Plants represent a significant part of the human diet. Humans have utilized every part of plants for survival, and seeds are no exception. Seeds offer high protein, unsaturated fats, fibre, essential vitamins, and minerals for various food applications. They are also a promising reservoir of bioactive compounds, where various phytochemicals, such as polyphenolic compounds, capable of maintaining and improving well-being, are present in abundant quantities. Plants from Malvaceae and Cannabaceae families are known for their fibre-rich stems that benefit humankind by serving numerous purposes. For many centuries they have been exploited extensively for various commercial and industrial uses. Their seeds, which are often regarded as a by-product of fibre processing, have been scientifically discovered to have an essential role in combating hypercholesterolemia, diabetes, cancer, and oxidative stress.
1. Development of Sustainable Functional Ingredients and Functional Foods from Plant By-Products
By-products generated from various stages of agricultural practices such as fruit peels, fruit pomace, seeds, and cereal brans are rich in bioactive phytonutrients that can alternatively be recovered for many applications (e.g., as antioxidant sources in food supplements and cosmetic products) rather than being discarded and polluting the environment
[1]. Polyphenols are among the many sources of phytonutrients in the plant kingdom that can be extracted through solvent extraction (e.g., water, ethanol, and supercritical fluid) or solvent-free extraction (e.g., microwave-assisted and ultrasonic-assisted) modes
[2][3]. Currently, there is a rising trend in using natural antioxidants as a replacement to synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butyl hydroquinone (TBHQ) in food preservation and packaging
[4]. Although synthetic antioxidants are relatively cheaper and more stable than their natural counterparts, the safety of these chemicals is a matter of great concern
[5]. The use of synthetic antioxidants has been associated with a broad spectrum of environmental pollution (e.g., water contamination and poor air quality) and health hazards (e.g., allergies, oxidative stress, and DNA damage) following immoderate exposure
[6][7].
Currently, there are various eco-friendly methods that have been developed and can be employed for the efficient extraction of antioxidants from natural matrices such as plants, for example using deep eutectic solvents (DES) in addition to microwave-assisted (MAE), ultrasound-assisted (UAE), and supercritical fluid extractions (SFE). The microwave-assisted extraction approach is beneficial in increasing extraction yield and antioxidant activity of extracted phenolic compounds. It is also more efficient (i.e., shorter extraction time and lower temperature) than conventional extraction methods. Weremfo et al.
[8] compared MAE (using 58% ethanol for 5 min at 400 W) to conventional extraction (employing 56% ethanol for 23 min at 63 °C). Microwave-assisted extraction demonstrated increased total phenolic content (by 58.8%) and antioxidant activities according to 1-diphenyl-2-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays (by 21–25%) from avocado seeds. Moreover, phenolic compounds such as rutin, catechin, and syringic acid were also extracted from avocado seeds.
The supercritical fluid extraction (SFE) method is efficient and affordable; even just a tiny amount of phenolic compounds in plants can be retrieved. Furthermore, this process requires little or no solvent, and heat-sensitive bioactive constituents will be sustained throughout the extraction process. Buszewski et al.
[9] discovered many phenolic compounds from
Lupinus luteus seed extracts by utilizing carbon dioxide under supercritical conditions (Sc-CO
2). The extracts showed substantial antioxidant and antiradical properties, particularly rich in apigenin and fisetin. Furthermore, they were also non-cytotoxic and had antimicrobial characteristics.
In a recent study, Wu et al.
[10] extracted antioxidants from
Polygonum aviculare leaves using choline chloride and levulinic acid in a deep eutectic solvents-based ultrasonic-assisted extraction (ChCl-Lev-based UAE) method. As opposed to extractions by maceration, Soxhlet apparatus, and microwave, ChCl-Lev-based UAE extracted phenolics such as gallic acid, 5-caffeoylquinic acid, and 3-chlorogenic acid more efficiently. Deep eutectic solvents (DES) are more stable, less volatile, less toxic, and more biodegradable than conventional solvents such as methanol, acetone, and chloroform.
Contrasted with synthetic antioxidants, recovering polyphenols from natural sources does not involve using hazardous chemicals and emission of contaminants (e.g., greenhouse gases) into the atmosphere
[6]. High quantities of polar polyphenolic compounds can still be efficiently extracted from plant matrices by simply using water, the ‘greenest’ and cheapest solvent of all
[11]. Simple alcohols such as ethanol are also low in toxicity, biodegradable, low cost, and are thus an environmentally preferrable solvent
[12]. The advantages concerning health and safety of supercritical fluids such as water and carbon dioxide are prominent, as described by Knez et al.
