Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alicia Tosoni | -- | 2981 | 2022-10-21 06:51:05 | | | |
| 2 | Catherine Yang | Meta information modification | 2981 | 2022-10-21 07:40:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nunno, V.D.; Giannini, C.; Asioli, S.; Conti, A.; Furtner, J.; Balestrini, D.; Tosoni, A. Anaplastic (Malignant) Meningioma. Encyclopedia. Available online: https://encyclopedia.pub/entry/30634 (accessed on 07 February 2026).
Nunno VD, Giannini C, Asioli S, Conti A, Furtner J, Balestrini D, et al. Anaplastic (Malignant) Meningioma. Encyclopedia. Available at: https://encyclopedia.pub/entry/30634. Accessed February 07, 2026.
Nunno, Vincenzo Di, Caterina Giannini, Sofia Asioli, Alfredo Conti, Julia Furtner, Damiano Balestrini, Alicia Tosoni. "Anaplastic (Malignant) Meningioma" Encyclopedia, https://encyclopedia.pub/entry/30634 (accessed February 07, 2026).
Nunno, V.D., Giannini, C., Asioli, S., Conti, A., Furtner, J., Balestrini, D., & Tosoni, A. (2022, October 21). Anaplastic (Malignant) Meningioma. In Encyclopedia. https://encyclopedia.pub/entry/30634
Nunno, Vincenzo Di, et al. "Anaplastic (Malignant) Meningioma." Encyclopedia. Web. 21 October, 2022.
Copy Citation
Meningiomas are the most common primary central nervous system malignancies accounting for 36% of all intracranial tumors. However, only 1% of meningioma is classified as malignant (anaplastic) meningioma. Due to their rarity, clinical management of these tumors presents several gaps.
meningioma
anaplastic meningioma
1. Introduction
Meningiomas are primary central nervous system (CNS) tumors originating from the arachnoid cells located on the inner surface of the dura [1]. These are the most common primary CNS malignancies accounting for 36% of all intracranial tumors [2] and are in most cases benign (CNS WHO grade 1). By contrast, anaplastic (malignant) meningiomas (malignant meningiomas: MMs) are rare tumors, representing 1% of all meningiomas [3]. Different from low-grade meningiomas that occur most often in women and are associated with a relatively good outcome, MMs are more frequent in men and have a poor prognosis with reported five-year survival rates of 28–61% [4].
Patients with a grade 3 meningioma can be divided into two groups: patients with primary MM who receive a diagnosis of MM at their first surgery and patients with secondary MM in whom the MM is the result of transformation from a lower grade tumor [5][6]. The prognosis of patients with primary MM has been shown to be favorable compared to those with secondary MM in multiple retrospective series [5][7][8][9].
2. Pathology
Meningiomas are the most common intracranial/extraparenchymal tumors [10] and include a large spectrum of tumors with varying histopathological features ranging from benign (CNS World Health Organization/WHO grade 1) tumors to atypical (CNS WHO grade 2) and anaplastic (malignant) tumors (CNS WHO grade 3). Anaplastic (malignant) meningiomas, CNS WHO grade 3, are the least common, accounting for 1–3% of meningiomas (WHO CNS 2021) and include three different subtypes: anaplastic (malignant), rhabdoid, and papillary (WHO CNS 2021). Similar to other tumors in the 2021 WHO CNS tumors classification, they can be diagnosed either based on histopathological findings or a combination of morphological and molecular findings.
2.1. Histopathological Diagnosis
Anaplastic (malignant) meningiomas are defined as meningiomas, which show (1) markedly elevated mitotic activity (20 or more mitoses in 10 consecutive high-power fields each of 0.16 mm2, at least 12.5 per 1 mm2); (2) frank malignant cytology, resembling carcinoma, melanoma, or sarcoma; (3) harbor TERT (telomerase reverse transcriptase [1]) promoter mutation; and (4) harbor CDKN2A (cyclin-dependent kinase inhibitor [1]) and/or CDKN2B homozygous deletion [1]. Extensive necrosis is frequently observed in aplastic (malignant) meningioma, as is parenchymal brain invasion. Malignant features can be present either at first resection or at recurrence.
The diagnosis of meningioma can be confirmed by immunohistochemical stains since most meningiomas express EMA (epithelial membrane antigen, [11]), progesterone receptor, and vimentin. Both EMA and progesterone receptor stains, however, can be faint, focal, or absent, particularly in high-grade subtypes [11]. A stain which can be quite helpful is somatostatin receptor 2A (SSTR2A, [11]), which shows strong and diffuse positivity in most meningiomas and whose expression is typically retained in grade 3 examples. This stain, however, needs to be evaluated carefully in the context of the overall histopathological, immunohistochemical, and molecular findings since SSTR2A can be expressed in a variety of other tumors occurring in the meninges, including solitary fibrous tumor.
Assessment of proliferative activity can be facilitated by the Ki67 stain, which highlights the most proliferative foci and therefore facilitates mitotic count. One caveat is, however, the presence of macrophages and tumor-infiltrating lymphocytes (TIL), which can spuriously increase the Ki67 counts [12]. Compared to mitotic counts, the assessment of frank anaplasia as a criterion for the diagnosis of anaplastic (malignant) meningioma is subject to greater interobserver variability and lower reproducibility. Extreme anaplasia and true sarcomatous (metaplastic) differentiation may make the diagnosis extremely challenging [11]. In both cases, molecular studies (see below) can help to identify a molecular signature supportive of the diagnosis.
