Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | TAMANNA JAHAN MONY | -- | 3347 | 2022-09-28 09:58:05 | | | |
| 2 | Rita Xu | Meta information modification | 3347 | 2022-09-28 11:08:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mony, T.J.; Elahi, F.; Choi, J.W.; Park, S.J. Neuropharmacological Effects of Terpenoids. Encyclopedia. Available online: https://encyclopedia.pub/entry/27809 (accessed on 12 March 2026).
Mony TJ, Elahi F, Choi JW, Park SJ. Neuropharmacological Effects of Terpenoids. Encyclopedia. Available at: https://encyclopedia.pub/entry/27809. Accessed March 12, 2026.
Mony, Tamanna Jahan, Fazle Elahi, Ji Woong Choi, Se Jin Park. "Neuropharmacological Effects of Terpenoids" Encyclopedia, https://encyclopedia.pub/entry/27809 (accessed March 12, 2026).
Mony, T.J., Elahi, F., Choi, J.W., & Park, S.J. (2022, September 28). Neuropharmacological Effects of Terpenoids. In Encyclopedia. https://encyclopedia.pub/entry/27809
Mony, Tamanna Jahan, et al. "Neuropharmacological Effects of Terpenoids." Encyclopedia. Web. 28 September, 2022.
Copy Citation
Terpenoids are widely distributed in nature, especially in the plant kingdom, and exhibit diverse pharmacological activities. In recent years, screening has revealed a wide variety of new terpenoids that are active against different psychiatric disorders.
neuropharmacological effect
terpenoid
preclinical
animal model
1. Introduction
Terpenoids are widely distributed in plants, microorganisms, fungi, marine organisms, animals, sedimentary rocks, and oils [1]. These compounds are structurally diverse, biosynthetically formed natural products and are sometimes referred to as “terpenes.” The term “terpenoid” denotes a compound that contains an integral number of C5 units and is derived from the basic branch C5 unit isoprene (2-methyl-1,3-butadiene) [2]. The diverse structural variations among terpenoids give this large group of molecules a wide range of potent biological activities in areas such as cell membrane construction, signal transduction, immunomodulation, inflammation control, antioxidation, and inhibition of several enzymes [1][3][4].
Terpenoids and their semisynthetic derivatives may represent promising neuroprotective agents against several neurological and cognitive dysfunctions. Celastrol, ginsenosides, oleanolic acid, ursolic acid, asiatic acid, erythrodiol, and some triterpenoid saponins have been studied for years and have shown efficacy in protecting the brain against processes including neuroinflammation and oxidative stress [5][6]. Many more have also attracted interest in recent years, including lupeol, rosmarinic acid, resveratrol, betulinic acid, pomolic acid, maslinic acid, uvaol, tormentic acid, and erythrodiol [5]. These compounds are found in higher plants, including common edible and nonedible plants, and may occur as free compounds, conjugates, or saponins (with one or more sugar units) [5][7][8]. These terpenoids have been used for centuries in traditional medicine to improve memory and cognitive function. Some of them are in preclinical or clinical trials, and some have already been approved for use in humans. The diverse structures and functions of terpenoids have sparked interest in investigating the commercial use of these compounds, emphasizing their practical importance as alternative medicines for psychiatric disorders.
Psychiatric disorders are patterns of behavioral alterations that cause significant distress or impairment of personal functions. The characteristic alterations in behavior and emotional state associated with malfunction and structural damage of the central nervous system have differentiated this class of pathology from common neurological disorders. Researchers used the references of some of the major categories of disorders described in the Diagnostic and Statistical Manual of Mental Disorders (DSM) [9], the most widely used system for classifying psychiatric disorders and standardizing their diagnostic criteria. Psychiatric disorders are intertwined with multiple pathophysiological conditions, including oxidative stress, mitochondrial dysfunction, neuroinflammation, neuronal degeneration, and synaptic loss [10]. The selectivity and potency of terpenoids make them highly promising potential candidates for use in psychosis studies. In Figure 1, researchers describe the common pathophysiological changes associated with psychotic disorders and, in Figure 2, the overall major mechanisms of action of terpenoids.
