
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Justyna Srebro | -- | 5528 | 2022-10-27 14:08:27 | | | |
| 2 | Rita Xu | -2 word(s) | 5526 | 2022-10-31 02:51:23 | | | | |
| 3 | Rita Xu | + 2 word(s) | 5528 | 2022-10-31 09:10:46 | | |
Video Upload Options
Proton Pump Inhibitors, also known as PPIs, belong to a group of antisecretory drugs. Since their introduction to pharmacotherapy, PPIs have been widely used in the treatment of numerous diseases manifested by excessive secretion of gastric acid. There are still unmet needs regarding their availability for patients of all age groups. Their poor stability hinders the development of formulations in which dose can be easily adjusted.
1. Introduction
Proton pump inhibitors have been found to be very effective in suppressing gastric acid secretion. They share the same mechanism of action, although there are slight differences in their chemical structure [1]. PPIs inhibit the activity of the enzyme H+/K+-ATPase, also named gastric proton pump, located in the parietal cells of the stomach. Proton pump inhibitors are inactive compounds (often simply but incorrectly called ‘prodrugs’), which require activation in the low pH of parietal cells, to suppress the activity of the proton pump. Therefore, to avoid premature activation in the stomach after oral administration, they must be protected from gastric acid, e.g., with enteric coating [2].
2. The Most Important Issues to Be Considered in the Formulation of Medicinal Products with PPIs
2.1. Physicochemical Properties of PPIs
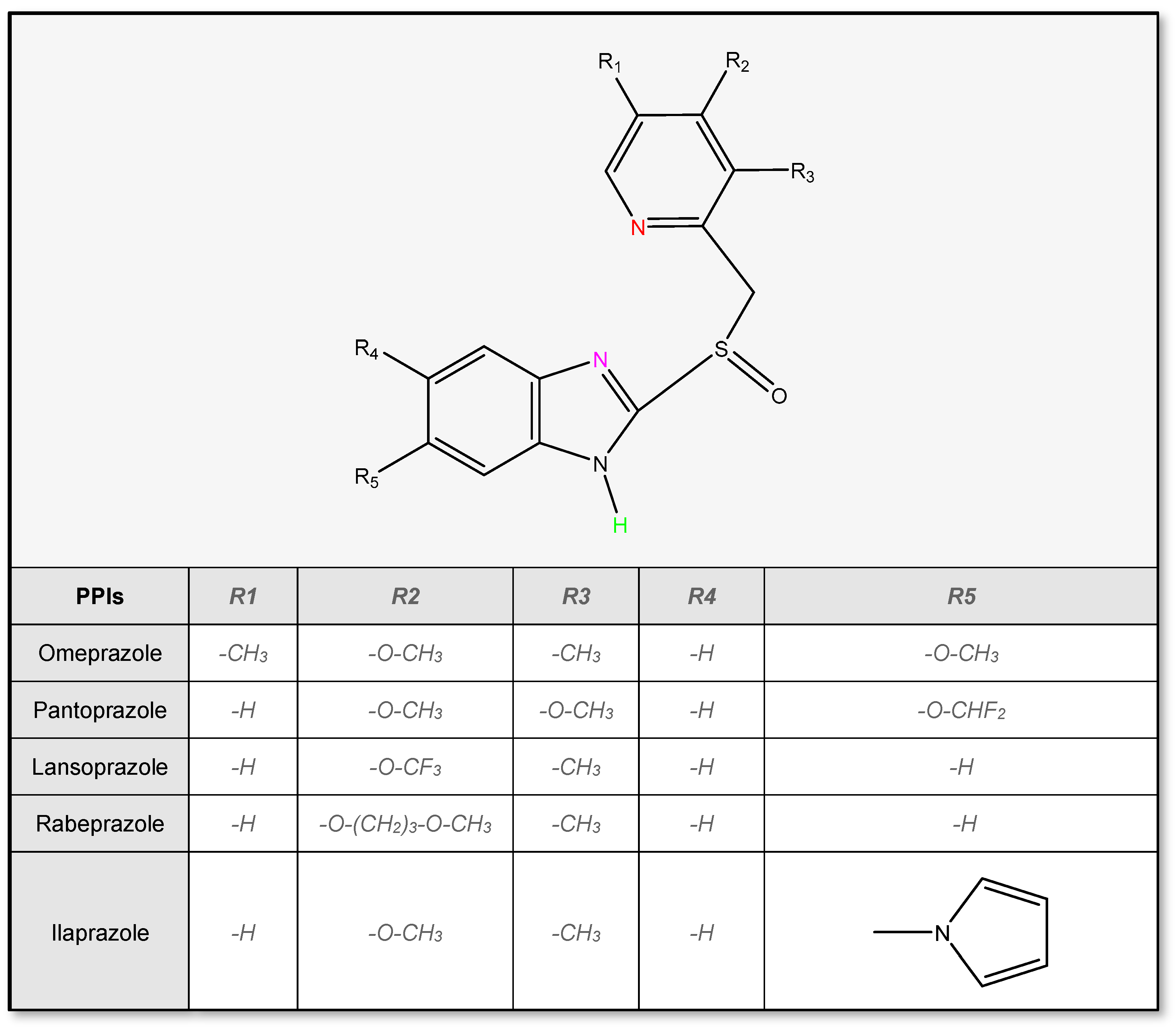
2.2. Stability of Proton Pump Inhibitors
2.2.1. Stability in Solutions
2.2.2 Influence of Temperature
2.2.3 Influence of Salts
2.2.4 Influence of Light
2.3 Interaction of Enteric Polymers with PPIs and Its Effect on the Stability
3. PPIs’ Pharmaceutical Formulations Available on the Market
PPIs are administered by two different routes: oral or intravenous. Currently manufactured dosage forms for oral administration include enteric-coated capsules, enteric coated tablets, multiple-unit pellet system (MUPS), and suspensions with microparticulates. For intravenous administration, PPIs are available as lyophilized powders for reconstitution [25]. There are a large number of manufactured brand and generic products, among which are:
- Delayed-Release Tablets, including MUPS and ODT formulations,
- Delayed-Release Capsules,
- Oral Suspensions,
- Powders for Injections or Infusions,
- Fixed-Dose Combinations.
It is worth mentioning, that proton pump inhibitors can be administered to adult and pediatric patients who require enteral nutrition via a feeding tube. However, factors such as the risk of clogging the tube or adhering the drug to the walls of the tube or the possibility of drug degradation should be carefully considered before administration . The most convenient dosage forms for application via feeding tube are those composed of pellets or granules that can be easily dispersed in water or other vehicles. These include tablets or capsules containing delayed-release pellets, as well as granules for oral suspensions [28]. PPIs should be administered via feeding tube only in accordance with the manufacturer’s recommendations.