Loss of H3 (histone 3, [13][14][15]) p.K28me3 (K27me3) has been reported in 10–20% of anaplastic (malignant) meningiomas, and it could be associated with decreased overall survival [13][14][15]. However, a recently published study failed to show a significant OS difference between patients with retained or lost H3-K27me3 [16].
2.2. Rhabdoid and Papillary Meningiomas
While in previous WHO classification rhabdoid and papillary meningiomas were considered malignant (WHO grade 3) simply based on their histological features, for both tumors now the presence of additional features, which fulfil criteria for classification as anaplastic (malignant) meningioma, irrespective of the rhabdoid or papillary phenotype, is required for a CNS WHO grade 3 designation.
Rhabdoid meningioma shows the presence of rhabdoid cells, which are plump cells with eccentric nuclei, open chromatin, macronucleoli, and prominent eosinophilic paranuclear inclusions [17]. Most of them are highly proliferative and usually fulfil the criteria for anaplastic meningioma grade 3 according to CNS WHO 2021.
Vaubel RA et al., showed that some meningiomas may show rhabdoid features only focally or lack high mitotic activity, and the behavior of these tumors is more in line with their histologic grade than with the rhabdoid appearance; they should therefore be graded similarly to non-rhabdoid meningiomas. The presence of rhabdoid features should, however, still be reported as some of these tumors may still behave aggressively. Close follow-up of these patients is required [18].
Papillary meningioma is characterized by a predominant perivascular papillary/pseudopapillary pattern [1][19]. The tumor cells typically are arranged in a perivascular pseudorosette-like pattern and at times show rhabdoid morphology. The presence of focal papillary architecture and/or the absence of other high-grade features in a meningioma with papillary architecture is not sufficient for designating the tumors as CNS WHO grade 3 [1]. Rhabdoid and papillary meningiomas can occur both in children and adult patients.
BAP1 (BRCA1-associated protein, [20]) mutations resulting in loss of BAP1 and loss of nuclear expression have been reported both in rhabdoid and papillary meningioma and can be associated with BAP1 tumor predisposition syndrome. In the study by Shankar et al., patients whose tumors were BAP1 negative had reduced time to recurence and required intensive clinical management [20].
2.3. Anaplastic (Malignant) Meningioma
The most frequent genetic alteration occurring in meningioma is the inactivation in the neurofibromatosis 2 genes (merlin) on chromosome 22q, which occurs in approximately 50% of meningiomas.
Mutations occurring in the non-NF2 (neurofibromatosis type 2 gene, [21])-mutated meningioma include TRAF7 (Tumor Necrosis Factor Receptor Associated Factor 7, [21]), AKT (protein kinase B), SMO (smoothened frizzled class receptor, [21]), and PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha, [21]) genes, which are strongly related to the meningioma subtypes and are typically associated with low-grade (CNS WHO grade 1) meningiomas [21].
Allelic losses in 22q12.2 regions, encoding the NF2 gene, are the most common abnormalities in this group of tumors (40–60% of cases). 22q loss of heterozygosity incidence increases with meningioma grade (75–85% in grade 3 meningioma) [22][23]. A double-hit mechanism is involved in the inactivation of merlin (69-kDa moesin–ezrin–radixin-like protein encoded by NF2 gene): 22q loss of heterozygosity followed by a second hit on the remaining gene (nonsense or frameshift or missense mutations or affecting splice sites, or interstitial deletions).
According to the CNS WHO classification, more than 30% of NF2-mutated meningiomas are grade 2–3 and recur more frequently than the others. NF2-mutated meningiomas show high chromosome instability during progression.
The accumulation of copy number losses, including 1p, 6p/q, 10q, 14q, and 18p/q and, less frequently, 2p/q, 3p, 4p/q, 7p, 8p/q, and 9p, compatible with instability is restricted to NF2-mutated meningioma. Recurrent genomic alterations, mainly involving CDKN2A/CDKN2B locus loss on 9p, are found frequently in meningiomas at recurrence as well as at progression and are associated with prognosis. CDKN2A and/or CDKN2B homozygous deletion is now considered sufficient for a CNS WHO grade 3 designation [24][25].
Gains of chromosomal arms on 1q/9q/12q/15q/17q/20q are less common and mostly found in specific low-grade subtype meningiomas [1].
TERT promoter mutations also occur mostly (although not exclusively) in NF2-altered meningioma and, although uncommon, are highly associated with grade and decreased time to recurrence/progression [26]. In particular, hotspot mutations (C228T and C250T) in the TERT promoter were detected in 20% of WHO grade 3 meningiomas compared to 1.7% and 5.7% of grade 1 and 2 meningiomas, respectively [1][26], and in 6.4% in a large cohort of meningiomas [27].
Maier et al. [28] reported that TERT promoter mutations can occur independently of malignant progression in meningioma. TERT promoter mutation was most often present from the primitive tumor tissue across recurrences in a consecutive single-center cohort of malignant meningioma.
Assessment of TERT promoter status has now been added as a criterion for a diagnosis of CNS WHO grade 3 meningioma independent of the histopathological findings.
