
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daiki Hayashi | -- | 1784 | 2022-10-25 10:17:27 | | | |
| 2 | Vivi Li | Meta information modification | 1784 | 2022-10-27 04:02:20 | | |
Video Upload Options
The drastic increase in the number of patients with diabetes and its complications is a global issue. Diabetic nephropathy, the leading cause of chronic kidney disease, significantly affects patients’ quality of life and medical expenses. Furthermore, there are limited drugs for treating diabetic nephropathy patients. Impaired lipid signaling, especially abnormal protein kinase C (PKC) activation by de novo-synthesized diacylglycerol (DG) under high blood glucose, is one of the causes of diabetic nephropathy. DG kinase (DGK) is an enzyme that phosphorylates DG and generates phosphatidic acid, i.e., DGK can inhibit PKC activation under diabetic conditions. Indeed, it has been proven that DGK activation ameliorates diabetic nephropathy.
1. Diabetes and Diabetic Nephropathy
2. Hyperglycemia Impairs Lipid Signaling
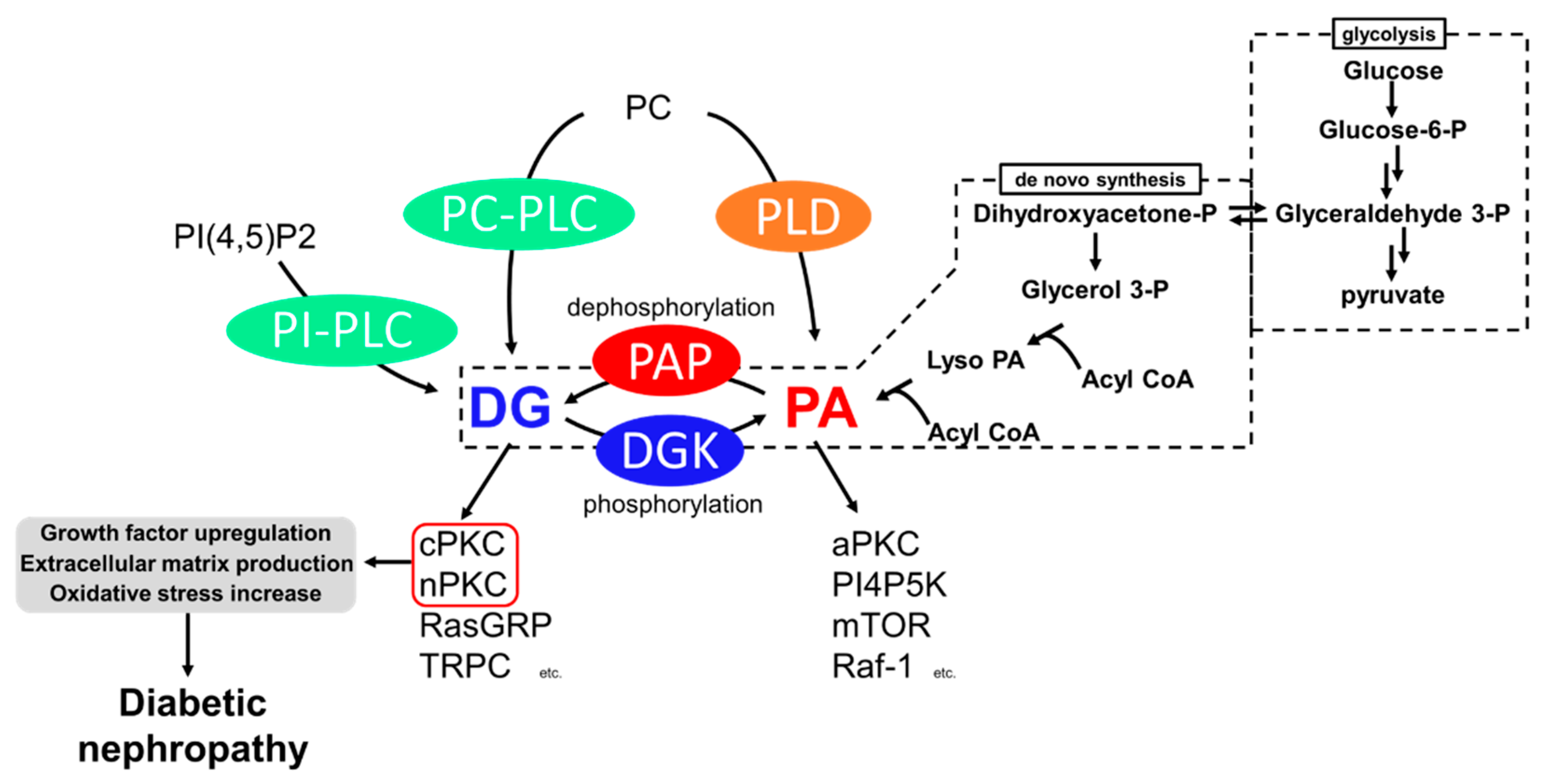
3. Diabetic Nephropathy and PKC
4. Diabetic Nephropathy and DGK
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119.