4. Development of New Formulations with PPIs
Proton pump inhibitors have been marketed worldwide for more than 30 years [29][30]. At this time, many pharmaceutical solutions have been proposed to improve their acceptability, stability, safety, and efficacy. The most popular route of administration of PPIs is oral, which is the most common for all medicinal products. Formulations with PPIs include numerous dosage forms, starting from simple enteric-coated tablets or pellets encapsulated in hard gelatine capsules, through the other novel form of tablets, and ending with many different forms of micro- or nanoparticulates. There have also been some approaches to the administration of PPIs through alternative routes of administration, such as transdermal or rectal.
The detailed information on PPIs’ formulations described in the literature have been collected in table 1.
| Formulation | PPI | Development Stage | Description |
|---|---|---|---|
| Nanoparticles | Omeprazole | In vitro In vivo antiulcer activity (rats) |
|
| In vitro In vivo antiulcer activity (rats) |
|
||
| Pantoprazole | In vitro |
|
|
| In vitro |
|
||
| Pantoprazole + Aceclofenac | In vitro In vivo (rats) |
|
|
| Lansoprazole | In vitro |
|
|
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Lansoprazole + curcumin | In vitro |
|
|
| Esomeprazole | In vitro Ex vivo permeability study In vivo PK and PD studies (rats) |
|
|
| Microparticles | Omeprazole | In vitro In vivo PK study (rabbits) |
|
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Omeprazole + piperine | In vivo PK and bioavailability studies (rabbits) |
|
|
| Omeprazole + clarithromycin | In vitro |
|
|
| Pantoprazole | In vitro |
|
|
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro In vivo antiulcer activity (rats) |
|
||
| In vitro In vivo antiulcer activity (rats) |
|
||
| In vitro In vivo antiulcer activity (rats) |
|
||
| In vitro In vivo antiulcer activity (rats) |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vivo bioavailability study (dogs) |
|
||
| In vitro In vivo antiulcer activity (rats) |
|
||
| In vitro |
|
||
| Lansoprazole | In vitro In vivo PK and antiulcer activity studies (rats) |
|
|
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Rabeprazole | In vitro In vivo antiulcer activity (rats) |
|
|
| In vitro |
|
||
| In vitro In vivo floating study (rabbits) |
|
||
| Rabeprazole + amoxicillin | In vitro In vivo antiulcer activity and radiographic study (rats) |
|
|
| Esomeprazole | In vitro |
|
|
| Minitablets | Omeprazole | In vitro |
|
| Pantoprazole | In vitro |
|
|
| Pellets | Omeprazole | In vitro In vivo PK and gastro-resistance studies (dogs/rats) |
|
| In vitro In vivo PK and bioequivalence studies (rabbits) |
|
||
| In vitro In silico (ANN, modelling tablet properties) |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Pantoprazole | In vitro |
|
|
| Lansoprazole | In vitro |
|
|
| In vitro |