While this brief discussion is limited to tumors which are diagnosed as anaplastic (malignant) meningioma in 2021 WHO CNS tumor classification, as it pertains to the full spectrum of meningioma, a critical clinical need is how to distinguish those patients with low or no risk of recurrence from those with an intermediate risk among tumors at present in the spectrum of CNS WHO grade 1 and 2 meningiomas. Integrated morphological and mostly molecularly based meningioma classifications incorporating copy number mutational profile and whole-genome methylation profile are being developed to better predict patient outcomes and inform clinical decision-making [27][28][29].
3. Radiological Features
On computed tomography (CT), meningiomas present usually as slightly hyperdense, extra-axial, well-circumscribed, dura-based masses that may show intratumoral calcifications, especially in slow-growing subtypes, frequently discovered incidentally. Adjacent remodeling of the skull or hyperostosis can be found [30][31].
To further characterize the tumoral lesion, gadolinium-enhanced magnetic resonance imaging (MRI) is the method of choice for the diagnosis and response assessment in meningioma patients [32][33].
Herein, meningiomas in general are hypo- to isointense on T1-weighted images and hypo- to hyperintense on T2-weighted images showing homogenous vivid contrast enhancement after intravenous application of contrast enhancement. Central necrosis, cysts, or perifocal oedema are not indicative to determine the tumor grade as they can occur in both benign and malignant meningiomas. In meningiomas, the adjacent dura is often thickened, and contrast-enhancing is known as dural tail, and a CSF (cerebrospinal fluid) cleft is often seen between the extra-axial tumoral mass and the adjacent brain cortex, which, however, can also be seen in other extra-axial masses such as dural metastases or solitary fibrous tumors [33].
Anaplastic (malignant) meningiomas are characterized by aggressive behavior presenting as loss of the CSF cleft, the missing demarcation between the tumoral mass and the adjacent brain parenchyma, and invasion of the surrounding tissues [34]. An example of a CNS WHO grade 3 meningioma is illustrated in Figure 1.
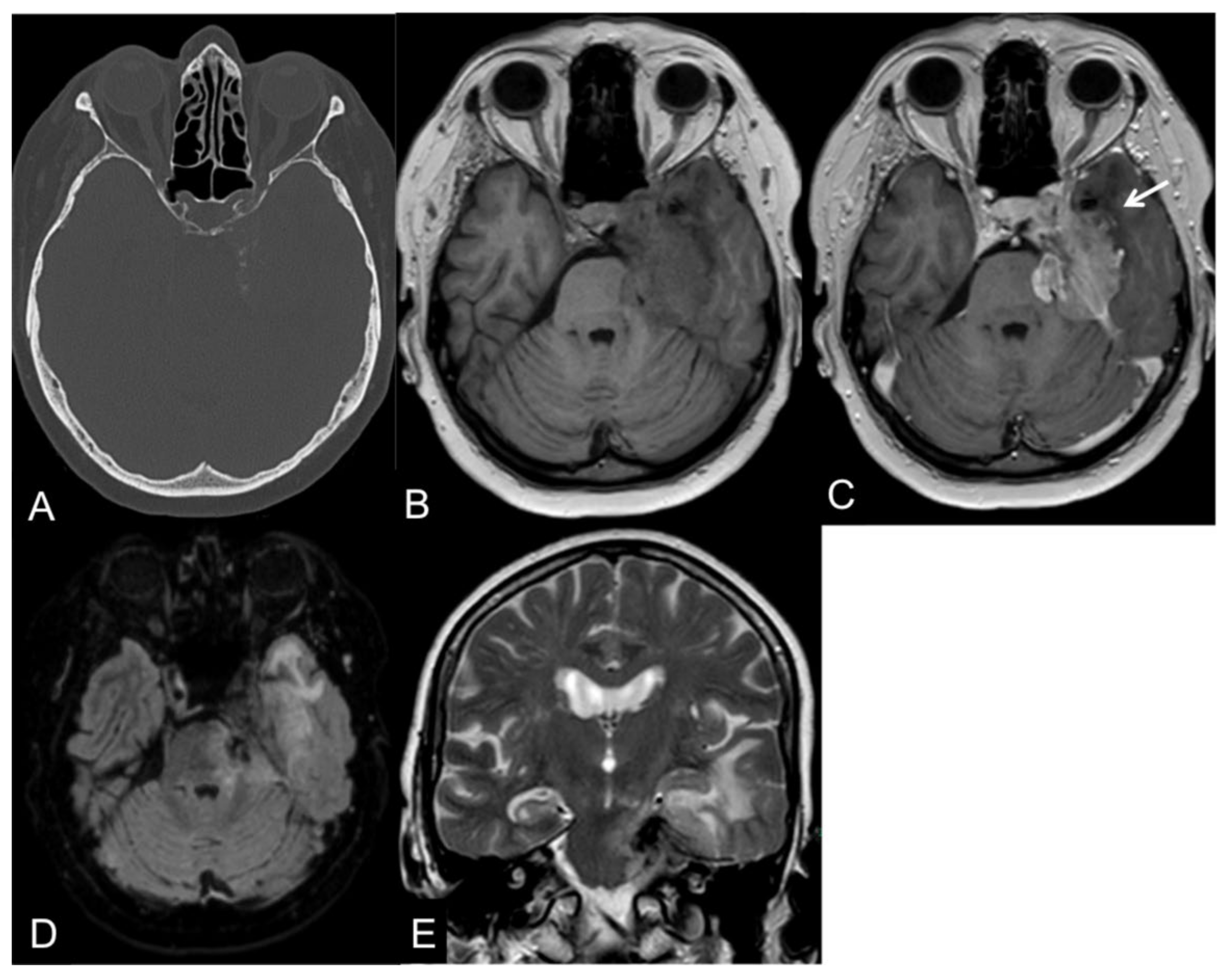
Figure 1. CT and MR images of a 69-year-old female patient with an anaplastic petroclival meningioma (III). The tumor shows calcifications in the CT scan (A). On T1-weight images, the intratumoral signal is iso- to hypointense in comparison to the gray matter (B) with a vivid contrast enhancement after intravenous gadolinium-based contrast enhancement (C) that extends into the adjacent brain parenchyma, representing parenchymal infiltration (arrow). On fluid-attenuated inversion recovery (FLAIR; (D)) and T2-weighted images (E), the tumor presents a central low signal intensity representing the intratumoral calcifications, an absence of the CSF cleft, and a moderate perifocal edema.
