
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arthur Richard Horrocks | + 3495 word(s) | 3495 | 2020-09-28 10:22:18 |
Video Upload Options
Organobromine flame retardants have been well-established for many years but because of environmental concerns have been under significant pressures to reduce their usage. However, these retardants are most always used in the presence of synergists, primarily those based on antimony compounds such as antimony III oxide, which also is associated with toxicological issues. This entry compares this with current available and potentially more environmentally sustainable synergists such as the zinc stannates and the more recently studied zinc tungstate, both of which also offer smoke suppressing properties.
1. Introduction
Organobromine-based flame retardants (BrFRs) for bulk polymers and textiles are generally considered to be the most abundantly used after aluminium trihydrate. Environmental concerns expressed during the last 20 years have resulted in certain BrFRs being banned or regulated and has ensured that few if any new ones have been developed during this period [1][2]. While the world market for and use of flame retardants continues to grow, the environmental pressures on BrFR usage, however, especially during the last 10 years, have been such as to see their gradual replacement by phosphorus- and/or nitrogen-containing species in the main, although recently, the potential environmental toxicity of selected organophosphorus-based retardants has also come under scrutiny [3]. It is likely, therefore, that in the foreseeable future, their share will decline, although the possibility of the development of bio-based BrFRs has recently been proposed.
2. Organobromine Flame Retardant Synergists
In addition to the above-mentioned toxicological concerns raised regarding currently available BrFRs and their use, since they are almost always in the presence of a synergist, notably those based on antimony compounds, which have known toxicological properties [4][5], these too have fallen under environmental scrutiny. The principal antimony-containing synergist is antimony III oxide, which although extremely effective in terms of flame retardancy and cost, increases smoke and toxic gas development during a fire. Current commercial alternatives such as the zinc stannates are available, which while being more selective in terms of their effectiveness with BrFRs, they have no known toxicological properties and offer smoke suppressing properties. However, they are more expensive than Sb2O3, and so are often used in high value applications, including engineering polymers. Recent research has also demonstrated the synergistic and smoke suppressing properties of zinc tungstate. Below is a brief overview of how antimony III oxide functions, both in terms of its advantageous and disadvantageous characteristics, and how this compares with the properties of alternatives available, which now should be more effectively considered because of associated environmental concerns.
2.1. Antimony III Oxide
While it has been recognised that a number of antimony compounds act as synergists for halogen-containing flame retardants, antimony III oxide, Sb2O3, is that most commonly used, largely based on its lower cost and its apparent properties of being independent of BrFR compound chemical structure and polymer type in which it is present. The history of its use as a potential flame retardant in its own right goes back over a century [6], although its use as a synergist in combination with chlorine-containing species stems from World War II and work by the US Army in 1944, as described fully by Little later in 1947 in what must have been one of the first texts describing the flame retarding of textile fabrics [7]. This early work identified the role of hydrogen chloride as being of major importance. At the same time, Coppick of the American Viscose Corporation [8] identified a number of important features regarding the effect of antimony III oxide (ATO or Sb2O3) in the presence of a neoprene rubber coated on to cotton for use as military tentage including:
- the effectiveness of the Sb2O3/neoprene combination in regard to HCl generation;
- the identification of an optimum Sb2O3/Cl ratio equivalent to antimony oxyhalide, SbOCl, formation as an intermediate;
- the use of additional boric acid, zinc borate or an ammonium phosphate as afterglow retardants; and
- the combination of the neoprene/Sb2O3 combination alone was wash durable, but not afterglow retardant.
Since that time a significant amount of work has been undertaken to understand the mechanism of chlorine-containing flame retardant-Sb2O3 synergism, notably by Costa et al. [9][10], which has identified a series of chlorination reactions of the synergist via a series of complex volatile antimony oxyhalides leading eventually to the formation of SbOCl and then SbCl3. Parallel work showed that for decabromodiphenyl ether (DecaBDE)-Sb2O3 combinations, a similar sequence of reactions occurred with the generally more rapid formation of SbBr3 [11]. This most likely explains why in antimony-bromine flame retardants generally, including those used in textile coating and back-coating applications [12], the optimum antimony: bromine molar ratio = 1:3. The main flame retarding activity of both Sb-Cl and Sb-Br formulations involves reaction or either SbCl3 or SbBr3 with the major flame propagating radicals H·, O·, OH·, and HO2· and derived hydrogen halides, HX, via a series of complex chain reactions [13][14]. The principal propagation and termination reactions may be more simply summarised as:
|
SbX3 + H· → HX + SbX2· |
(1) |
|
SbX2· + H· → SbX· + HX |
(2) |
|
SbX· + H· → Sb + HX |
(3) |
|
HX + H· → H2 + X· (leading to flame inhibition) |
(4) |
|
X· + HO2· → HX + O2 |
(5) |
|
HX + OH· → H2O + X· |
(6) |
Hastie also proposed other termination/flame inhibition reactions based on reactions of the type:
|
SbOH + H· → SbO + H2 |
(7) |
|
SbO + H· → SbOH |
(8) |
where the formation of antimony oxy-species derives from reaction with atomic oxygen O· or OH· radicals. These stages are similar to those proposed also for the flame extinguishing effects of tin compounds as shall be discussed in the next section.