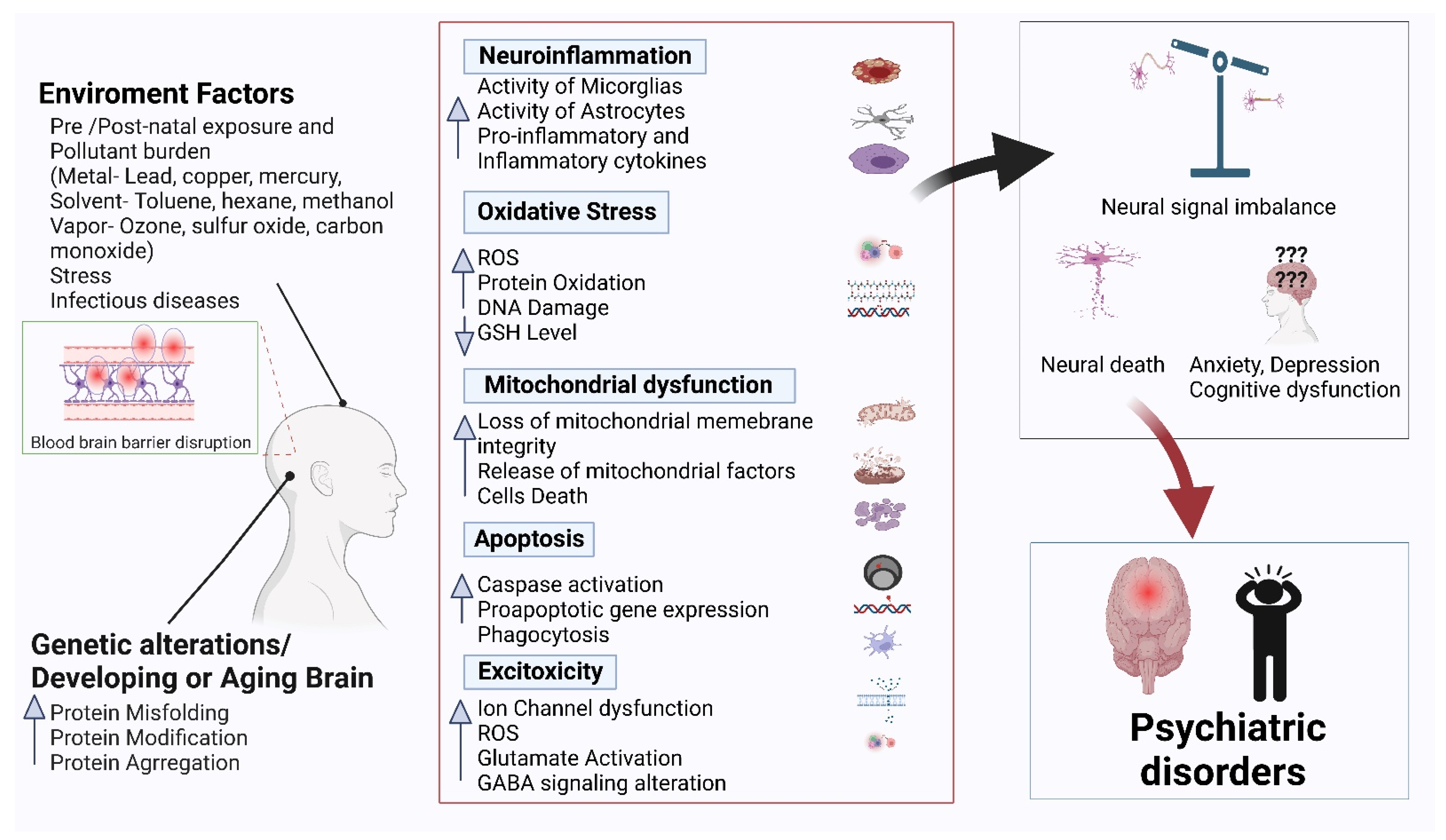
Figure 1. Common pathophysiology of psychiatric disorders.