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222.
- Carey, I.M.; Critchley, J.A.; Dewilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared with the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521.
- Hovind, P.; Tarnow, L.; Rossing, P.; Jensen, B.R.; Graae, M.; Torp, I.; Binder, C.; Parving, H.H. Predictors for the Development of Microalbuminuria and Macroalbuminuria in Patients with Type 1 Diabetes: Inception Cohort Study. Br. Med. J. 2004, 328, 1105–1108.
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed. Res. Int. 2021, 2021, 1497449.
- Secrist, J.P.; Karnitz, L.; Abraham, R.T. T-Cell Antigen Receptor Ligation Induces Tyrosine Phosphorylation of Phospholipase C-Γ1. J. Biol. Chem. 1991, 266, 12135–12139.
- Rhee, S.G.; Bae, Y.S. Regulation of Phosphoinositide-Specific Phospholipase C Isozymes. J. Biol. Chem. 1997, 272, 15045–15048.
- Monick, M.M.; Carter, A.B.; Gudmundsson, G.; Mallampalli, R.; Powers, L.S.; Hunninghake, G.W. A Phosphatidylcholine-Specific Phospholipase C Regulates Activation of P42/44 Mitogen-Activated Protein Kinases in Lipopolysaccharide-Stimulated Human Alveolar Macrophages. J. Immunol. 1999, 162, 3005–3012.
- Carman, G.M.; Han, G.S. Phosphatidic Acid Phosphatase, a Key Enzyme in the Regulation of Lipid Synthesis. J. Biol. Chem. 2009, 284, 2593–2597.
- Carman, G.M.; Han, G.-S. Roles of Phosphatidate Phosphatase Enzymes in Lipid Metabolism. Trends Biochem. Sci. 2006, 31, 694–699.
- Exton, J.H. Phospholipase D: Enzymology, Mechanisms of Regulation, and Function. Physiol. Rev. 1997, 77, 303–320.
- Craven, P.A.; Davidson, C.M.; DeRubertis, F.R. Increase in Diacylglycerol Mass in Isolated Glomeruli by Glucose from de Novo Synthesis of Glycerolipids. Diabetes 1990, 39, 667–674.
- Thorens, B.; Mueckler, M. Glucose Transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 2010, 298, 141–145.
- Jiang, X.; Yang, F.; Brailoiu, E.; Jakubowski, H.; Dun, N.J.; Schafer, A.I.; Yang, X.; Durante, W.; Wang, H. Differential Regulation of Homocysteine Transport in Vascular Endothelial and Smooth Muscle Cells. Diabetes 1993, 42, 80–89.
- Nishizuka, Y. Intracellular Signaling by Hydrolysis of Phospholipids and Activation of Protein Kinase, C. Science 1992, 258, 607–614.
- Newton, A.C. Regulation of Protein Kinase, C. Curr. Opin. Cell Biol. 1997, 9, 161–167.
- Ebinu, J.O.; Bottorff, D.A.; Chan, E.Y.W.; Stang, S.L.; Dunn, R.J.; Stone, J.C. RasGRP, a Ras Guanyl Nucleotide- Releasing Protein with Calcium- and Diacylglycerol-Binding Motifs. Science 1998, 280, 1082–1086.