Generally, Sb2O3 appears to be equally efficient with the current commercial range of chlorinated and brominated flame retardants available, thus suggesting that the above chemical mechanisms are little affected by the chemical character of the halogenated flame retardant component or the polymer matrix they are present in or textile material they are applied to. Furthermore, while the gas phase mechanisms as outlined above are considered to be the major, if not sole flame retardant mechanism operating in a given polymer matrix, since there is evolution of either HCl or HBr, these as acids, will promote condensed phase char-forming reactions in polymers like cellulose, poly(vinyl alcohol) and poly(vinyl acetate) (and related copolymers), via dehydration of the pendant –OH groups present. In coating and back-coating formulations, this char-forming character of the resin component present is crucial in offering an additional char barrier to the exposed textile substrate. This is particularly important if the underlying textile is simply fusible and not char-forming like polypropylene and polyester, commonly used in both domestic and contract furnishing fabrics. In poly(vinyl chloride)-based coatings for textiles, the need for the presence of a plasticiser, which reduces the inherent flame retardant effect of the chlorine content, requires the presence of Sb2O3 to restore it to an acceptable level. Because of its very low particle size (see Table 1), antimony III oxide may be easily extruded as a dispersed additive in fibres and so when included in modacrylic fibres, which typically comprise a copolymer based on acrylonitrile and vinylidene chloride, their inherent flame retardancy is enhanced, although the presence of these fine particles may change the fibre lustre and aesthetics of the resulting fabrics.
Table 1. Properties of commercial grades of antimony III oxide and zinc stannates.
|
Antimony III Oxide, Sb2O3 |
Zinc Hydroxystannate (ZHS), Zn Sn(OH) 6 |
Zinc Stannate (ZS), ZnSn03 |
|
White powder |
White powder |
White powder |
|
% Antimony 83.5 |
% Tin 41.0–43.0 |
% Tin 53.0–56.0 |
|
|
% Zinc 22.0–23.5 |
% Zinc 26.2–27.5 |
|
Water solubility 0.001g/100 mL water, 25 °C |
% Moisture 0.7 max |
% Moisture 0.5 max |
|
Average particle size 0.4–1.8 microns |
Average particle size (d50) 1.4–2.2 microns |
Average particle size (d50) 1.4–2.2. microns |
However, because of the very effective gas phase flame inhibiting reactions of these systems exemplified in Equations 4, 7 and 8 above, incomplete combustion obviously occurs with increased concentrations of toxic fire gases [15] and especially smoke [16] being the outcomes, both of which are the major causes of loss of life in fires. In addition to the above flame inhibiting reactions, the concentration of complex particulate materials, including complex polynuclear aromatic hydrocarbons [17], will be enhanced by the debromination and dehydrobromination of the BrFRs present. Thus antimony-bromine flame retardant formulations have significantly increased smoke and carbon monoxide levels associated with them relative to the pure polymer matrix during burning.
Furthermore, afterglow may also be a problem as first documented many years ago, as was its suppression by the addition of ammonium phosphate and zinc borate. Zinc borate not only is a smoke suppressant but also interacts positively with antimony III oxide in its catalytic activity [18]. However, while the toxicity of zinc borate did not cause concern 20 years ago [19], more recently it has been classed as a Category 2 Reproductive Toxicant according to the Global Harmonized System (GHS) for Classification and Labelling of Chemicals and is toxic to aquatic life [20]. Not surprisingly, these factors have added to the ecotoxicological concerns regarding the use of halogen-containing flame retardants in recent years.