[13]. They have also been affirmed as ‘generally recognized as safe’ (GRAS). Many novel methods applying solvent-free extractions have been used widely in agri-food and nutraceutical industries. The efforts to preserve natural antioxidants are seen to accelerate progress in meeting Goal 13 of the SDGs on climate action, that is, to take action to combat climate change and its impacts. Therefore, valorisation of underexploited plant sources for their value-added polyphenols could catalyse the global agenda to achieve not only Goal 13 but also the rest of the SDGs.
Seeds are not just essential for plants to produce the next generation but also contain commercial values because of their high nutritional values and functional properties. These properties rendered the usage of seeds into a variety of valuable products such as food ingredients for humans and animal feeds in the forms of seed oil, seed meal, seed milk, and more
[14][15]. For that reason, they have become a subject of interest in food science studies as part of the attempts to manage agricultural by-products while meeting nutritional needs and food demands from growing global population
[16]. Wide-ranging studies have been conducted to investigate the beneficial health effects of plant seeds. For example, referring to Ros and Hu
[17], higher consumption of plant seeds as well as grains, nuts, legumes, cocoa, and coffee beans can reduce the risk of chronic diet-related ailments such as cardiovascular diseases (CVD) and type 2 diabetes mellitus (T2DM). An abundance of plant seeds has been discovered to participate in chronic disease prevention, attributed to their antitumor, antidiabetic, antioxidant, and antiproliferative effects
[18].
2. Seeds: An Excellent Source of Polyphenolic Compounds
Polyphenolic compounds or polyphenols are naturally occurring elements with phenol units found exclusively and abundantly in plant-based foods (fruits, vegetables, cereals, grains, legumes, oilseeds, coffee, and tea), alongside their essential nutrients. They are secondary metabolites of plants with antioxidant and antiviral capabilities, produced to protect themselves against pathogens, support cell growth and division processes, and stimulate photosynthesis
[19]. Polyphenols are among other secondary metabolites (i.e., terpenoids and isoprenoids, alkaloids, and glucosinolates) biosynthesized in plants by the seven-step shikimic pathway usually occurring in the chloroplast
[20]. They are naturally present in specialized cells that are not required for primary photosynthesis or cellular respiration metabolism but are hypothesized to be imperative for plant survival in the environment. Secondary metabolites in plants defend against external factors such as ultra-violet radiation, pathogens, predators, oxidative stress, and extreme climatic conditions. Plants, unlike animals, cannot escape their biotic and abiotic stimuli because their root system attaches them to the soil. Plant phenolics can also be classified into two types: preformed phenolics or induced phenolics. Preformed phenolics are synthesized during normal plant tissue development, whereas plants synthesize induced phenolics in response to the aforementioned external stressors
[21].
In addition to performing roles in physiological processes in plants, polyphenols also contribute to their sensorial characteristics, particularly colour, flavour, aroma, and astringency. For instance, flavonoid-type polyphenols are the agents that impart a specific colour to different parts of plants, making them widely known as natural pigments
