
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Lualdi | -- | 1832 | 2022-10-04 16:04:33 | | | |
| 2 | Beatrix Zheng | -5 word(s) | 1827 | 2022-10-04 16:16:05 | | |
Video Upload Options
The growing number of patients affected by neurodegenerative disorders represents a huge problem for healthcare systems, human society, and economics. In this context, omics strategies are crucial for the identification of molecular factors involved in disease pathobiology, and for the discovery of biomarkers that allow early diagnosis, patients’ stratification, and treatment response prediction. The integration of different omics data is a required step towards the goal of personalized medicine. The Italian proteomics community is actively developing and applying proteomics approaches to the study of neurodegenerative disorders; moreover, it is leading the mitochondria-focused initiative of the Human Proteome Project, which is particularly important given the central role of mitochondrial impairment in neurodegeneration.
1. Introduction
2. Mitochondrial Proteomics and Neurodegeneration
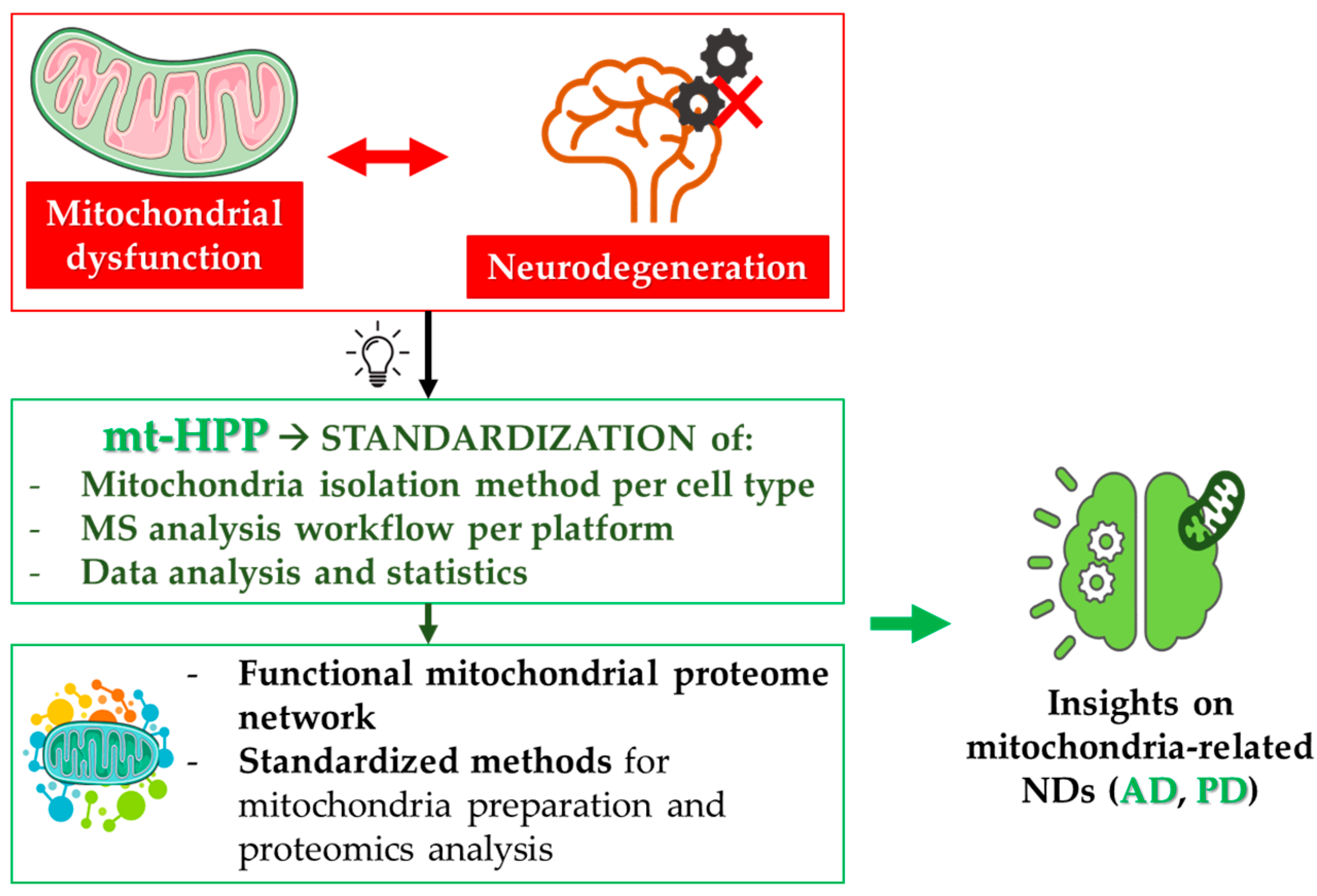
2.1. The Italian mt-HPP Initiative
2.2. Mitochondrial Proteomics in Alzheimer’s Disease
2.3. Mitochondrial Proteomics in Parkinson’s Disease
References
- Armstrong, R. What Causes Neurodegenerative Disease? Folia Neuropathol. 2020, 58, 93–112.