While a possible ban or restriction of use of antimony III oxide and other antimony-based synergists may be called for in the future, there are conflicting reports with regard to its commercial importance in any case. For example, market predictions suggest that at the present time about 50% of antimony used in the world is as synergists for halogen-containing polymers and flame retardants with the likelihood of continued growth in total global usage [21]. This also suggests a continuing increase in the use of halogen-containing flame retardants and polymers in spite of increasing environmental pressures. On the other hand and in spite of the previously mentioned toxicological concerns, there have been pessimistic predictions about its continuing availability as a globally sourced mineral to the extent that unless serious recycling of antimony III oxide is undertaken, the world’s known reserves will have become sufficiently depleted to be uneconomically viable before 2050 [22].
2.2. Zinc stannates
At the present time, the only commercial alternatives to antimony III oxide as an effective synergist are zinc hydroxystannate (ZnHS) and zinc stannate (ZnS) and their development since their origins in about 1990 have been reviewed [23]. Their general characteristics are also listed in Table 1. They are generally assumed to behave in a manner similar to antimony III oxide in their synergistic activity with halogenated polymers and flame retardants but in addition, they have considerable smoke and carbon monoxide suppressing activity [24] and may also promote char formation. Both ZnHS and ZnS are used successfully in applications involving key polymers like PVC coatings, polyamide engineering plastics and unsaturated polyester resins used in combination with fibre and textile reinforcing elements. However, unlike antimony III oxide, both ZnHS and ZnS are genuine mixed oxides where the zinc and tin atoms are built into a crystal lattice rather than as simple oxide blends. It is the chemical arrangement of the zinc and the tin within the crystal structure which gives these materials their fire protection performance in combination with halogen-containing species, although differing is some respects from the chemically simpler Sb2O3 in terms of selectivity of synergistic efficiency with different BrFRs and polymer or textile substrates (see below).
Whether or not ZnHS or ZnS is chosen for a particular application depends on the processing temperatures used, since in the former its hydroxyl groups are driven off by heat at about 180°C in the initial stages of a fire, thereby giving additional cooling and slowing the combustion reaction. Zinc stannate, however, is stable up to 400 °C and so can withstand processing in melt extrusion and similar processes in polymers that may melt at temperatures approaching 300°C. A major feature is that at the present time, both stannates have had no undesirable toxicological properties identified and so they are considered to be more environmentally sustainable than ATO. Furthermore, unlike the latter, which shows no flame retardant activity when used alone unless present in a halogenated polymer like PVC, both ZnHS and ZnS can be used alone in non-halogenated polymeric systems as char-promoters and smoke suppressants.
In fibre-forming polymers like polypropylene [25] and polyamides 6 and 6.6 [26] synergistic behaviour of the zinc stannates with halogenated additives has been demonstrated, although because of the relatively high levels of flame retardant required, acceptable fibres have yet to be reported.
Initial mechanistic work by Cusack [27] proposed that an ideal mole ratio Sn:halogen = 1:4 is considered to be the optimal ratio based on the observed volatility of tin or even 6:1, if zinc is included, with the intermediates SnX4 and ZnX2 being analogues to SbX3 intermediate formation observed with antimony-halogen systems. However, while vapour or gas phase activity of zinc stannate-halogen systems is considered to be the more significant flame retardant mechanism, chars may still contain considerable fractions of tin and zinc [28] [29]. Increased char formation has been observed in parallel with smoke suppression [30].
As stated above, Hastie as observed for antimony synergised systems , had earlier proposed that tin compounds functioned via H· radical interactions of the type:
|
SnOH + H· → SnO + H2 |
(9) |
|
SnO + H· → SnOH |
(10) |
|
SnO2 + H2 → SnO + H2O |
(11) |
which were later corroborated by Cusack et al. [31]. However, later work by Kicko-Walczak [32][33] reported the mechanistic studies of action of zinc hydroxystannate in brominated unsaturated polyester resin matrices as being a multi-stage degradation giving rise to the initial formation of tin II and IV bromides over the range 240–340°C.