- Lucas, P.; Ukhanov, K.; Leinders-Zufall, T.; Zufall, F. A Diacylglycerol-Gated Cation Channel in Vomeronasal Neuron Dendrites Is Impaired in TRPC2 Mutant Mice: Mechanism of Pheromone Transduction. Neuron 2003, 40, 551–561.
- Limatola, C.; Schaap, D.; Moolenaar, W.H.; van Blitterswijk, W.J. Phosphatidic Acid Activation of Protein Kinase C-Zeta Overexpressed in COS Cells: Comparison with Other Protein Kinase C Isotypes and Other Acidic Lipids. Biochem. J. 1994, 304, 1001–1008.
- Jones, D.R.; Sanjuan, M.A.; Mérida, I. Type Iα Phosphatidylinositol 4-Phosphate 5-Kinase Is a Putative Target for Increased Intracellular Phosphatidic Acid. FEBS Lett. 2000, 476, 160–165.
- Ghosh, S.; Strum, J.C.; Sciorra, V.A.; Daniel, L.; Bell, R.M. Raf-1 Kinase Possesses Distinct Binding Domains for Phosphatidylserine and Phosphatidic Acid: Phosphatidic Acid Regulates the Translocation of Raf-1 in 12-O-Tetradecanoylphorbol-13-Acetate-Stimulated Madin-Darby Canine Kidney Cells. J. Biol. Chem. 1996, 271, 8472–8480.
- Fang, Y.; Vilella-Bach, M.; Bachmann, R.; Flanigan, A.; Chen, J. Phosphatidic Acid-Mediated Mitogenic Activation of MTOR Signaling. Science 2001, 294, 1942–1945.
- Newton, A.C. Protein Kinase C: Perfectly Balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230.
- Parker, P.J.; Coussens, L.; Totty, N.; Rhee, L.; Young, S.; Chen, E.; Stabel, S.; Waterfield, M.D.; Ullrich, A. The Complete Primary Structure of Protein Kinase C—The Major Phorbol Ester Receptor. Science 1986, 233, 853–859.
- Knopf, J.L.; Lee, M.H.; Sultzman, L.A.; Kriz, R.W.; Loomis, C.R.; Hewick, R.M.; Bell, R.M. Cloning and Expression of Multiple Protein Kinase C CDNAs. Cell 1986, 46, 491–502.
- Ohno, S.; Akita, Y.; Konno, Y.; Imajoh, S.; Suzuki, K. A Novel Phorbol Ester Receptor/Protein Kinase, NPKC, Distantly Related to the Protein Kinase C Family. Cell 1988, 53, 731–741.
- Ono, Y.; Fujii, T.; Ogita, K.; Kikkawa, U.; Igarashi, K.; Nishizuka, Y. The Structure, Expression, and Properties of Additional Members of the Protein Kinase C Family. J. Biol. Chem. 1988, 263, 6927–6932.
- Nakanishi, H.; Exton, J.H. Purification and Characterization of the ζ Isoform of Protein Kinase C from Bovine Kidney. J. Biol. Chem. 1992, 267, 16347–16354.
- Craven, P.A.; DeRubertis, F. Protein Kinase C Is Activated in Glomeruli from Streptozotocin Diabetic Rats. Possible Mediation by Glucose. J. Clin. Investig. 1989, 83, 1667–1675.
- Inoguchi, T.; Battan, R.; Handler, E.; Sportsman, J.R.; Heath, W.; King, G.L. Preferential Elevation of Protein Kinase C Isoform Beta II and Diacylglycerol Levels in the Aorta and Heart of Diabetic Rats: Differential Reversibility to Glycemic Control by Islet Cell Transplantation. Proc. Natl. Acad. Sci. USA 1992, 89, 11059–11063.
- Ayo, S.H.; Radnik, R.; Garoni, J.A.; Troyer, D.A.; Kreisberg, J.I. High Glucose Increases Diacylglycerol Mass and Activates Protein Kinase C in Mesangial Cell Cultures. Am. J. Physiol. Physiol. 1991, 261, F571–F577.
- Koya, D.; King, G.L. Protein Kinase C Activation and the Development of Diabetic Complications. Diabetes 1998, 47, 859–866.