Diffusion-weighted imaging (DWI) is reported to aid in the differentiation between benign and malign meningiomas representing decreased apparent diffusion coefficient (ADC) values in the high-grade subtypes; however, the results are controversial [35][36][37].
While intratumoral relative cerebral blood volume (rCBV) does not differentiate between benign and malignant meningiomas, MR perfusion is increased in the perifocal oedema in malignant meningiomas due to the local infiltration of tumor cells [38].
In MR-spectroscopy, meningiomas are characterized by increased choline and alanine peaks, whereas N-acetyl aspartate and creatine peaks are reduced [30].
Recently, radiomics is increasingly gaining importance in neuro-oncological imaging correlating quantitative radiological features with, e.g., histopathological or molecular tumor subtypes. Several studies have reported a potential role of radiographic features, e.g., shape or texture in predicting tumor grade in noninvasive meningioma (WHO grade I meningioma) [39][40][41].
The Response Assessment in Neuro-Oncology (RANO) Meningioma Working Group proposed response criteria, especially for clinical trials in meningioma patients based on the standardized Brain Tumor Imaging Protocol including a 3D T1-weighted contrast-enhanced MR sequence with a slice thickness of ≤1.5 mm [33][42]. These response criteria are solely eligible for fast-growing meningiomas (showing a 15% increase in the sum of the products of perpendicular diameters within the last 6 months) or if a new lesion has developed. For slow-growing meningiomas, more sensitive indicators of response, such as a change in the rate of growth, may be more appropriate [33].
Complete response (CR) is defined as an absence of all contrast-enhancing lesions for at least 8 weeks. Given the low rate of response expected, particularly in grade I meningiomas, the category minor response (MR) is determined as a reduction in the product of the maximum perpendicular diameters of 25% or more but less than 50% has been added. If the decrease exceeds 50%, it is characterized as partial response (PR). In either case, the reduction has to sustain for at least 8 weeks. An increase by ≥25% in the sum of the product of perpendicular diameters of target lesions compared with the smallest tumor measurement, any new lesion, or considerable progression of nontarget lesions are specified as progressive disease (PD). If none of the above-mentioned criteria is suitable, it is defined as a stable disease (SD). Besides the radiological criteria, the usage of corticosteroids and the clinical status has to be taken into account [33].
4. Systemic Treatments
Although several agents have been tested in meningiomas refractory to surgery and radiation therapy, none of them suggested a clear clinical efficacy. Thus, to date, there is not a standard of care for systemic management of the disease, and the inclusion of these patients in clinical trials remains the best therapeutic option.
Temozolomide and irinotecan have been assessed in small phase II trials showing a modest efficacy [43][44]. More recently, trabectedin was investigated in a randomized phase II trial [45]. The comparator arm was the local standard of care and patients with recurrent grade 2 or 3 meningiomas. In this trial, there was no additional benefit in terms of OS and PFS with the administration of trabectedin. Of note, this study confirmed that the DNA methylation class of meningiomas was an independent prognostic factor for OS [45].
Meningiomas often express somatostatin receptors; therefore, somatostatin analogues and receptor radionuclide therapy have been tested in these tumors [46][47][48]. Trials investigating somatostatin analogues demonstrated a modest clinical activity of these compounds mainly resulting in reduced tumor growth. Octreotide shows to reduce cell proliferation but does not induce apoptosis of cancer cells [46][47][48]. In addition, peptide receptor radionuclide therapy failed to show a tumor shrinkage. Nonetheless, the use of 177Lu-DOTATOC was associated with a high percentage of stable disease [49].
In a meta-analysis carried out by Mirian C et al., 111 patients with treatment-refractory meningiomas received somatostatin receptor-targeted radiopeptide therapy [50]. Of the 19 patients with grade 3 meningioma included, the 6-month PFS was 0%, while the 1-year OS rate was 52% [50].
The evidence that meningioma is a largely vascularized tumor has led to the investigation of agents targeting angiogenesis. Inhibitors of the vascular endothelial growth factor (VEGF) such as bevacizumab [51][52] and the VEGF receptor (VEGFR) such as sunitinib and vatalanib [53] did not result in tumor responses but reached a high grade of 6 months PFS. In particular, sunitinib [54] administration in patients with refractory grade 2 or 3 meningiomas was associated with a 6-month PFS of 42% and a median OS of 24.6 months [54].