At higher temperatures (340–420°C), the final char structure is formed and the vapour phase flame retardant reactions take place. The released tin bromides are considered to be hydrolysed in the flame to tin II oxide and hydrogen bromide, with both products then inhibiting flame reactions as outlined above and as formerly proposed by Hastie:
|
SnBr2 + H2O → SnO + 2HBr |
(12) |
The above suggested mechanisms, coupled with their inherent, little understood, smoke suppression activity, clearly indicate that zinc stannate-halogen flame retardant interactions are not as straightforward as those reported for Sb-Br systems. In this respect, it also is noteworthy that recent work [34] has reported that zinc stannate in combination with poly(pentabromobenzyl acrylate), BrPBz, in polyamide 6.6 does not show the above expected vapour phase activity with volatile tin II and IV bromides, tin (II) oxide and interactions between the latter and released hydrogen radicals as being the most significant mechanisms. In fact a considerable amount of bromine is trapped within the char that would otherwise be expected to have been released into the vapour phase and in addition the interaction of bromine is primarily with zinc present in ZnS and not with tin, as might be expected from earlier studies referred to above.
2.3. Metal Tungstates
With no real advances during the last 30 years towards seeking a replacement for antimony III oxide apart from the zinc stannates, recent research [35] has investigated over 150 metal complexes for their ability either to promote char and/or demonstrate synergistic activity with brominated flame retardants in an engineering polymer typified by polyamide 6.6 (PA66) where BrFRs are often preferred [36] and the application can withstand the added cost. Initial studies with zinc oxalate in combination with BrPBz showed positive, possibly synergistic interactions in terms of reduction in cone calorimetric peak heat release rate (PHRR) and increased residual char levels [37]. Of the 150 metal complexes screened, aluminium (AlW), tin (II) (SnW) and zinc (ZnW) tungstates increased char formation and reduced PHRR values when present alone in PA66. Furthermore, when also in the presence of the phosphorus-containing FRs, aluminium diethyl phosphinate (AlPi) and AlPi in the presence of melamine polyphosphate, they increased their respective flame retardant behaviours and, in the case of ZnW, reduced smoke formation [38]. This work was subsequently extended to study potential synergistic interactions with the BrFRs, brominated polystyrene and poly(pentabromobenzyl acrylate) [39].
In this latter work, each tungstate was compounded alone (at 5 wt%) and with either of the two BrFRs, BrPS and BrPBz (at 10 wt% bromine levels) in PA66 and compared for fire performance with each BrFR alone, present also at 10 wt% Br levels. These results are summarised with respect to limiting oxygen index, LOI, and cone calorimetric peak heat release rate, PHRR, percentage reduction in PHRR, RPHRR, and total smoke smoke release, TSR, values in Table 2.
Table 2. Formulations and principal flammability parameters for tungstate/bromine-containing formulations in polyamide 6.6 (PA66) (adapted from ref. 39).
|
Sample |
Composition (%) |
Flammability parameters |
|||||
|
|
PA66 |
MC* |
PolyBrFR |
LOI, Vol.% |
PHRR, kW/m2 |
TSR, m2/m2 |
RPHRR, % |
|
PA66 |
100 |
- |
- |
22.6 |
1644 |
609 |
- |
|
BrPS |
90 |
- |
10 |
22.9 |
1049 |
1821 |
36.2 |
|
BrPBz |
90 |
- |
10 |
22.3 |
1206 |
1447 |
26.6 |
|
AlW ** |
95 |
5 |
- |
23.0 |
1156 |
927 |
29.7 |
|
SnW ** |
95 |
5 |
- |
21.5 |
954 |
939 |
42.0 |
|
ZnW ** |
95 |
5 |
- |
22.0 |
1190 |
638 |
27.6 |
|
AlW-BrPS |
85 |
5 |
10 |
23.3 |
999 |
1789 |
39.2 |
|
AlW-BrPBz |
85 |
5 |
10 |
22.3 |
1174 |
1246 |
28.6 |
|
SnW-BrPS |
85 |
5 |
10 |
26.7 |
546 |
1973 |
66.8 |
|
SnW-BrPBz |
85 |
5 |
10 |
26.7 |
802 |
1766 |
51.2 |
|
ZnW-BrPS |
85 |
5 |
10 |
26.2 |
485 |
949 |
70.5 |
|
ZnW-BrPBz |
85 |
5 |
10 |
28.5 |
896 |
1186 |
45.5 |
Notes: BrPS = brominated polystyrene; BrPBz = poly(pentabromobenzyl acrylate); AlW, SnW and ZnW = aluminium, tin (II) and zinc tungstates respectively; MC* indicates each metal compound; ** values from ref. 35; PHRR is peak heat release; TSR is total smoke release; RPHRR % is the percentage reduction in peak heat release rate, PHRR, with respect to PA66
It is evident that the addition of the three tungstates alone has little effect on LOI values, although reductions in PHRR values are noted with respect to the PA66 control. However, when tin II and zinc tungstates were added to the respective BrFR-containing formulations in PA66, there were significant increases in LOI indicating possible synergy. The ZnW-BrPBz formulation achieved the highest LOI value of 28.5 vol% with aluminium tungstate showing a minimal effect. However, the post-ignition parameter, PHRR, shows signification reductions for both SnW- and ZnW-BrFR formulations, with the ZnW-BrPS showing the highest percentage reduction (RPHRR = 70.5%). As expected, significant increases in TSR values occur when each BrFR is present alone in PA66, but considerable reductions with respect to these values are observed for both zinc tungstate-BrFR formulations.