[22]. Flavonoids have also been reported to provide positive health effects, for example in an in vivo study by Zhu et al.
[23], it was demonstrated that flavonoids obtained from guava leaves (guaijaverin and avicularin) had antihyperglycemic, antihypercholesterolemic, and hepatic protective effects in diabetic mice. These beneficial effects were linked with inhibition of dipeptidyl peptidase IV by guaijaverin and reduction of glucose uptake (through glucose transporter type 4) by avicularin.
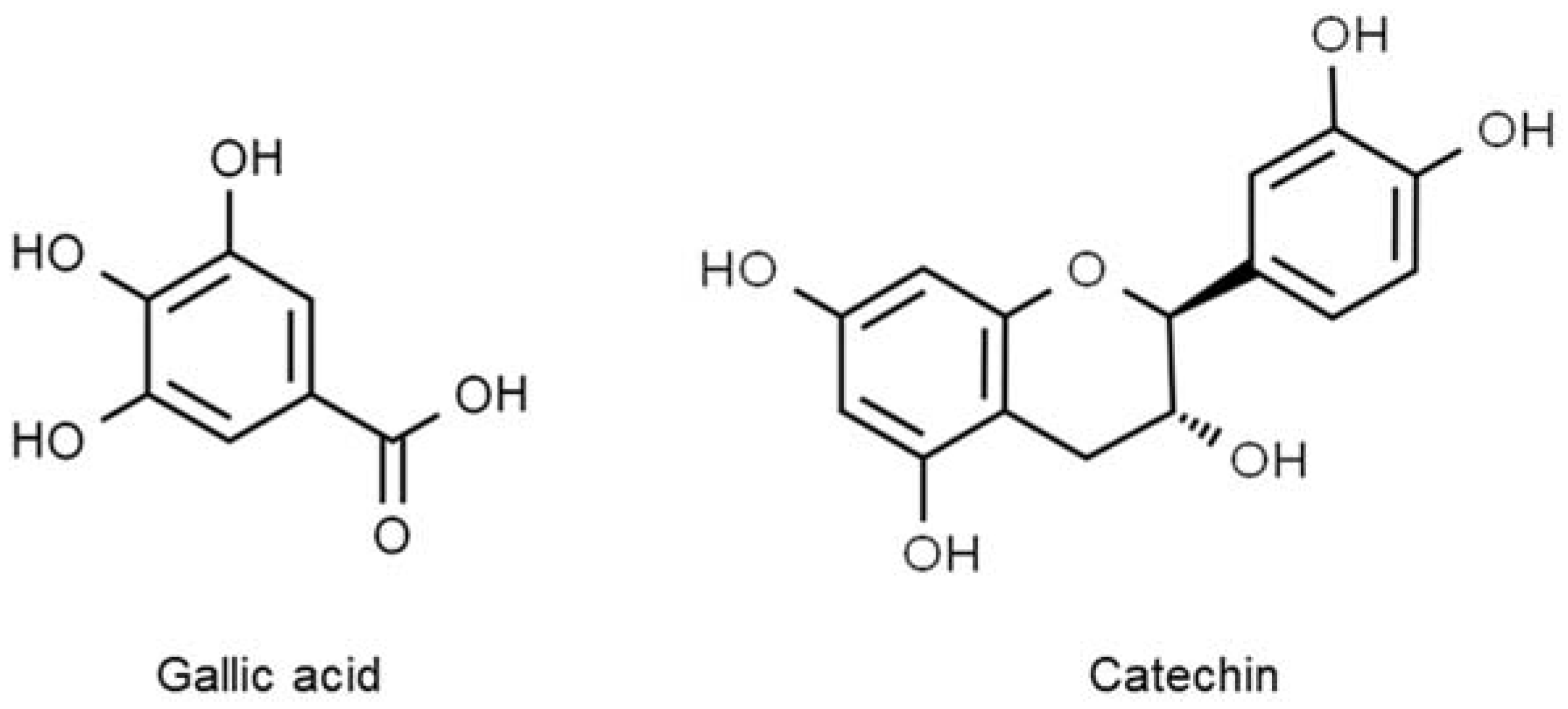
Polyphenols are broadly diverse in structure. They are all characterized by multiple phenol rings of at least three to five units and several hydroxyl groups. Several examples of polyphenols are gallic acid, isoflavones, tannins, and anthocyanins, which are commonly present in most plants (
Figure 1). They have been extensively exploited for many purposes, such as in the manufacture of dyes, ink, and supplements. The number of phenol rings and the type of linkages between the rings determine which class they belong to, either phenolic acids (non-flavonoids), flavonoids, stilbenes, or lignans
[24].
Figure 1. The structures of the most common polyphenols. Gallic acid, C7H6O5 (left), belongs to the phenolic acid class, whereas catechin, C15H14O6 (right), is part of the flavonoid class.
The functions of polyphenols in biological reactions and physiological processes are also wide-ranging. They have been well-investigated to confer numerous health benefits, especially in curbing the global health burden of non-communicable diseases (NCDs) by primarily acting as antioxidants. Antioxidants have pharmacological properties such as anti-inflammatory, anti-ageing, and anticancer, thus playing crucial roles as health-protecting compounds. They work by breaking harmful chain reactions and excessive cell proliferation occurring in people's body through the actions of unstable molecules called free radicals and pro-oxidants. Antioxidant molecules donate some of their electrons to neutralize and inactivate the free radicals from damaging other body cells, leading to the pathogenesis of many chronic diseases
[25].
Table 1 presents the major classes of polyphenols, selected polyphenolic compounds, the health benefits they render, and sources from underutilized seeds.
The agricultural and food-processing industries produce a huge number of unused by-products that can be spared and transformed into high-quality food products for the benefit of both animals and humans. In recent years, the global attempts to valorise agricultural wastes have been soaring as there has been a growing body of research that shows that polyphenols are also concentrated in agricultural residues such as fruit peels and seed meals. When consumed correctly and not reaching toxic levels, these bioactive compounds have essential roles in disease prevention, maintenance, and treatment
[42]. Thus, they are relevant to people's daily diet for optimal well-being. Despite that, studies on the bioavailability of polyphenols in whole plant-based foods are still scarce, and the metabolic fate of these antioxidants in the body is not fully known. Polyphenols are isolated and purified from plants (fruits, vegetables, and agricultural by-products) and transformed into supplements
[22]. Hence, acquiring optimum health benefits from polyphenols in the food matrix would require people to eat a balanced, moderate, and varied diet
[43][44].