Other target molecules inhibiting the epidermic growth factor receptor (EFGFR) or the stem cell factor receptor (KIT) showed modest clinical efficacy in phase II mono-arm studies [55][56].
Meningiomas express the inactivation of the NF2 in about 50% of cases. The inactivation of NF2 resulted in overexpression of the mammalian target of rapamycin complex 1 (mTORC1). The mTOR inhibitor everolimus has been tested in combination with bevacizumab and octreotide [57][58]. The CEVOREM trial assessed the combination between everolimus and octreotide. In this trial, the 1-year OS detected was 75%. Notably, about 80% of patients reported a decrease in the tumor growth rate of more than 50% [57].
Immune-checkpoint inhibitors (ICIs) are monoclonal antibodies able to restore an inhibited immune response against tumor cells. Two agents targeting the programmed death receptor 1 (PD-1) have been tested in refractory grade 2 or 3 meningiomas [59][60]. Pembrolizumab has been recently investigated in a small phase 2 trial on 25 patients [59]. This trial reaches its primary endpoint with a 6-month PFS of 48%. The PD-1 inhibitor nivolumab failed to meet its primary endpoint and reach a 6-month PFS of 42.4% [60]; however, nivolumab administration was associated with a median OS of 30.9 months and led to a long-course radiographic response. Notably, both these studies reported that a subgroup of patients with refractory meningioma could be more likely to benefit from ICIs administration [59][60].
There are several novel molecules and treatments under investigation in refractory and grade 3 meningiomas. The only phase 3 trial assessing systemic agents which are currently recruiting patients is the POPLAR-NF2 trial (NCT05130866). This trial has a placebo as a comparator arm and is investigating the pan-histone deacetylase inhibitor REC 2282 in patients with germinal or sporadic NF2 mutated meningioma. The other two histone deacetylase inhibitors are under investigation in patients with refractory meningioma. These are Panobinostat (in combination with radiation therapy, NCT01324635) and AR-42 (NCT02282917). The mTORC 1/2 dual inhibitor vistusertib showed promising clinical activity in preclinical [61] studies and is under investigation in two phase II clinical trials (NCT03071874, NCT02831257). The mitogen-activated protein kinase (MEK) inhibitor selumetinib (NCT03095248) and trametinib (NCT03631953) in combination with the PI3K inhibitor alpelisib and the cyclin-dependent kinase ribociclib (NCT02933736) are other agents under investigation. Tazemetostat is an (Enhancer Of Zeste 2 Polycomb Repressive Complex 2 Subunit) EZH2 inhibitor which is currently under investigation in patients with BAP-1 mutated meningioma (NCT02860286). Preclinical studies suggest that NF2 mutated meningiomas could be more sensitive to the inhibition of the focal adhesion kinase (FAK) [62]; thus, a clinical trial involving a FAK inhibitor is currently ongoing (NCT02523014). Finally, other studies investigating ICIs are still ongoing and will respond to the clinical efficacy of PD-1 and CTLA-4 (cytotoxic T-lypmhocyte antigen 4) combination therapy (NCT02648997) as well as a combination of proton therapy and PD-1 inhibition with avelumab (NCT03267836). Notably, none of the mentioned trials is tailored for patients with grade 3 meningiomas, but about all of them allow the inclusion of patients with refractory meningiomas.
The Forkhead box M1 (FOXM1) transcription factor is an oncogenic driver often altered in high-grade meningioma and tumor aggressiveness [63]. Targets of FOXM1 have shown promising activity in patients with solid tumors, including gliomas [64][65]. It could be possible that these agents could be also assessed in MM patients in the coming future. NF2 mutated meningioma can often express the oncogene receptor FGFR (fibroblast growth factor receptor) and could be targeted by specific FGFR inhibitors [66]. To date, the FGFR inhibitor pemigatinib is under assessment in different primary central nervous system malignancies harboring activating FGFR alterations (NCT05267106).
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-oncology 2021, 23, 1231–1251.
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro-oncology 2015, 17 (Suppl. 4), iv1–iv62.
- Garzon-Muvdi, T.; Yang, W.; Lim, M.; Brem, H.; Huang, J. Atypical and anaplastic meningioma: Outcomes in a population based study. J. Neurooncol. 2017, 133, 321–330.
- Hanft, S.; Canoll, P.; Bruce, J.N. A review of malignant meningiomas: Diagnosis, characteristics, and treatment. J. Neurooncol. 2010, 99, 433–443.
- Peyre, M.; Gauchotte, G.; Giry, M.; Froehlich, S.; Pallud, J.; Graillon, T.; Bielle, F.; Cazals-Hatem, D.; Varlet, P.; Figarella-Branger, D.; et al. De novo and secondary anaplastic meningiomas: A study of clinical and histomolecular prognostic factors. Neuro-oncology 2018, 20, 1113–1121.
- Zhang, G.J.; Zhang, Y.S.; Zhang, G.B.; Li, D.; Zhang, L.W.; Wu, Z.; Zhang, J.T. Prognostic factors and the management of anaplastic meningioma. Clin. Neurol. Neurosurg. 2018, 170, 13–19.