|
||
| In vitro |
|
||
| In vitro In vivo PK study (dogs) |
|
||
| In vitro |
|
||
| In vitro In vivo bioavailability study (dogs) |
|
||
| Rabeprazole | In vitro |
|
|
| In vitro |
|
||
| In vitro |
|
||
| Esomeprazole | In vitro In vivo PK study (rats) IVIVC |
|
|
| In vitro In silico (ANN, coating process) |
|
||
| Tablets | Omeprazole | In vitro |
|
| In vitro |
|
||
| In vitro |
|
||
| Omeprazole + domperidone | In vitro |
|
|
| Pantoprazole | In vitro |
|
|
| In vitro In vivo antiulcer activity (rats) |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Lansoprazole | In vitro |
|
|
| In vitro |
|
||
| In vitro In vivo absorption studies (dogs), disintegration time in the mouth (human) |
|
||
| In vitro |
|
||
| In vivo (human) Clinical trials |
|
||
| In vivo (human) |
|
||
| In vivo bioequivalence studies (human) |
|
||
| In vitro In vivo (human) |
|
||
| In vitro |
|
||
| Rabeprazole | In vitro In vivo PK studies (beagle dogs) |
|
|
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Esomeprazole | In vitro |
|
|
| In vitro |
|
||
| In vitro Ex vivo permeation studies (porcine mucosa) In vivo pharmacokinetics studies (rats) |
|
||
| In vitro |
|
||
| In vitro |
|
||
| Dexlansoprazole | In vitro |
|
|
| Tenatoprazole | In vitro |
|
|
| In vitro |
|
||
| Ilaprazole | In vitro |
|
|
| In vitro |
|
||
| Fixed-dose combination products | Esomeprazole + naproxen | In vitro |
|
| Bilayer tablets | Lansoprazole + amoxycillin | In vitro |
|
| Esomeprazole + aceclofenac | In vitro |
|
|
| Esomeprazole + clarithromycin | In vitro |
|
|
| Esomeprazole + levosulpiride | In vitro |
|
|
| Floating tablets | Pantoprazole | In vitro |
|
| In vitro |
|
||
| Lansoprazole | In vitro |
|
|
| In vitro |
|
||
| Rabeprazole | In vitro In vivo pharmacokinetic and antiulcer activity studies (rats) |
|
|
| Hydrogel formulations | Pantoprazole | In vitro |
|
| In vitro |
|
||
| In vitro In vivo studies on hydrogel gastro-retention (mice) |
|
||
| Rabeprazole | In vitro |
|
|
| Mucoadhesive tablets | Omeprazole | In vitro |
|
| In vitro In vivo studies on absorption from the oral cavity and tablets adhesion to the oral mucosa (human) |
|
||
| In vitro In vivo pharmacokinetic studies (hamsters) |
|
||
| In vitro In vivo pharmacokinetic studies (hamster), mucoadhesive force measurement (human) |
|
||
| In vitro |
|
||
| Pantoprazole | In vitro |
|
|
| Oral liquid suspensions | Omeprazole | In vitro |
|
| In vitro |
|
||
| In vitro In vivo preliminary toxicity and antiulcer activity studies (mice) |
|
||
| Transdermal delivery | Omeprazole | In vivo PK study (human) |
|
| Lansoprazole | Ex vivo penetration study (pigs) In vivo PK study (rats) |
|
|
| Rabeprazole | Ex vivo penetration study (snake) |
|
|
| Suppositories | Omeprazole | In vitro |
|
| Clinical trial (efficacy, PK) |
|
||
| Intravenous formulations | Omeprazole | In vitro |
|
| In vitro |
|
5. Future Perspectives
References
- Strand, D.S.; Kim, D.; Peura, D.A.; 25 years of proton pump inhibitors: A comprehensive review. Gut Liver 2017, 11, 27–37, https://doi.org/10.5009/gnl15502.