Plant seeds are one of the richest sources of polyphenols, either concentrated in the seed coat or the cotyledon or both. Interestingly, several seeds have been labelled as “specialty seeds” or “super seeds” due to their remarkable biological activity, attributed to high levels of bioactivity and bioavailability. These tiny seeds are high in polyphenols and rich in monounsaturated fats, protein, vitamins, minerals, and fibre. Black cumin, chia, hemp, flax, perilla, pumpkin, quinoa, and sesame seeds are the specialty seeds that have been reported to be the most typically utilized in human diets for generations
[45]. Furthermore, some of these seeds are included in the dietary guidelines of nations such as the United States, Australia, and Qatar as part of the healthy dietary selection
[46][47][48]. Seeds are versatile because they may be consumed in several ways, including appetizers, cuisines (e.g., incorporated into cereals, salads, and various main meals), and food products such as bread and spreads. Other plant seeds and polyphenols detected in plants are summarized in
Table 1.
3. Seeds from Fibre Crops That Are Potential Sources of Polyphenolic Compounds
Okra, cotton, hemp, and kenaf plants are some examples of promising sources for natural polyphenols. They have been described as equally crucial as sustainable sources of natural fibre with many potential uses
[49]. However, the industrial fibre processing from these plants generates a large number of waste materials, including seeds that can alternatively be recovered and utilized for the addition of values (nutritional qualities, oxidative stability, and sensory properties), instead of being discarded
[12][50][51]. Today, many studies have demonstrated the widespread food applications of these seeds and their derivatives, attributable to the significant dietary constituents (protein, healthy fats, dietary fibre, vitamins, and minerals) essential for many bodily processes, as well as numerous dietary polyphenolic compounds that confer physiological benefits beyond essential nutrition
[51].
3.1 Nutritional Properties and Polyphenolic Contents of Okra Seeds against Diseases
Seeds of okra are made up of 20–35% protein content with reasonable amounts of amino acids comparable to those of soybeans. The Protein Efficiency Ratio (PER) of okra seeds is remarkably better than soybeans
[52][53][54]. The oil content in okra seeds is almost similar to other oilseeds, which is in the range of 20–40%, and the oil abundantly consists of PUFA such as linoleic acid. The seeds are also an essential source of phenolic constituents such as oligomeric catechins (2.5 mg/g of seeds) and flavonol derivatives (3.4 mg/g of seeds)
[55][56].
Okra seeds have been claimed to deliver beneficial health effects in many studies, as they have been found to have potential antidiabetic, antifatigue, antistress, and anticancer activities due to their generous amount of numerous phytochemicals
[56][57][58][59]. Those health-beneficial phytochemicals include isoquercitrin and quercetin-3-O-gentiobiose
[58]. The potential of okra seed extract as an antidepressant was scientifically proven in Xia et al.
[60] investigating male ICR mice. In the experiment, mice treated with aqueous okra seed extract up to 600 mg/kg body weight underwent a series of behavioural tests. They showed positive results in suppressing depression signs, namely hypothalamic–pituitary–adrenal (HPA) axis hyperactivity, oxidative stress, and imbalance of neurotransmitter levels in the hippocampus and frontal cortex of the brain. In support of these outcomes, applying UPLC-DAD/Q-TOF MS technology identified various phenolic compounds in okra seed extract and quantified them, constituting almost 30% of the extract. The main compounds were identified as catechin and quercetin derivatives. Therefore, it can be concluded that the antidepressant activity of okra seed extract might be due to the antioxidant activities of these flavonoids, which assist in reducing the effects of oxidative damage in the brain
[60].