- Zhao, P.; Hu, M.; Zhao, M.; Ren, X.; Jiang, Z. Prognostic factors for patients with atypical or malignant meningiomas treated at a single center. Neurosurg. Rev. 2015, 38, 101–107, discussion 107.
- Moliterno, J.; Cope, W.P.; Vartanian, E.D.; Reiner, A.S.; Kellen, R.; Ogilvie, S.Q.; Huse, J.T.; Gutin, P.H. Survival in patients treated for anaplastic meningioma. J. Neurosurg. 2015, 123, 23–30.
- Champeaux, C.; Jecko, V. World Health Organization grade III meningiomas. A retrospective study for outcome and prognostic factors assessment. Neurochirurgie 2016, 62, 203–208.
- Low, J.T.; Ostrom, Q.T.; Cioffi, G.; Neff, C.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. Primary brain and other central nervous system tumors in the United States (2014–2018): A summary of the CBTRUS statistical report for clinicians. Neurooncol. Pract. 2022, 9, 165–182.
- Lucas, C.G.; Devine, P.; Solomon, D.A.; Giannini, C.; Reifenberger, G.; Dahiya, S.; Caccamo, D.; Perry, A. Sarcomatous Meningioma: Diagnostic Pitfalls and the Utility of Molecular Testing. J. Neuropathol. Exp. Neurol. 2021, 80, 764–768.
- Bale, T.A.; Benhamida, J.; Roychoudury, S.; Villafania, L.; Wrzolek, M.A.; Bouffard, J.P.; Bapat, K.; Ladanyi, M.; Rosenblum, M.K. Infarction with associated pseudosarcomatous changes mimics anaplasia in otherwise grade I meningiomas. Mod. Pathol. 2020, 33, 1298–1306.
- Gauchotte, G.; Peyre, M.; Pouget, C.; Cazals-Hatem, D.; Polivka, M.; Rech, F.; Varlet, P.; Loiseau, H.; Lacomme, S.; Mokhtari, K.; et al. Prognostic Value of Histopathological Features and Loss of H3K27me3 Immunolabeling in Anaplastic Meningioma: A Multicenter Retrospective Study. J. Neuropathol. Exp. Neurol. 2020, 79, 754–762.
- Katz, L.M.; Hielscher, T.; Liechty, B.; Silverman, J.; Zagzag, D.; Sen, R.; Wu, P.; Golfinos, J.G.; Reuss, D.; Neidert, M.C.; et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018, 135, 955–963.
- Nassiri, F.; Wang, J.Z.; Singh, O.; Karimi, S.; Dalcourt, T.; Ijad, N.; Pirouzmand, N.; Ng, H.K.; Saladino, A.; Pollo, B.; et al. Loss of H3K27me3 in meningiomas. Neuro-oncology 2021, 23, 1282–1291.
- Maier, A.D.; Brøchner, C.B.; Mirian, C.; Haslund-Vinding, J.; Bartek, J., Jr.; Ekström, T.J.; Poulsen, F.R.; Scheie, D.; Mathiesen, T. Loss of H3K27me3 in WHO grade 3 meningioma. Brain Tumor Pathol. 2022.
- Perry, A.; Scheithauer, B.W.; Stafford, S.L.; Abell-Aleff, P.C.; Meyer, F.B. “Rhabdoid” meningioma: An aggressive variant. Am. J. Surg. Pathol. 1998, 22, 1482–1490.
- Vaubel, R.A.; Chen, S.G.; Raleigh, D.R.; Link, M.J.; Chicoine, M.R.; Barani, I.; Jenkins, S.M.; Aleff, P.A.; Rodriguez, F.J.; Burger, P.C.; et al. Meningiomas With Rhabdoid Features Lacking Other Histologic Features of Malignancy: A Study of 44 Cases and Review of the Literature. J. Neuropathol. Exp. Neurol. 2016, 75, 44–52.
- Ludwin, S.K.; Rubinstein, L.J.; Russell, D.S. Papillary meningioma: A malignant variant of meningioma. Cancer 1975, 36, 1363–1373.
- Shankar, G.M.; Abedalthagafi, M.; Vaubel, R.A.; Merrill, P.H.; Nayyar, N.; Gill, C.M.; Brewster, R.; Bi, W.L.; Agarwalla, P.K.; Thorner, A.R.; et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro-oncology 2017, 19, 535–545.
- Yuzawa, S.; Nishihara, H.; Tanino, M.; Kimura, T.; Moriya, J.; Kamoshima, Y.; Nagashima, K.; Tanaka, S. A case of cerebral astroblastoma with rhabdoid features: A cytological, histological, and immunohistochemical study. Brain Tumor Pathol. 2016, 33, 63–70.
- Peyre, M.; Kalamarides, M. Molecular genetics of meningiomas: Building the roadmap towards personalized therapy. Neurochirurgie 2018, 64, 22–28.
- Suppiah, S.; Nassiri, F.; Bi, W.L.; Dunn, I.F.; Hanemann, C.O.; Horbinski, C.M.; Hashizume, R.; James, C.D.; Mawrin, C.; Noushmehr, H.; et al. Molecular and translational advances in meningiomas. Neuro-oncology 2019, 21, i4–i17.
- Boström, J.; Meyer-Puttlitz, B.; Wolter, M.; Blaschke, B.; Weber, R.G.; Lichter, P.; Ichimura, K.; Collins, V.P.; Reifenberger, G. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am. J. Pathol. 2001, 159, 661–669.