- Shin, J.M.; Sachs, G.; Pharmacology of proton pump inhibitors. Curr. Gastroenterol. Rep. 2008, 10, 528–534, https://doi.org/10.1007/s11894-008-0098-4.
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A.; Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019, https://doi.org/10.1016/j.ejps.2019.105019.
- Brändström, A.; Bergman, N.-Å.; Grundevik, I.; Johansson, S.; Tekenbergs-Hjelte, L.; Ohlson, K.; Chemical reactions of omeprazole and omeprazole analogues. III. Protolytic behaviour of compounds in the omeprazole system. Acta Chem. Scand. 1989, 43, 569–576.
- DrugBank Online . DrugBank. Retrieved 2022-10-27
- Andersson, T.; Weidolf, L.; Stereoselective disposition of proton pump inhibitors. Clin. Drug. Investig. 2008, 28, 263–279, https://doi.org/10.2165/00044011-200828050-00001.
- Hancu, G.; Modroiu, A.; Chiral Switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals 2022, 15, 240, https://doi.org/10.3390/ph15020240.
- Geng, L.; Han, L.; Huang, L.; Wu, Z.; Wu, Z.; Qi, X. High anti-acid omeprazole lightweight capsule for gastro-enteric system acid-related disorders treatment. J. Clin. Gastroenterol. Treat. 2019, 5, 1–11.
- Kan, S.-L.; Lu, J.; Liu, J.-P.; Zhao, Y. Preparation and in vitro/in vivo evaluation of esomeprazole magnesium-modified release pellets. Drug. Deliv. 2016, 23, 866–873.
- He, W.; Yang, M.; Fan, J.H.; Feng, C.X.; Zhang, S.J.; Wang, J.X.; Guan, P.P.; Wu, W. Influences of sodium carbonate on physicochemical properties of lansoprazole in designed multiple coating pellets. AAPS PharmSciTech 2010, 11, 1287–1293.
- Wu, C.; Sun, L.; Sun, J.; Yang, Y.; Ren, C.; Ai, X.; Lian, H.; He, Z. Profiling biopharmaceutical deciding properties of absorption of lansoprazole enteric-coated tablets using gastrointestinal simulation technology. Int. J. Pharm. 2013, 453, 300–306.
- Lee, S.-H.; Kim, J.-E. Quality by design applied development of immediate-release rabeprazole sodium dry-coated tablet. Pharmaceutics 2021, 13, 259.
- de Campos, D.R.; Klein, S.; Zoller, T.; Vieria, N.R.; Barros, F.A.P.; Meurer, E.C.; Coelho, E.C.; Marchioretto, M.A.; Pedrazzoli, J. Evaluation of pantoprazole formulations in different dissolution apparatus using biorelevant medium. Arzneimittelforschung 2010, 60, 42–47.
- Mathew, M.; das Gupta, V.; Bailey, R.E. Stability of omeprazole solutions at various pH values as determined by high-performance liquid chromatography. Drug. Dev. Ind. Pharm. 1995, 21, 965–971, https://doi.org/10.3109/03639049509026660.
- DellaGreca, M.; Iesce, M.R.; Previtera, L.; Rubino, M.; Temussi, F.; Brigante, M.; of lansoprazole and omeprazole in the aquatic environment.. Chemosphere 2006, 63, 1087–1093, https://doi.org/10.1016/j.chemosphere.2005.09.003.
- Garcia, C.V.; Nudelman, N.S.; Steppe, M.; Schapoval, E.E.S. Structural elucidation of rabeprazole sodium photodegradation products. J. Pharm. Biomed. Anal. 2008, 46, 88–93, https://doi.org/10.1016/j.jpba.2007.09.002.
- Mahadik, M.; Bhusari, V.; Kulkarni, M.; Dhaneshwar, S. LC-UV and LC-MS evaluation of stress degradation behaviour of tenatoprazole. J. Pharm. Biomed. Anal. 2009, 50, 787–793, https://doi.org/10.1016/j.jpba.2009.06.026.