Evidence strongly supports the notion that dietary polyphenols are beneficial in treating diabetes mellitus. Due to the excellent composition of polyphenolic compounds in okra seed extract, its antidiabetic property has also been investigated and confirmed in many studies. By employing in vitro α-glucosidase enzyme inhibition assay, okra seed extract was observed to confer an equivalent antidiabetic effect with acarbose, an antidiabetic medication
[61]. The efficacy of okra seed extract as an inhibitor to α-glucosidase activity could dampen carbohydrate digestion into glucose, thus lowering postprandial glucose and insulin levels in diabetic patients
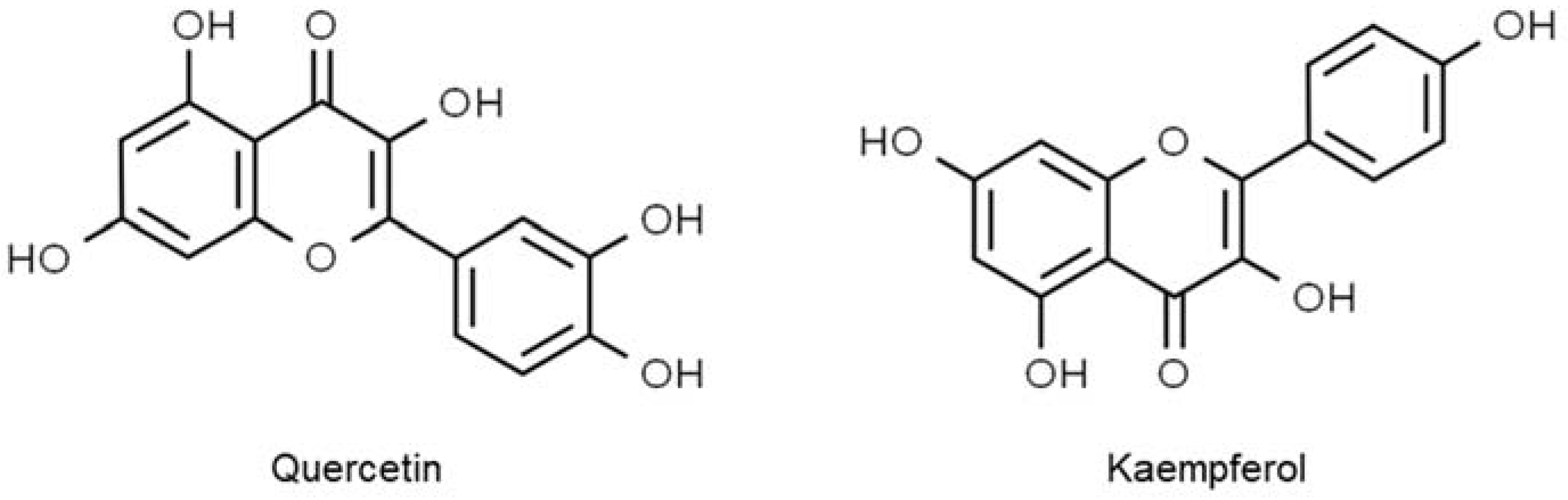
[62]. Among other polyphenols that were detected in okra seed extract, derivatives of quercetin (quercetin 3-O-(malonyl)-glucose and quercetin-3-o-glucose-xylose) were the ones with the highest concentrations, indicating that the antidiabetic effect in the study was explicitly contributed by these compounds (
Figure 2)
[62].
Figure 2. Polyphenolic compounds in okra seed.
In the study by Ong et al.
[61], polyphenol-rich extract from okra seed also has been demonstrated to be potentially vasoprotective. The promising vasoprotective effect of okra seed extract was achieved via several mechanisms. Quercetin in okra seed extract exhibited strong antioxidative and cytoprotective effects towards endothelial cells (HMEC-1) from the reactivity of hydrogen peroxide radicals, thereby improving impaired endothelial function. In addition to that is relief from vascular inflammation induced by an inflammatory cytokine, tumour necrosis factor-α (TNF-α). Inhibition of TNF-α efficiently reduced the expression of genes of two cell adhesion molecules, vascular cell adhesion molecule and E-selectin, which usually increased in untreated inflammation. As previously reported, quercetin also impeded the expression of other inflammatory factors, which are interleukin-1β (IL-1β) and interleukin-6 (IL-6) in vitro
[63]. Prevention of vascular oxidative stress can decrease the likelihood of contracting the risk factors of cardiovascular diseases such as hypertension, hyperglycaemia, and hypercholesterolemia, to name a few. As okra seed extract is antioxidative, vasoprotective, and possibly cardioprotective, it may be helpful as a potential complementary and alternative treatment in managing cardiovascular diseases. Many traditional societies and cultures have utilized okra and its parts for health maintenance and disease treatments
[64].
Uniquely, the intake of okra seed extract containing high levels of polyphenols can also produce an antifatigue effect in addition to its known capability as an antioxidant source. In one study, it was reported that okra seed extract showed highly remarkable in vitro antioxidant activities as measured through DPPH, FRAP, and reducing power assays as compared to extracts from okra pods and skins
[58]. Further, it was elucidated that the antifatigue property of okra was also contributed by its seeds, where polyphenols, namely isoquercitrin and quercetin-3-O-gentiobiose are found ubiquitously, in comparison to other okra constituents. The parameters associated with oxidative stress-related fatigue, such as levels of blood lactic acid (BLA) and blood urea nitrogen (BUN) and liver levels of malondialdehyde (MDA), are reduced considerably following okra seed extract treatment up to 0.6 g/kg body weight for 21 consecutive days in male ICR mice. On the other hand, hepatic glycogen (HG), total superoxide dismutase (SOD), and glutathione peroxidase (GSH) levels positively increased. Hence, these are the possible mechanisms on how quercetin and its derivatives in okra seed extract can mitigate fatigue, the results of which are similar to those found in a previous study
[65]. Moreover, recent literature also demonstrated that quercetin exhibits good antifatigue capacity through the enhancement of antioxidant activities, glycogen storage, and muscle function of male BALB/c mice after 6 weeks of 0.005% quercetin supplementation
[66].