In previous work [40], the relative flammability properties of BrPS and BrPBz formulations containing either antimony III oxide or zinc stannate, ZnS, in PA66 were compared and these results for total smoke release, TSR, have been combined with those in Table 2 to produce the differential smoke (ΔTSR%) bar charts in Figure 1. ΔTSR% represents the percentage changes in smoke release caused by the addition of Sb2O3, ZnS or tin II and zinc tungstates to each BrFR-containing formulation in PA66.
Not surprisingly, the formulations containing antimony III oxide (ATO) as the synergist show significant increases in smoke generation with only SnW behaving in a similar but much less severe manner. In contrast, the well-documented smoke suppressing property of zinc stannate, in the presence of each PolyBrFR is evident. However, the greatest reduction in TSR is observed when ZnW is in the presence of either BrPS or BrPBz, which suggests that ZnW is comparable to zinc stannate as a smoke suppressant. It is interesting to note that the effectiveness of ZnW compared to ZnS with the two BrFRs investigated is reversed, in that ZnW is the more effective smoke suppressant with BrPS, while ZnS is the more effective with BrPBz.
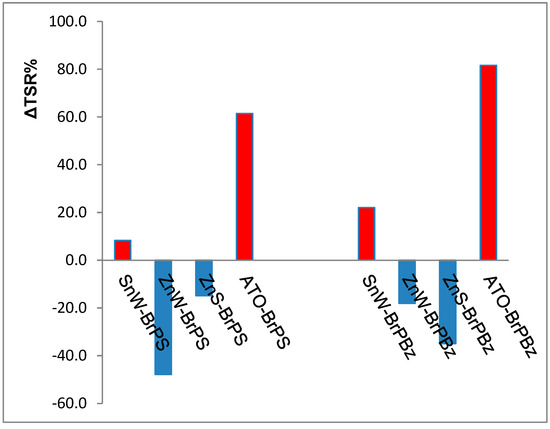
Figure 1. Percentage changes in smoke generation where the percentage change in total smoke release, ΔTSR% = (TSR(MC/BrFR)/TSR(BrFR) – 1) × 100 and TSR(MC/BrFR) and TSR(BrFR are the respective smoke release values for each synergist and tungstate (MC) in combination with each BrFR and each BrFR alone in PA66: Red columns indicate an increase and blue columns a decrease in smoke generation with respect to that from either brominated polystyrene, BrPS, or poly(pentabromobenzyl acrylate), BrPBz, present in PA66 alone. SnW and ZnW = tin (II) and zinc tungstates respectively; ATO = antimony (III) oxide and ZnS = zinc stannate, reproduced from ref. 39.
Analysis for metallic and bromine residues in cone calorimetric chars showed that generally there was a loss of bromine to the vapour phase as expected, as indicated by a clear reduction in the Br:W molar ratios present. However, while losses of Zn and Sn were also observed, surprisingly the former was significantly greater. Furthermore, higher Br:W ratios were observed for the ZnW-containing samples, which would suggest that more bromine was retained in the condensed phase than with SnW-containing formulations. These char results indicated that respective metal/bromine losses to the volatile phase are not simply comparable for the two tungstates and that condensed phase activity of ZnW is significant in its role as both a synergist and a smoke suppressant, especially with brominated polystyrene.
If metal tungstates and in particular zinc tungstate are to be considered as possible replacements to antimony III oxide, it is essential that their properties in combination with a larger range of BrFRs present in a wider range of polymers and/or applied to textile fabrics of various types should be studied. Given that the zinc stannates are very selective in regard to their synergistic behaviour, it would not be surprising if zinc tungstate behaved in a similar manner based on the above results.