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413.
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2016, 108, djv377.
- Goutagny, S.; Nault, J.C.; Mallet, M.; Henin, D.; Rossi, J.Z.; Kalamarides, M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014, 24, 184–189.
- Maier, A.D.; Stenman, A.; Svahn, F.; Mirian, C.; Bartek, J., Jr.; Juhler, M.; Zedenius, J.; Broholm, H.; Mathiesen, T. TERT promoter mutations in primary and secondary WHO grade III meningioma. Brain Pathol. 2021, 31, 61–69.
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852.
- Buhl, R.; Nabavi, A.; Wolff, S.; Hugo, H.H.; Alfke, K.; Jansen, O.; Mehdorn, H.M. MR spectroscopy in patients with intracranial meningiomas. Neurol. Res. 2007, 29, 43–46.
- Zeng, L.; Liang, P.; Jiao, J.; Chen, J.; Lei, T. Will an Asymptomatic Meningioma Grow or Not Grow? A Meta-analysis. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2015, 76, 341–347.
- Maggio, I.; Franceschi, E.; Tosoni, A.; Nunno, V.D.; Gatto, L.; Lodi, R.; Brandes, A.A. Meningioma: Not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021, 10, Cns72.
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the Response Assessment in Neuro-Oncology Working Group. Neuro-oncology 2019, 21, 26–36.
- O’Leary, S.; Adams, W.M.; Parrish, R.W.; Mukonoweshuro, W. Atypical imaging appearances of intracranial meningiomas. Clin. Radiol. 2007, 62, 10–17.
- Filippi, C.G.; Edgar, M.A.; Uluğ, A.M.; Prowda, J.C.; Heier, L.A.; Zimmerman, R.D. Appearance of meningiomas on diffusion-weighted images: Correlating diffusion constants with histopathologic findings. AJNR Am. J. Neuroradiol. 2001, 22, 65–72.
- Nagar, V.A.; Ye, J.R.; Ng, W.H.; Chan, Y.H.; Hui, F.; Lee, C.K.; Lim, C.C. Diffusion-weighted MR imaging: Diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am. J. Neuroradiol. 2008, 29, 1147–1152.
- Santelli, L.; Ramondo, G.; Della Puppa, A.; Ermani, M.; Scienza, R.; d’Avella, D.; Manara, R. Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir. 2010, 152, 1315–1319, discussion 1319.
- Zhang, H.; Rödiger, L.A.; Shen, T.; Miao, J.; Oudkerk, M. Perfusion MR imaging for differentiation of benign and malignant meningiomas. Neuroradiology 2008, 50, 525–530.
- Chen, C.; Guo, X.; Wang, J.; Guo, W.; Ma, X.; Xu, J. The Diagnostic Value of Radiomics-Based Machine Learning in Predicting the Grade of Meningiomas Using Conventional Magnetic Resonance Imaging: A Preliminary Study. Front. Oncol. 2019, 9, 1338.
- Morin, O.; Chen, W.C.; Nassiri, F.; Susko, M.; Magill, S.T.; Vasudevan, H.N.; Wu, A.; Vallières, M.; Gennatas, E.D.; Valdes, G.; et al. Integrated models incorporating radiologic and radiomic features predict meningioma grade, local failure, and overall survival. Neurooncol. Adv. 2019, 1, vdz011.
- Park, Y.W.; Oh, J.; You, S.C.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Lee, S.K. Radiomics and machine learning may accurately predict the grade and histological subtype in meningiomas using conventional and diffusion tensor imaging. Eur. Radiol. 2019, 29, 4068–4076.
- Ellingson, B.M.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro-oncology 2015, 17, 1188–1198.
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Temozolomide for treatment-resistant recurrent meningioma. Neurology 2004, 62, 1210–1212.
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J. Neurooncol. 2006, 78, 271–276.
- Preusser, M.; Silvani, A.; Le Rhun, E.; Soffietti, R.; Lombardi, G.; Sepulveda, J.M.; Brandal, P.; Brazil, L.; Bonneville-Levard, A.; Lorgis, V.; et al. Trabectedin for recurrent WHO grade 2 or 3 meningioma: A randomized phase 2 study of the EORTC Brain Tumor Group (EORTC-1320-BTG). Neuro-oncology 2021, 24, 755–767.
- Chamberlain, M.C.; Glantz, M.J.; Fadul, C.E. Recurrent meningioma: Salvage therapy with long-acting somatostatin analogue. Neurology 2007, 69, 969–973.
- Norden, A.D.; Ligon, K.L.; Hammond, S.N.; Muzikansky, A.; Reardon, D.A.; Kaley, T.J.; Batchelor, T.T.; Plotkin, S.R.; Raizer, J.J.; Wong, E.T.; et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 2015, 84, 280–286.
- Simó, M.; Argyriou, A.A.; Macià, M.; Plans, G.; Majós, C.; Vidal, N.; Gil, M.; Bruna, J. Recurrent high-grade meningioma: A phase II trial with somatostatin analogue therapy. Cancer Chemother. Pharmacol. 2014, 73, 919–923.