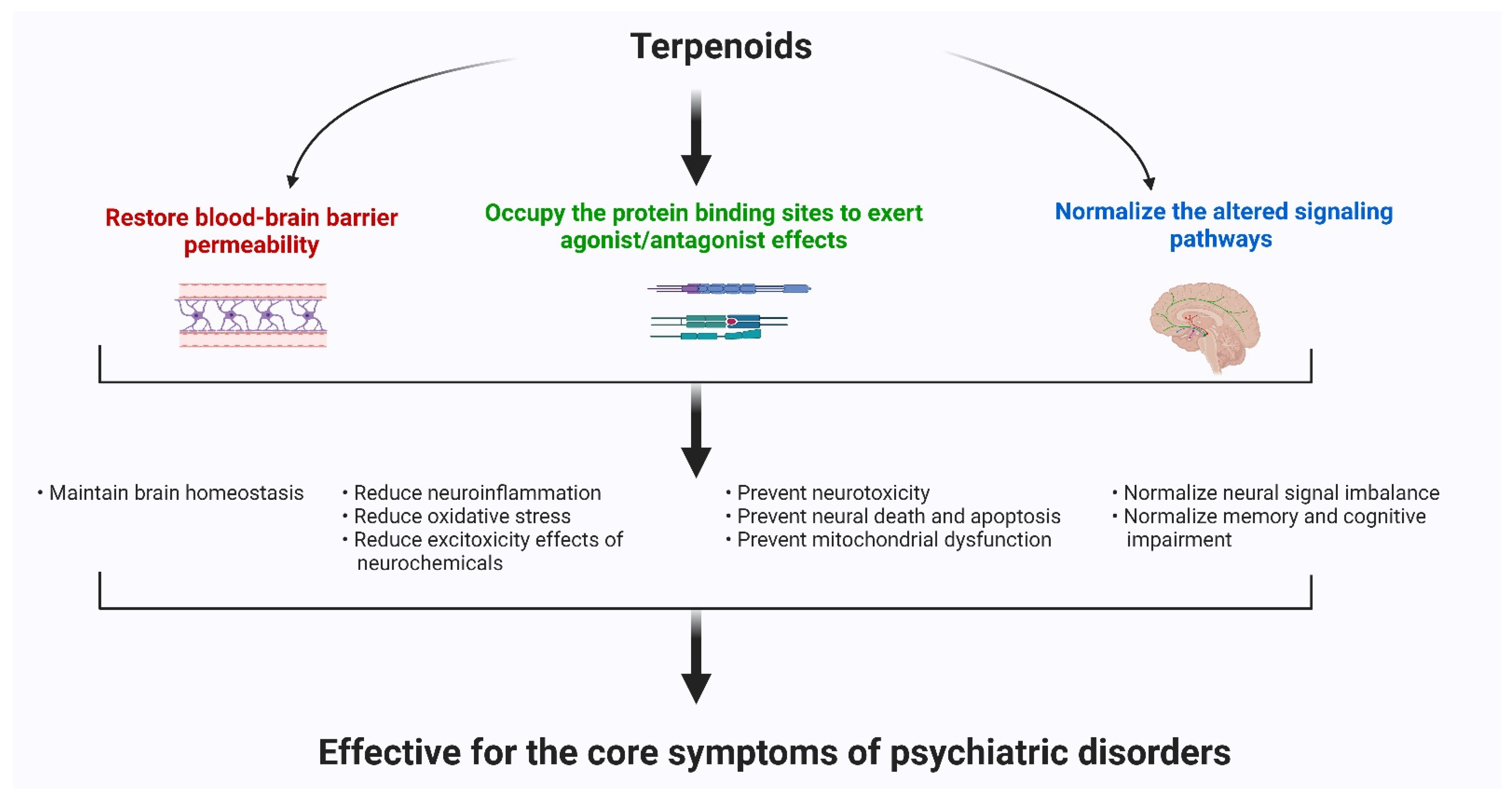
Figure 2. The major mechanisms of action of terpenoids.