- Menne, J.; Park, J.K.; Boehne, M.; Elger, M.; Lindschau, C.; Kirsch, T.; Meier, M.; Gueler, F.; Fiebeler, A.; Bahlmann, F.H.; et al. Diminished Loss of Proteoglycans and Lack of Albuminuria in Protein Kinase C-α-Deficient Diabetic Mice. Diabetes 2004, 53, 2101–2109.
- Ohshiro, Y.; Ma, R.C.; Yasuda, Y.; Hiraoka-Yamamoto, J.; Clermont, A.C.; Isshiki, K.; Yagi, K.; Arikawa, E.; Kern, T.S.; King, G.L. Reduction of Diabetes-Induced Oxidative Stress, Fibrotic Cytokine Expression, and Renal Dysfunction in Protein Kinase Cβ-Null Mice. Diabetes 2006, 55, 3112–3120.
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The Effect of Ruboxistaurin on Nephropathy in Type 2 Diabetes. Diabetes Care 2005, 28, 2686–2690.
- Shiba, T.; Inoguchi, T.; Sportsman, J.R.; Heath, W.F.; Bursell, S.; King, G.L. Correlation of Diacylglycerol Level and Protein Kinase C Activity in Rat Retina to Retinal Circulation. Am. J. Physiol. 1993, 265, E783–E793.
- Derubertis, F.R.; Craven, P.A. Activation of Protein Kinase C in Glomerular Cells in Diabetes: Mechanisms and Potential Links to the Pathogenesis of Diabetic Glomerulopathy. Diabetes 1994, 43, 1–8.
- Babazono, T.; Kapor-Drezgic, J.; Dlugosz, J.A.; Whiteside, C. Altered Expression and Subcellular Localization of Diacylglycerol- Sensitive Protein Kinase C Isoforms in Diabetic Rat Glomerular Cells. Diabetes 1998, 47, 668–676.
- Chiarelli, F.; Gaspari, S.; Marcovecchio, M.L. Role of Growth Factors in Diabetic Kidney Disease. Horm. Metab. Res. 2009, 41, 585–593.
- Reeves, W.B.; Andreoli, T.E. Transforming Growth Factor β Contributes to Progressive Diabetic Nephropathy. Proc. Natl. Acad. Sci. USA 2000, 97, 7667–7669.
- Arora, M.K.; Singh, U.K. Molecular Mechanisms in the Pathogenesis of Diabetic Nephropathy: An Update. Vascul. Pharmacol. 2013, 58, 259–271.
- Koya, D.; Jirousek, M.R.; Lin, Y.W.; Ishii, H.; Kuboki, K.; King, G.L. Characterization of Protein Kinase C β Isoform Activation on the Gene Expression of Transforming Growth Factor-β, Extracellular Matrix Components, and Prostanoids in the Glomeruli of Diabetic Rats. J. Clin. Investig. 1997, 100, 115–126.
- Hayashida, T.; Schnaper, H.W. High Ambient Glucose Enhances Sensitivity to TGF-Β1 via Extracellular Signal-Regulated Kinase and Protein Kinase Cδ Activities in Human Mesangial Cells. J. Am. Soc. Nephrol. 2004, 15, 2032–2041.
- Riser, B.L.; Denichilo, M.; Cortes, P.; Baker, C.; Grondin, J.M.; Yee, J.; Narins, R.G. Regulation of Connective Tissue Growth Factor Activity in Cultured Rat Mesangial Cells and Its Expression in Experimental Diabetic Glomerulosclerosis. J. Am. Soc. Nephrol. 2000, 11, 25–38.
- Khamaisi, M.; Schrijvers, B.F.; De Vriese, A.S.; Raz, I.; Flyvbjerg, A. The Emerging Role of VEGF in Diabetic Kidney Disease. Nephrol. Dial. Transplant. 2003, 18, 1427–1430.
- Hoshi, S.; Tomari, S.; Hoshi, S.; Nomoto, K.I.; Kuromitsu, J.; Nagata, M. High Glucose Induced VEGF Expression via PKC and ERK in Glomerular Podocytes. Biochem. Biophys. Res. Commun. 2002, 290, 177–184.