- Marincek, N.; Radojewski, P.; Dumont, R.A.; Brunner, P.; Müller-Brand, J.; Maecke, H.R.; Briel, M.; Walter, M.A. Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: Long-term results of a phase II clinical trial. J. Nucl. Med. 2015, 56, 171–176.
- Mirian, C.; Duun-Henriksen, A.K.; Maier, A.; Pedersen, M.M.; Jensen, L.R.; Bashir, A.; Graillon, T.; Hrachova, M.; Bota, D.; van Essen, M.; et al. Somatostatin Receptor-Targeted Radiopeptide Therapy in Treatment-Refractory Meningioma: Individual Patient Data Meta-analysis. J. Nucl. Med. 2021, 62, 507–513.
- Nayak, L.; Iwamoto, F.M.; Rudnick, J.D.; Norden, A.D.; Lee, E.Q.; Drappatz, J.; Omuro, A.; Kaley, T.J. Atypical and anaplastic meningiomas treated with bevacizumab. J. Neurooncol. 2012, 109, 187–193.
- Lou, E.; Sumrall, A.L.; Turner, S.; Peters, K.B.; Desjardins, A.; Vredenburgh, J.J.; McLendon, R.E.; Herndon, J.E., 2nd; McSherry, F.; Norfleet, J.; et al. Bevacizumab therapy for adults with recurrent/progressive meningioma: A retrospective series. J. Neurooncol. 2012, 109, 63–70.
- Raizer, J.J.; Grimm, S.A.; Rademaker, A.; Chandler, J.P.; Muro, K.; Helenowski, I.; Rice, L.; McCarthy, K.; Johnston, S.K.; Mrugala, M.M.; et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J. Neurooncol. 2014, 117, 93–101.
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; DeAngelis, L.M.; Gavrilovic, I.; et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro-oncology 2015, 17, 116–121.
- Norden, A.D.; Raizer, J.J.; Abrey, L.E.; Lamborn, K.R.; Lassman, A.B.; Chang, S.M.; Yung, W.K.; Gilbert, M.R.; Fine, H.A.; Mehta, M.; et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J. Neurooncol. 2010, 96, 211–217.
- Wen, P.Y.; Yung, W.K.; Lamborn, K.R.; Norden, A.D.; Cloughesy, T.F.; Abrey, L.E.; Fine, H.A.; Chang, S.M.; Robins, H.I.; Fink, K.; et al. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08). Neuro-oncology 2009, 11, 853–860.
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557.
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B., 3rd; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A., 3rd; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J. Neurooncol. 2016, 129, 281–288.
- Brastianos, P.K.; Kim, A.E.; Giobbie-Hurder, A.; Lee, E.Q.; Wang, N.; Eichler, A.F.; Chukwueke, U.; Forst, D.A.; Arrillaga-Romany, I.C.; Dietrich, J.; et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat. Commun. 2022, 13, 1325.
- Bi, W.L.; Nayak, L.; Meredith, D.M.; Driver, J.; Du, Z.; Hoffman, S.; Li, Y.; Lee, E.Q.; Beroukhim, R.; Rinne, M.; et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results. Neuro-oncology 2022, 24, 101–113.
- Beauchamp, R.L.; James, M.F.; DeSouza, P.A.; Wagh, V.; Zhao, W.N.; Jordan, J.T.; Stemmer-Rachamimov, A.; Plotkin, S.R.; Gusella, J.F.; Haggarty, S.J.; et al. A high-throughput kinome screen reveals serum/glucocorticoid-regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget 2015, 6, 16981–16997.
- Shapiro, I.M.; Kolev, V.N.; Vidal, C.M.; Kadariya, Y.; Ring, J.E.; Wright, Q.; Weaver, D.T.; Menges, C.; Padval, M.; McClatchey, A.I.; et al. Merlin deficiency predicts FAK inhibitor sensitivity: A synthetic lethal relationship. Sci. Transl. Med. 2014, 6, 237ra268.
- Kim, H.; Park, K.J.; Ryu, B.K.; Park, D.H.; Kong, D.S.; Chong, K.; Chae, Y.S.; Chung, Y.G.; Park, S.I.; Kang, S.H. Forkhead box M1 (FOXM1) transcription factor is a key oncogenic driver of aggressive human meningioma progression. Neuropathol. Appl. Neurobiol. 2020, 46, 125–141.
- Tang, J.H.; Yang, L.; Chen, J.X.; Li, Q.R.; Zhu, L.R.; Xu, Q.F.; Huang, G.H.; Zhang, Z.X.; Xiang, Y.; Du, L.; et al. Bortezomib inhibits growth and sensitizes glioma to temozolomide (TMZ) via down-regulating the FOXM1-Survivin axis. Cancer Commun. 2019, 39, 81.
- Borhani, S.; Gartel, A.L. FOXM1: A potential therapeutic target in human solid cancers. Expert Opin. Ther. Targets 2020, 24, 205–217.
- Das, A.; Martinez Santos, J.L.; Alshareef, M.; Porto, G.B.F.; Infinger, L.K.; Vandergrift, W.A., 3rd; Lindhorst, S.M.; Varma, A.K.; Patel, S.J.; Cachia, D. In Vitro Effect of Dovitinib (TKI258), a Multi-Target Angiokinase Inhibitor on Aggressive Meningioma Cells. Cancer Investig. 2020, 38, 349–355.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
709
Revisions:
2 times
(View History)
Update Date:
21 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