- Ruffini, N.; Klingenberg, S.; Schweiger, S.; Gerber, S. Common Factors in Neurodegeneration: A Meta-Study Revealing Shared Patterns on a Multi-Omics Scale. Cells 2020, 9, 2642.
- Manzoni, C.; Lewis, P.A.; Ferrari, R. Network Analysis for Complex Neurodegenerative Diseases. Curr. Genet. Med. Rep. 2020, 8, 17–25.
- Urbani, A.; De Canio, M.; Palmieri, F.; Sechi, S.; Bini, L.; Castagnola, M.; Fasano, M.; Modesti, A.; Roncada, P.; Timperio, A.M.; et al. The Mitochondrial Italian Human Proteome Project Initiative (Mt-HPP). Mol. Biosyst. 2013, 9, 1984–1992.
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259.
- Chinnery, P.F.; Hudson, G. Mitochondrial Genetics. Br. Med. Bull. 2013, 106, 135–159.
- Tatsuta, T.; Langer, T. Quality Control of Mitochondria: Protection against Neurodegeneration and Ageing. EMBO J. 2008, 27, 306–314.
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality Control of the Mitochondrion. Dev. Cell 2021, 56, 881–905.
- Stotland, A.; Gottlieb, R.A. Mitochondrial Quality Control: Easy Come, Easy Go. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2802–2811.
- Monti, C.; Colugnat, I.; Lopiano, L.; Chiò, A.; Alberio, T. Network Analysis Identifies Disease-Specific Pathways for Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 370–381.
- Yang, D.; Ying, J.; Wang, X.; Zhao, T.; Yoon, S.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Hua, F. Mitochondrial Dynamics: A Key Role in Neurodegeneration and a Potential Target for Neurodegenerative Disease. Front. Neurosci. 2021, 15, 654785.
- Lezi, E.; Swerdlow, R.H. Mitochondria in Neurodegeneration. Adv. Exp. Med. Biol. 2012, 942, 269–286.
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Vafai, S.B.; Ong, S.-E.; Walford, G.A.; Sugiana, C.; Boneh, A.; Chen, W.K.; et al. A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell 2008, 134, 112–123.
- Alberio, T.; Pieroni, L.; Ronci, M.; Banfi, C.; Bongarzone, I.; Bottoni, P.; Brioschi, M.; Caterino, M.; Chinello, C.; Cormio, A.; et al. Toward the Standardization of Mitochondrial Proteomics: The Italian Mitochondrial Human Proteome Project Initiative. J. Proteome Res. 2017, 16, 4319–4329.
- Butterfield, D.A.; Perluigi, M.; Reed, T.; Muharib, T.; Hughes, C.P.; Robinson, R.A.S.; Sultana, R. Redox Proteomics in Selected Neurodegenerative Disorders: From Its Infancy to Future Applications. Antioxid. Redox Signal. 2012, 17, 1610–1655.
- Butterfield, D.A.; Perluigi, M. Redox Proteomics: A Key Tool for New Insights into Protein Modification with Relevance to Disease. Antioxid. Redox Signal. 2017, 26, 277–279.