- Xia, L.; Wang, H.; Munk, S.; Frecker, H.; Goldberg, H.J.; Fantus, I.G.; Whiteside, C.I. Reactive Oxygen Species, PKC-A1, and PKC-ζ Mediate High-Glucose-Induced Vascular Endothelial Growth Factor Expression in Mesangial Cells. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 1280–1288.
- Thallas-Bonke, V.; Thorpe, S.R.; Coughlan, M.T.; Fukami, K.; Yap, F.Y.T.; Sourris, K.C.; Penfold, S.A.; Bach, L.A.; Cooper, M.E.; Forbes, J.M. Inhibition of NADPH Oxidase Prevents Advanced Glycation End Product-Mediated Damage in Diabetic Nephropathy through a Protein Kinase C-α-Dependent Pathway. Diabetes 2008, 57, 460–469.
- Ishii, H.; Jirousek, M.R.; Koya, D.; Takagi, C.; Xia, P.; Clermont, A.; Bursell, S.E.; Kern, T.S.; Ballas, L.M.; Heath, W.F.; et al. Amelioration of Vascular Dysfunctions in Diabetic Rats by an Oral PKC β Inhibitor. Science 1996, 272, 728–731.
- Koya, D.; Haneda, M.; Nakagawa, H.; Isshiki, K.; Sato, H.; Maeda, S.; Sugimoto, T.; Yasuda, H.; Kashiwagi, A.; Ways, D.K.; et al. Amelioration of Accelerated Diabetic Mesangial Expansion by Treatment with a PKC β Inhibitor in Diabetic Db/Db Mice, a Rodent Model for Type 2 Diabetes. FASEB J. 2000, 14, 439–447.
- Sheetz, M.J.; Aiello, L.P.; Davis, M.D.; Danis, R.; Bek, T.; Cunha-Vaz, J.; Shahri, N.; Berg, P.H. The Effect of the Oral PKC β Inhibitor Ruboxistaurin on Vision Loss in Two Phase 3 Studies. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1750–1757.
- Meier, M.; Park, J.K.; Overheu, D.; Kirsch, T.; Lindschau, C.; Gueler, F.; Leitges, M.; Menne, J.; Haller, H. Deletion of Protein Kinase C-β Isoform in Vivo Reduces Renal Hypertrophy but Not Albuminuria in the Streptozotocin-Induced Diabetic Mouse Model. Diabetes 2007, 56, 346–354.
- Kanoh, H.; Yamada, K.; Sakane, F. Diacylglycerol Kinase: A Key Modulator of Signal Transduction? Trends Biochem. Sci. 1990, 15, 47–50.
- Sakane, F.; Imai, S.I.; Kai, M.; Yasuda, S.; Kanoh, H. Diacylglycerol Kinases: Why so Many of Them? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 793–806.
- Topham, M.K.; Prescott, S.M. Mammalian diacylglycerol kinases, a Family of Lipid Kinases with Signaling Functions. J. Biol. Chem. 1999, 274, 11447–11450.
- Van Blitterswijk, W.J.; Houssa, B. Properties and Functions of Diacylglycerol Kinases. Cell. Signal. 2000, 12, 595–605.
- Kanoh, H.; Yamada, K.; Sakane, F. Diacylglycerol Kinases: Emerging Downstream Regulators in Cell Signaling Systems. J. Biochem. 2002, 131, 629–633.
- Sakane, F.; Yamada, K.; Kanoh, H.; Yokoyama, C.; Tanabe, T. Porcine Diacylglycerol Kinase Sequence Has Zinc Finger and E-F Hand Motifs. Nature 1990, 344, 345–348.
- Klauck, T.M.; Xu, X.; Mousseau, B.; Jaken, S. Cloning and Characterization of a Glucocorticoid-Induced Diacylglycerol Kinase. J. Biol. Chem. 1996, 271, 19781–19788.
- Letwin, K.; Yee, S.P.; Pawson, T. Novel Protein-Tyrosine Kinase CDNAs Related to Fps/Fes and Eph Cloned Using Anti-Phosphotyrosine Antibody. Oncogene 1988, 3, 621–627.
- Imai, S.; Kai, M.; Yasuda, S.; Kanoh, H.; Sakane, F. Identification and Characterization of a Novel Human Type II Diacylglycerol Kinase, DGK Kappa. J. Biol. Chem. 2005, 280, 39870–39881.