3.2. Nutritional Properties and Polyphenolic Contents of Cotton Seeds against Diseases
There are two types of fibre which wrap the seeds of the cotton plant in a capsule called a ‘boll’, and a ‘ginning’ procedure can separate them. Cotton seeds have various uses as food due to their remarkable physicochemical and nutritional properties. Cottonseed protein is considerably high (20–25%). Its quality is outstanding, as measured by the amino acid composition and numerous functional properties. It was indicated that glandless cottonseed protein is nearly comparable or even better than that of soybean protein
[67][68]. Besides, the oil of the seeds (17–22%) is composed of fatty acids, namely palmitic acid (22%), oleic acid (20%), and linoleic acid (54%)
[69].
Gossypol is known to be toxic to the liver and reproductive organs
[70]. Nevertheless, many studies have also reported its desirable bioactivities towards health as an anti-inflammatory, anti-obesity, and antifungal agents, and as an anticancer drug for several cancer types
[71][72][73][74][75][76]. Promising anti-obesity activity of gossypol was exhibited in female laboratory rats, in which their body weight gain and appetite were curbed after 5 and 10 mg/kg body weight/day of gossypol treatment for 15 days
[77]. Further, in an in vitro assessment, cottonseed oil enriched with gossypol was also proven to control proliferation and adipogenesis of human pre-adipocytes. These positive health outcomes were attained through reductions in a few gene expressions, intracellular triglyceride level, and glycerol 3-phosphate dehydrogenase (GPDH) activity
[72]. Additionally, gossypol portrayed its potential in eliminating fungal pathogens through some possible modes of action. Two of these actions work by preventing fungal access to carbon and nitrogen, consequently inhibiting their cell wall formation, and obstructing the development of aflatoxisomes
[73]. On the other hand, gossypol performs potential cancer-fighting benefits by several vital processes such as altering a specific gene expression and inhibition of DNA synthesis so that cancer cells cannot proliferate
[78][79].
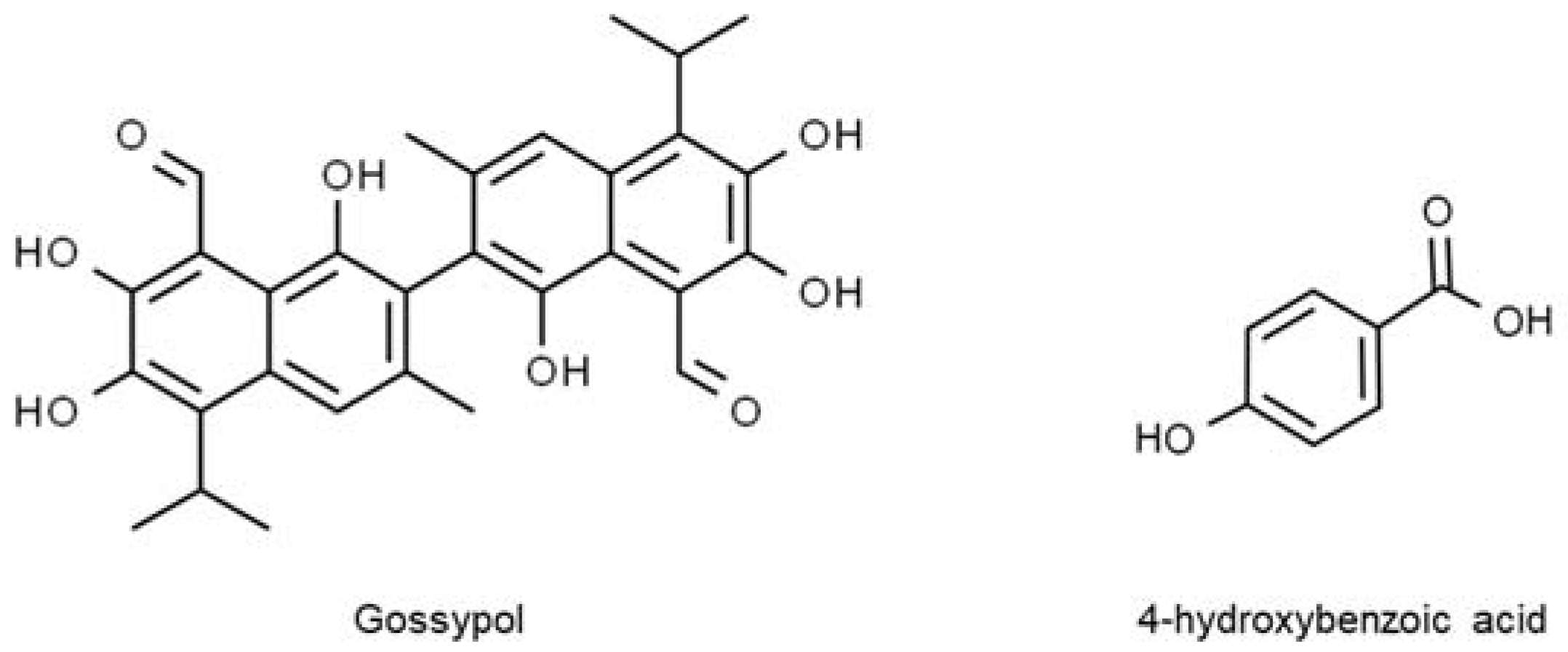
Ideally, glandless cottonseed devoid of dark spots (pigment glands) and the presence of gossypol exhibit safer and better health effects than glanded cottonseed. Moreover, other polyphenolic compounds with scientifically proven health benefits such as gallic acid, quercetin, flavonol glycosides, and 3,4-dihydroxybenzoic acid co-exist in cottonseed (