3. Conclusions
The conventionally used synergist antimony III oxide, generally used with bromine-containing flame retardants, functions with similar effectiveness and independently of both BrFR chemical structure and the polymer matrix or textile substrate present. Its replacement by the established non-toxic, but more expensive zinc stannates, which show greater BrFR selectivity in their ability to be similarly effective as synergists, may be offset against their smoke suppressing properties and the need to design tailored systems for high value engineering polymers like polyamide 6.6. In a similar manner, the recently reported synergistic and smoke suppressing effects of zinc tungstate in combination with selected polymeric brominated flame retardants compounded in polyamide 6.6 also offer opportunities for Sb2O3 replacement in the longer term.
References
- A Richard Horrocks; The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications—A Review. Polymers 2020, 12, 2160, 10.3390/polym12092160.
- Georlette, Pierre. Applications of halogen flame retardants in Fire Retardant Materials; Horrocks, A R; Price D, Eds.; Woodhead Publishing: Cambridge, UK, 2000; pp. 264.
- Arlene Blum; Mamta Behl; Linda S. Birnbaum; Miriam L. Diamond; Allison Phillips; Veena Singla; Nisha S. Sipes; Heather M. Stapleton; Marta Venier; Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers?. Environmental Science & Technology Letters 2019, 6, 638-649, 10.1021/acs.estlett.9b00582.
- Anon. Antimony trioxide in Toxicological Risks of Selected Flame-Retardant Chemicals; Anon, Eds.; National Academic Press: Washington, DC, USA, 2000; pp. 229.
- Report on Carcinogens Monograph on Antimony Trioxide. 2018. . US Department of Health and Human Services.. Retrieved 2020-10-21
- Perkin, W.H. Metal oxides as flame retardants to cellulose. US patent 844,042, 1907.
- Little R.W., Ed. Flame-proofing Textile Fabrics.; Reinhold Publishing Corporation, New York, USA, 1947.
- Coppick, S. Metallic oxide-chlorinated body type, a. fundamentals of processes. In Flame-proofing Textile Fabrics.; Little R.W., Ed.; Reinhold Publishing Corporation, New York, USA, 1947, pp.239-247.
- Costa, L.; Goberti, P.; Paganetto, G.; Camino, G.; Sgarzi, P. Thermal behaviour of chlorine-antimony fire-retardant systems. Polym Degrad Stab 1990, 30(1), 13-28.
- Costa, L.; Paganetto, G.; Bertelli, G.; Camino, G. Thermal decomposition of antimony oxyhalides I Oxychlorides. 1990, J Therm Anal 1990, 36(3), 1141-1153.
- Bertelli, G.; Costa, L.; Fenza, S.; Marchetti, E.; Camino, G.; Locatelli, R. Thermal behaviour of bromine-metal fire retardant systems. Poly Degrad Stab 1988, 20(3-4), 295-314.
- Horrocks, A.R. Overview of traditional flame-retardant solutions (including coating and back-coating). In Update on Flame Retardant Textiles: State of the Art, Environmental Issues and Innovative Solutions.; Alongi, J.; Horrocks, A.R.; Carosio. F.; Malucelli, G., Eds.; Smithers Rapra, Shawbury, UK, 2013, pp.152-171.
- Hastie, J.W. Molecular basis of flame inhibition. J Res Natl Bur Stds 1973, 77A (6), 733-754
- Hastie, J.W. Mass spectrometric studies of flame inhibition, Combust Flame 1973, 21, 49-54.
- Stec, A.A. Fire toxicity - The elephant in the room? Fire Saf J 2017, 19, 79–90.
- Purser, D. Toxicity of fire retardants in relation to life safety and environmental hazards. In Fire Retardant Materials.; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing, Cambridge, UK, 2001, pp.69-127.
- Pasternak, M.; Zinn, B.T.; Browner, R.F. Studies of the chemical mechanism of smoke particulates formation during the combustion of chlorinated polymers. Combust Sci Technol 1982, 28, 263-270.
- Shen, K.K.; Kochesfahani, S.H.; Jouffret, F. Boron-based flame retardants and flame retardancy. In Fire Retardancy of Polymeric Materials.; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press, Taylor & Francis Group, Boca Raton, Fl., USA, 2010, pp. 207-237.