- Stroyer, A.; McGinity, J.W.; Leopold, C.S. Solid state interactions between the proton pump inhibitor omeprazole and various enteric coating polymers. J. Pharm. Sci. 2006, 95, 1342–1353, https://doi.org/10.1002/jps.20450.
- Quercia, R.A.; Fan, C.; Liu, X.; Chow, M.S.S. Stability of omeprazole in an extemporaneously prepared oral liquid. Am. J. Health-Syst. Pharm. 1997, 54, 1833–1836.
- El-Badry, M.; Taha, E.I.; Alanazi, F.K.; Alsarra, I.A. Study of omeprazole stability in aqueous solution: Influence of cyclodextrins. J. Drug Deliv. Sci. Technol. 2009, 19, 347–351, https://doi.org/10.1016/S1773-2247(09)50072-X.
- Ekpe, A.; Jacobsen, T. Effect of various salts on the stability of lansoprazole, omeprazole, and pantoprazole as determined by high-performance liquid chromatography. Drug. Dev. Ind. Pharm. 1999, 25, 1057–1065, https://doi.org/10.1081/DDC-100102270.
- Raffin, R.P.; Colomé, L.M.; Guterres, S.S.; Pohlmann, A.R. Validação de metodologia analítica por cromatografia líquida para doseamento e estudo da estabilidade de pantoprazol sódico. Quim. Nova 2007, 30, 1001–1005, https://doi.org/10.1590/s0100-40422007000400041.
- Dhurke, R.; Kushwaha, I.; Desai, B.G. Improvement in photostability of pantoprazole sodium by microencapsulation. PDA J. Pharm. Sci. Technol. 2013, 67, 43–52, https://doi.org/10.5731/pdajpst.2013.00901.
- Raffin, R.P.; Colomé, L.M.; Schapoval, E.E.S.; Pohlmann, A.R.; Guterres, S.S. Increasing sodium pantoprazole photostability by microencapsulation: Effect of the polymer and the preparation technique. Eur. J. Pharm. Biopharm. 2008, 69, 1014–1018, https://doi.org/10.1016/j.ejpb.2008.01.024.
- Missaghi, S.; Young, C.; Fegely, K.; Rajabi-Siahboomi, A.R. Delayed release film coating applications on oral solid dosage forms of proton pump inhibitors: Case studies delayed release solid dosage forms of proton pump inhibitors. Drug. Dev. Ind. Pharm. 2010, 36, 180–189.
- Riedel, A.; Leopold, C.S. Degradation of omeprazole induced by enteric polymer solutions and aqueous dispersions: HPLC investigations. Drug. Dev. Ind. Pharm. 2005, 31, 151–160.
- Riedel, A.; Leopold, C.S. Quantification of omeprazole degradation by enteric coating polymers: An UV-VIS spectroscopy study. Pharmazie 2005, 60, 126–130.
- Wensel, T.M.; Administration of proton pump inhibitors in patients requiring enteral nutrition.. P T 2009, 34, 143–152.
- Olbe, L.; Carlsson, E.; Lindberg, P.; A proton-pump inhibitor expedition: The case histories of omeprazole and esomeprazole.. Nat. Rev. Drug. Discov. 2003, 2, 132–139, https://doi.org/10.1038/nrd1010.
- Shin, J.M.; Munson, K.; Vagin, O.; Sachs, G.; The gastric HK-ATPase: Structure, function, and inhibition.. Pflug. Arch.—Eur. J. Physiol. 2009, 457, 609–622, https://doi.org/10.1007/s00424-008-0495-4.
- Hunt, R.H.; Scarpignato, C.; Potent acid suppression with PPIs and P-CABs: What’s new?. Curr. Treat. Options Gastroenterol. 2018, 16, 570–590, https://doi.org/10.1007/s11938-018-0206-y.
- Scarpignato, C.; Hunt, R.H.; Proton pump inhibitors: The beginning of the end or the end of the beginning?. Curr. Opin. Pharmacol. 2008, 8, 677–684, https://doi.org/10.1016/j.coph.2008.09.004.
- Hunt, R.H.; Armstrong, D.; Yaghoobi, M.; James, C.; Chen, Y.; Leonard, J.; Shin, J.M.; Lee, E.; Tang-Liu, D.; Sachs, G.; et al. Predictable prolonged suppression of gastric acidity with a novel proton pump inhibitor, AGN 201904-Z. Aliment. Pharmacol. Ther. 2008, 28, 187–199, https://doi.org/10.1111/j.1365-2036.2008.03725.x.