Figure 3). Due to that, gossypol detoxification is essential so that the role of these polyphenols as free radical scavengers in the prevention of many chronic illnesses can be maximized
[80]. In one study involving in vitro assays, ethanolic extracts of cottonseed either with or without glands were non-deleterious towards mouse macrophages and adipocytes. In addition, glandless cottonseed extract also showed great potential as an anti-inflammatory agent by stimulating an RNA-binding protein, tristetraprolin (TTP), to inhibit inflammation in the cells
[71]. Therefore, glandless cottonseed, which is as vital as other oilseeds such as soybeans and sesame seeds, can be fully utilized and developed into various food products and would consequently help stimulate economic growth in cotton-producing countries
[81][82][83].
Figure 3. Polyphenolic compounds in cotton seed.
3.3. Nutritional Properties and Polyphenolic Contents of Hemp Seeds against Diseases
Hemp seeds have high nutritional and commercial values due to their protein, fatty acid, carbohydrate, fibre content, and therapeutic compounds. Hemp seed contains a good amount of protein, between 25% to 30%, with an amino acid profile close to soybean and egg white proteins. Despite its protein quality, much attention is given to hemp seed oil
[84]. Similarly, oil content in hemp seeds is about 25–30%, and it is rich in unsaturated fatty acids such as oleic acid, linoleic acid, α-linolenic acid, γ-linolenic acid, and stearidonic acid, which is reported to be more than 90%. Moreover, hemp seed has the ideal ratio of ω-6 to ω-3 fatty acids (between 2:1 and 3:1) to prevent many chronic ailments such as heart disease, cancer, and rheumatoid arthritis
[85]. On the other hand, saturated fatty acids, namely palmitic acid and stearic acid, are present only in meagre amounts compared with other oilseeds
[86].
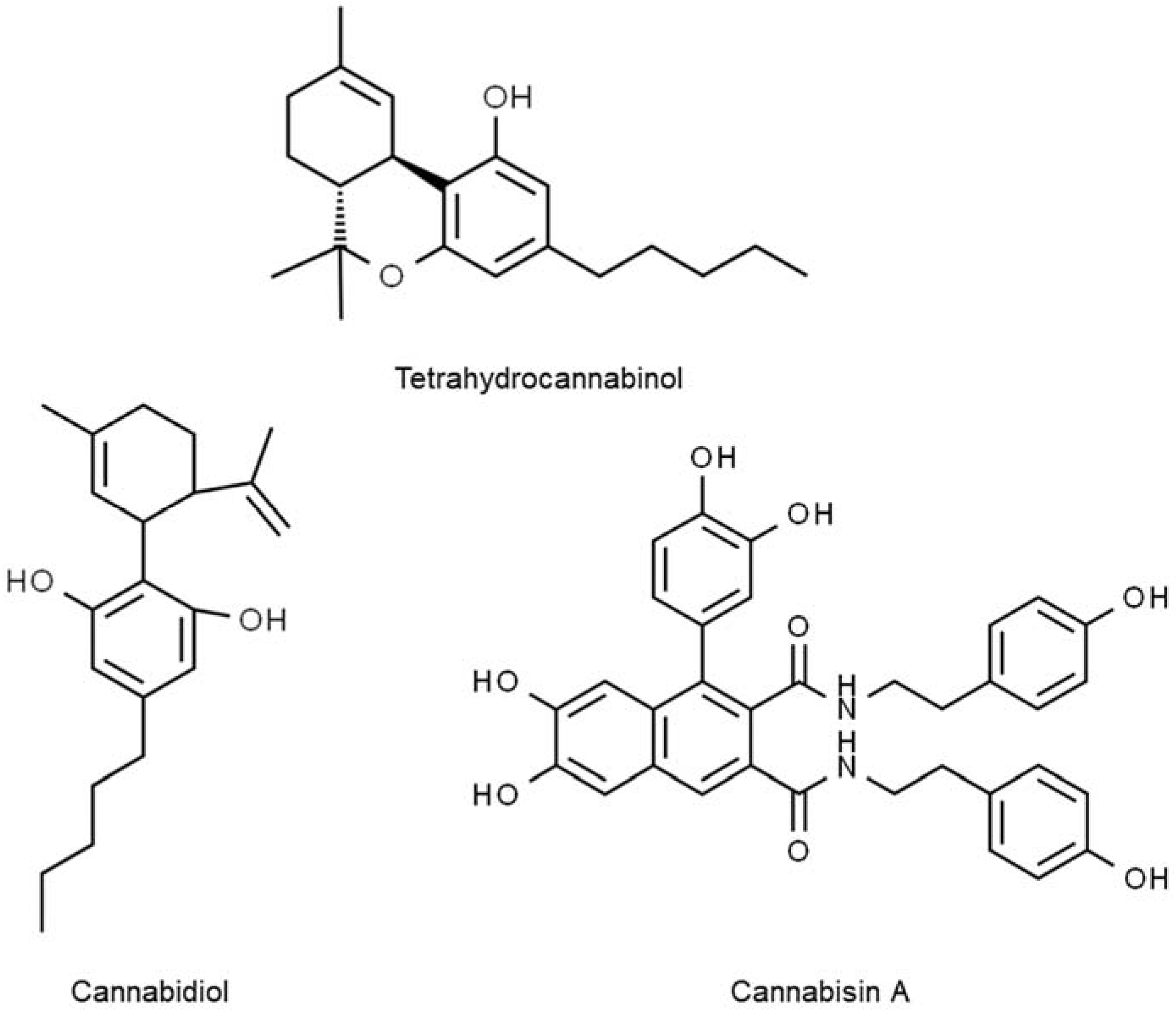
A group of chemical compounds that could give rise to psychoactive or hallucinogenic effects to people consuming it, identified as cannabinoids, are found in the flowers and fruits of hemp. Cannabinoids are terpene phenolics, a combination of terpenes and phenolic compounds exclusively synthesized in
C. sativa [84]. These compounds are also present in hemp seeds but in relatively smaller quantities than in the flowers and fruits. The first primary prevalent type of cannabinoids in hemp seeds is ∆-9-tetrahydrocannabinol (Δ-9-THC), as shown in