- Tang, W.; Bunting, M.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Molecular Cloning of a Novel Human Diacylglycerol Kinase Highly Selective for Arachidonate-Containing Substrates. J. Biol. Chem. 1996, 271, 10237–10241.
- Bunting, M.; Tang, W.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Molecular Cloning and Characterization of a Novel Human Diacylglycerol Kinase Zeta. J. Biol. Chem. 1996, 271, 10230–10236.
- Goto, K.; Kondo, H. A 104-KDa Diacylglycerol Kinase Containing Ankyrin-like Repeats Localizes in the Cell Nucleus. Proc. Natl. Acad. Sci. USA 1996, 93, 11196–11201.
- Ding, L.; Traer, E.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. The Cloning and Characterization of a Novel Human Diacylglycerol Kinase, DGKiota. J. Biol. Chem. 1998, 273, 32746–32752.
- Houssa, B.; Schaap, D.; Van Der Wal, J.; Goto, K.; Kondo, H.; Yamakawa, A.; Shibata, M.; Takenawa, T.; Van Blitterswijk, W.J. Cloning of a Novel Human Diacylglycerol Kinase (DGKθ) Containing Three Cysteine-Rich Domains, a Proline-Rich Region, and a Pleckstrin Homology Domain with an Overlapping Ras-Associating Domain. J. Biol. Chem. 1997, 272, 10422–10428.
- Rigotti, A. Absorption, Transport, and Tissue Delivery of Vitamin E. Mol. Asp. Med. 2007, 28, 423–436.
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15.
- Koya, D.; Lee, I.K.; Ishii, H.; Kanoh, H.; King, G.L. Prevention of Glomerular Dysfunction in Diabetic Rats by Treatment with D-Alpha-Tocopherol. J. Am. Soc. Nephrol. 1997, 8, 426–435.
- Tada, H.; Ishii, H.; Isogai, S. Protective Effect of D-α-Tocopherol on the Function of Human Mesangial Cells Exposed to High Glucose Concentrations. Metabolism 1997, 46, 779–784.
- Kakehi, T.; Yagi, K.; Saito, N.; Shirai, Y. Effects of Vitamin E and Its Derivatives on Diabetic Nephropathy in Rats and Identification of Diacylglycerol Kinase Subtype Involved in the Improvement of Diabetic Nephropathy. Funct. Foods Health Dis. 2017, 7, 816–832.
- Shirai, Y.; Segawa, S.; Kuriyama, M.; Goto, K.; Sakai, N.; Naoaki, S. Subtype-Specific Translocation of Diacylglycerol Kinase α and γ and Its Correlation with Protein Kinase C. J. Biol. Chem. 2000, 275, 24760–24766.
- Fukunaga-Takenaka, R.; Shirai, Y.; Yagi, K.; Adachi, N.; Sakai, N.; Merino, E.; Merida, I.; Saito, N. Importance of Chroman Ring and Tyrosine Phosphorylation in the Subtype-Specific Translocation and Activation of Diacylglycerol Kinase α by D-α-Tocopherol. Genes Cells 2005, 10, 311–319.
- Tasinato, A.; Boscoboinik, D.; Bartoli, G.M.; Maroni, P.; Azzi, A. D-α-Tocopherol Inhibition of Vascular Smooth Muscle Cell Proliferation Occurs at Physiological Concentrations, Correlates with Protein Kinase C Inhibition, and Is Independent of Its Antioxidant Properties. Proc. Natl. Acad. Sci. USA 1995, 92, 12190–12194.
- Pryor, W.A.; Cornicelli, J.A.; Devall, L.J.; Tait, B.; Trivedi, B.K.; Witiak, D.T.; Wu, M. A Rapid Screening Test To Determine the Antioxidant Potencies of Natural and Synthetic Antioxidants. J. Org. Chem. 1993, 58, 3521–3532.
- Atsumi, H.; Kitada, M.; Kanasaki, K.; Koya, D. Reversal of Redox-Dependent Inhibition of Diacylglycerol Kinase by Antioxidants in Mesangial Cells Exposed to High Glucose. Mol. Med. Rep. 2011, 4, 923–927.