- Anon, Zinc borate. In Toxicological Risks of Selected Flame-Retardant Chemicals.; National Academies Press, Washington, DC, USA, 2000, pp.149-191,
- Anon, Zinc Borate.; CAS No 10361-94-1, MSDS Datasheets,.; Sigma Aldrich, https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=14470&brand=SIAL&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsial%2F14470%3Flang%3Den (24/08/2020)
- Anon. Antimony Outlook to 2030, 14th Edition.; Roskill Information Services, London, UK, October 2020.
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. Metal scarcity and sustainability, analyzing the necessity to reduce the extraction of scarce metals. Res Conserv Recycl 2014, 93, 1–8.
- Horrocks, A.R.; Smart, G.; Price, D.; Kandola, B. Zinc stannates as alternative synergists in selected flame retardant systems. J Fire Sci 2009, 27, 495–521.
- Cusack, P.A.; Monk, A.W.; Pearce, J.A.; Reynolds, S.J. An investigation of inorganic tin flame retardants which suppress smoke and carbon monoxide emission from burning brominated polyester resins. Fire Mater 1989, 14, 23-29.
- Yukihiko, N.; Yasushi, K.; Misao, H.; Yasunori, K.; Hirofumi K. Flame retardant polyolefin compound having low smoking and toxicity. US Patent 5726231, 1998.
- Markezich, R.L.; Mundhenke, R.F. Bromine/chlorine synergism to flame retardant plastics and stability of flame retardant-polyamides. In Flame Retardants ’98.; Interscience Communications, London, UK, 1998, pp 93-102.
- Cusack, P.A. Tin chemicals as flame retardants. In Recent Advances in Flame Retardancy of Polymeric Materials, Vol. 2.; Lewin, M., Kirshenbaum, B.; Business Communications Inc, Norwalk, CT, USA, 1991, pp. 75-82.
- Cusack, P.A.; Heer, M.S.; Monk A.W. Zinc hydroxystannate: A combined flame retardant and smoke suppressant for halogenated polyesters. Polym Degrad Stab 1991, 32, 177-190.
- Jung, H.C.; Kim, W.N.; Lee, C.R.; Suh, K.S.; Kim, S.R. Properties of flame-retarding blends of polycarbonate and poly(acrylonitrile-butadiene-styrene). J Polym Eng 1998, 18(1/2), 115-130.
- Andre, F.; Cusack, P.A.; Monk, A.W.; Seangpraserkij, S. The effect of zinc hydroxystannate and zinc stannate on the fire properties of polyester resins containing additive-type flame retardants. Polym Degrad Stab1993, 40, 267-273.
- Bains, R.S.; Cusack, P.A.; Monk, A.W. A comparison of the fire retardant properties of zinc hydroxystannate and antimony trioxide in brominated polyester resins containing inorganic fillers. Eur Polym J 1990, 26(11), 1221-1227.
- Kicko-Walczak E. Flame retarded halogenated unsaturated polyester resins. Thermal decomposition study. J Polym Eng 2003, 23, 149-161.
- Kicko-Walczak, E. Studies on the mechanisms of thermal decomposition of unsaturated polyester resins with reduced flammability. Polym Polym Comp 2004, 12, 127-134.
- Ismaeili, N. Mechanistic study of synergism of inorganic synergists with flame retardants. Ph.D. Thesis, University of Bolton, Bolton, UK, 2019.
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Synthesis and thermal analytical screening of metal complexes as potential novel fire retardants in polyamide 6.6. Polym Degrad Stab 2017, 144, 420–433.
- Weil, E.D.; Levchik, S. Current practice and recent commercial developments in flame retardancy of polyamides. J Fire Sci 2004, 22, 251-264.
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The potential of metal oxalates as novel flame retardants and synergists for engineering polymers. Polym Degrad Stab 2014, 110, 290–297.
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Novel metal complexes as potential synergists with phosphorus-based flame retardants in polyamide 6.6. Polym Degrad Stab 2020, 179, 109220.
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Potential synergism between novel metal complexes and polymeric brominated flame retardants in polyamide 6.6. Polymers 2020 1543; doi.org/10.3390/polym12071543
- Horrocks, A.R.; Smart, G.; Kandola, B.K.; Holdsworth, A.F.; Price, D. Zinc stannate interactions with flame retardants in polyamides; Part 1: Synergies with organobromine-containing flame retardants in polyamides 6 (PA6) and 6.6 (PA6.6). Polym Degrad Stab 2012, 97, 2503–2510.





