zhang, T.; Pan, D.; Yang, Y.; Jiang, X.; Zhang, J.; Zeng, X.; Wu, Z.; Sun, Y.; Guo, Y. Effect of Lactobacillus acidophilus CICC 6074 S-Layer Protein on Colon Cancer HT-29 Cell Proliferation and Apoptosis. J. Agric. Food Chem. 2020, 68, 2639–2647.
- Moriyama, K.; Nishida, O. Targeting Cytokines, Pathogen-Associated Molecular Patterns, and Damage-Associated Molecular Patterns in Sepsis via Blood Purification. Int. J. Mol. Sci. 2021, 22, 8882.
- Eletto, D.; Mentucci, F.; Voli, A.; Petrella, A.; Porta, A.; Tosco, A. Helicobacter pylori Pathogen-Associated Molecular Patterns: Friends or Foes? Int. J. Mol. Sci. 2022, 23, 3531.
- Li, M.; Li, S.; Zhou, H.; Tang, X.; Wu, Y.; Jiang, W.; Tian, Z.; Zhou, X.; Yang, X.; Wang, Y. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat. Commun. 2020, 11, 1126.
- Mamou, G.; Corona, F.; Cohen-Khait, R.; Housden, N.G.; Yeung, V.; Sun, D.; Sridhar, P.; Pazos, M.; Knowles, T.J.; Kleanthous, C.; et al. Peptidoglycan maturation controls outer membrane protein assembly. Nature 2022, 606, 953–959.
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016, 7, 10515.
- Li, Y.; Ma, X.; Yue, Y.; Zhang, K.; Cheng, K.; Feng, Q.; Ma, N.; Liang, J.; Zhang, T.; Zhang, L.; et al. Rapid Surface Display of mRNA Antigens by Bacteria-Derived Outer Membrane Vesicles for a Personalized Tumor Vaccine. Adv. Mater. 2022, 34, e2109984.
- Zou, M.-Z.; Li, Z.-H.; Bai, X.-F.; Liu, C.-J.; Zhang, X.-Z. Hybrid Vesicles Based on Autologous Tumor Cell Membrane and Bacterial Outer Membrane To Enhance Innate Immune Response and Personalized Tumor Immunotherapy. Nano Lett. 2021, 21, 8609–8618.
- Jayasinghe, M.K.; Pirisinu, M.; Yang, Y.; Peng, B.; Pham, T.T.; Lee, C.Y.; Tan, M.; Vu, L.T.; Dang, X.T.T.; Pham, T.C.; et al. Surface-engineered extracellular vesicles for targeted delivery of therapeutic RNAs and peptides for cancer therapy. Theranostics 2022, 12, 3288–3315.
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin. B 2020, 10, 1534–1548.
- Hamann, L.; El-Samalouti, V.; Ulmer, A.J.; Flad, H.-D.; Rietschel, E.T. Components of gut bacteria as immunomodulators. Int. J. Food Microbiol. 1998, 41, 141–154.
- Divyashree, M.; Prakash, S.K.; Aditya, V.; Aljabali, A.A.; Alzahrani, K.J.; Azevedo, V.; Góes-Neto, A.; Tambuwala, M.M.; Barh, D. Bugs as drugs: Neglected but a promising future therapeutic strategy in cancer. Future Oncol. 2022, 18, 1609–1626.
- Adnan, M.; Khan, S.; Al-Shammari, E.; Patel, M.; Saeed, M.; Hadi, S. In pursuit of cancer metastasis therapy by bacteria and its biofilms: History or future. Med. Hypotheses 2017, 100, 78–81.
- Diaz, L.A.; Cheong, I.; Foss, C.A.; Zhang, X.; Peters, B.A.; Agrawal, N.; Bettegowda, C.; Karim, B.; Liu, G.; Khan, K.; et al. Pharmacologic and Toxicologic Evaluation of C. novyi-NT Spores. Toxicol. Sci. 2005, 88, 562–575.
- Umer, B.; Good, D.; Anné, J.; Duan, W.; Wei, M.Q. Clostridial Spores for Cancer Therapy: Targeting Solid Tumour Microenvironment. J. Toxicol. 2012, 2012, 862764.
- Lower, B.H.; Bazylinski, D.A. The Bacterial Magnetosome: A Unique Prokaryotic Organelle. J. Mol. Microbiol. Biotechnol. 2013, 23, 63–80.
- Uebe, R.; Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637.
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-Garcia, J.; Das, R.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H.; Orue, I.; et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small 2019, 15, e1902626.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
31 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