- Castegna, A.; Aksenov, M.; Aksenova, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic Identification of Oxidatively Modified Proteins in Alzheimer’s Disease Brain. Part I: Creatine Kinase BB, Glutamine Synthase, and Ubiquitin Carboxy-Terminal Hydrolase L-1. Free Radic. Biol. Med. 2002, 33, 562–571.
- Celi, P.; Gabai, G. Oxidant/Antioxidant Balance in Animal Nutrition and Health: The Role of Protein Oxidation. Front. Vet. Sci. 2015, 2, 48.
- Butterfield, D.A.; Palmieri, E.M.; Castegna, A. Clinical Implications from Proteomic Studies in Neurodegenerative Diseases: Lessons from Mitochondrial Proteins. Expert Rev. Proteom. 2016, 13, 259–274.
- Sultana, R.; Baglioni, M.; Cecchetti, R.; Cai, J.; Klein, J.B.; Bastiani, P.; Ruggiero, C.; Mecocci, P.; Butterfield, D.A. Lymphocyte Mitochondria: Towards Identification of Peripheral Biomarkers in Progression of Alzheimer Disease. Free Radic. Biol. Med. 2013, 65, 595–606.
- Bosetti, F.; Brizzi, F.; Barogi, S.; Mancuso, M.; Siciliano, G.; Tendi, E.A.; Murri, L.; Rapoport, S.I.; Solaini, G. Cytochrome c Oxidase and Mitochondrial F1F0-ATPase (ATP Synthase) Activities in Platelets and Brain from Patients with Alzheimer’s Disease. Neurobiol. Aging 2002, 23, 371–376.
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s Disease: Genetics and Pathogenesis. Annu. Rev. Pathol. 2011, 6, 193–222.
- Deng, H.; Wang, P.; Jankovic, J. The Genetics of Parkinson Disease. Ageing Res. Rev. 2018, 42, 72–85.
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4.
- Pingale, T.; Gupta, G.L. Classic and Evolving Animal Models in Parkinson’s Disease. Pharmacol. Biochem. Behav. 2020, 199, 173060.
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome Analysis of Human Substantia Nigra in Parkinson’s Disease. Proteomics 2004, 4, 3943–3952.
- De Iuliis, A.; Grigoletto, J.; Recchia, A.; Giusti, P.; Arslan, P. A Proteomic Approach in the Study of an Animal Model of Parkinson’s Disease. Clin. Chim. Acta 2005, 357, 202–209.
- Alberio, T.; Bondi, H.; Colombo, F.; Alloggio, I.; Pieroni, L.; Urbani, A.; Fasano, M. Mitochondrial Proteomics Investigation of a Cellular Model of Impaired Dopamine Homeostasis, an Early Step in Parkinson’s Disease Pathogenesis. Mol. Biosyst. 2014, 10, 1332–1344.
- Lualdi, M.; Ronci, M.; Zilocchi, M.; Corno, F.; Turilli, E.S.; Sponchiado, M.; Aceto, A.; Alberio, T.; Fasano, M. Exploring the Mitochondrial Degradome by the TAILS Proteomics Approach in a Cellular Model of Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 195.
- Kleifeld, O.; Doucet, A.; Prudova, A.; auf dem Keller, U.; Gioia, M.; Kizhakkedathu, J.N.; Overall, C.M. Identifying and Quantifying Proteolytic Events and the Natural N Terminome by Terminal Amine Isotopic Labeling of Substrates. Nat. Protoc. 2011, 6, 1578–1611.
- Marshall, N.C.; Klein, T.; Thejoe, M.; von Krosigk, N.; Kizhakkedathu, J.; Finlay, B.B.; Overall, C.M. Global Profiling of Proteolysis from the Mitochondrial Amino Terminome during Early Intrinsic Apoptosis Prior to Caspase-3 Activation. J. Proteome Res. 2018, 17, 4279–4296.
- Martinelli, P.; Rugarli, E.I. Emerging Roles of Mitochondrial Proteases in Neurodegeneration. Biochim. Biophys. Acta (BBA)-Bioenerg. 2010, 1797, 1–10.