Figure 4. It is one of the commonly used drugs worldwide for medicinal purposes of alleviating stress and depression. However, Δ-9-THC can be intoxicating with excessive and long-term use. An enormous controversy centres around the human consumption of hemp seed and its derived food products. As a result of advances in food technology, hemp seed cultivation and consumption have become legal in countries such as Australia, Canada, and the United States, provided that the THC level is below 0.3%
w/
w [87]. The other prominent cannabinoid, cannabidiol (CBD), is non-psychoactive. Due to its polyphenolic nature and potent antioxidant activities, CBD has been declared safe and used to treat brain disorders such as epilepsy. Epilepsy is characterized by unusual brain activity, repeated seizures or erratic behaviour episodes, and reduced awareness. The roles of CBD as an antiepileptic agent involve controlling inflammation reaction, preventing nerve damage, and regulating neurogenesis in the brain
[88]. Furthermore, Gray and Whalley
[89] suggested that CBD can curb epileptic symptoms by antagonizing the G protein-coupled receptor-55 (GPR55) receptors, suppressing Transient receptor potential vanilloid-1 (TRPV1) receptors, and blocking adenosine transport into cells.
Figure 4. Polyphenolic compounds in hemp seed.
Other than flavonoids and phenolic acids, the most abundant polyphenols, lignanamides, are also found in hemp seeds. Lignanamides are a subclass of lignan and are a highly potent antioxidant. In earlier scientific reports, the total lignanamides represented by hemp seed is 77 mg/100 g dry weight and they are mostly composed of cannabisin A, cannabisin B, and cannabisin M
[90][91]. A laboratory study reported that lignanamides isolated from hemp seed possessed the ability to block the action of acetylcholinesterase
[92]. The role of this enzyme is to break down acetylcholine that functions as a critical neurotransmitter in both central and peripheral nervous systems. Therefore, to treat neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases, balanced levels of acetylcholine can be maintained through acetylcholinesterase inhibitors. Lignanamides are various pharmacologically active compounds extracted from natural sources tested against acetylcholinesterase activity
[93]. Moreover, Irakli et al.
[94] pointed out that the antioxidant capacities of hemp seed extract are mainly contributed by polyphenolic compounds instead of other antioxidative compounds such as tocopherols and carotenoids.