The number of known terpenoids is currently exploding due to advances in isolation techniques, synthetic methods, and a plethora of biochemical diversity. Currently, symptomatic treatment does not alter the underlying course of neuropsychiatric disorders. Effective therapies for intricate psychiatric disorders are also limited by their extrapyramidal side effects. Terpenoids or terpenes have been used in traditional medicine for years as anti-inflammatory, antibacterial, anti-plasmodium, anticancer, and antioxidative medicine. These groups have also shown a positive contributions that have been proven in several preclinical studies on anxiety, depression and mood disorders. Therefore, new therapeutic approaches suitable for practical uses are urgently needed. Animal studies are invaluable to inform clinical research, to assess the necessity of further studies on a given topic and, most importantly, to assess the environmental exposure hazard in the evaluation of toxicological studies.
2. The Common Neuroprotective Mechanisms of Action of Terpenoids
2.1. Terpenoids Exert Neuroprotective Effects by Restoring Blood–Brain Barrier Permeability
The blood–brain barrier (BBB) is the primary metabolic interface between the peripheral blood supply and neural tissues or their fluid spaces. The key function of the BBB is to maintain central nervous system homeostasis and prevent undesirable harmful particles and chemicals from entering the brain [11]. The BBB protects the central nervous system from circulating pathogens, toxins, and xenobiotics, as well as restricts the migration of leukocytes and monocytes [12]. Several transporters and metabolites, such as P-glycoprotein and cytochrome P450 enzymes, located at the BBB, also protect the CNS [13]. These features greatly limit the transcellular and paracellular movement of molecules and cells across the cerebral microvasculature [11]. A highly specialized interconnected network of junctional complexes between adjacent endothelial cells along with high transendothelial electrical resistance (TEER) and relatively low pinocytotic activity confer the unique characteristics and functions of the BBB [14]. The loss of BBB vascular integrity increases the penetration of undesirable solutes, fluids, cells, pathogens, and toxins from peripheral circulation to the central nervous system. As a consequence, cerebral edema, cerebral hyperexcitability, and neuroinflammation occur and contribute to the clinical complications of many diseases [11]. BBB breakdown occurs in many neurological and neuropsychiatric disorders. The major routes for molecular transportation through the BBB include the transcellular and paracellular routes [13]. The structural disruption of the paracellular route is an important mechanism in the pathological state of BBB interruption. Therefore, disruptions in the assembly of junctional complexes and central endothelial cells decrease the integrity of the BBB and expose the CNS to systemic circulation. Pathological stimuli induce dysregulation of the structure of junctional complexes (both tight junctions and adherence junctions) and ultimately damage their specialized gating function [15][16]. Therefore, BBB integrity plays a pivotal role in the pathophysiological alteration of neuropsychiatric disorders.
Many inducers and mediators are involved in BBB breakdown, such as TNF-α, IL-1β, bradykinin, VEGF, MMPs, and oxidative stress [17][18][19][20]. Moreover, these disruptions can be mediated through the activation of PI3K, PKC, MAPK, and NF-κB, as well as the modulation of PPAR signaling transduction cascades [21][22][23]. The barrier properties of the BBB are of clinical significance from a neuropharmacological point of view for choosing the appropriate drug, as the barrier is largely seen as an obstacle for therapeutic compounds to reach their targets in the brain [24][25][26]. By targeting the abovementioned detrimental inducers and related signaling cascades, the stability of the BBB can be restored. Hence, the maintenance of BBB integrity is an excellent therapeutic target for neuropsychiatric drugs. Some novel therapeutics have been developed that show clinical efficacy and are able to restore the BBB. Numerous methods have been developed to aid the delivery of drugs to the CNS [26]. Most synthetic compounds at very high doses cause greater systemic off-target side effects. Therefore, nature-based products that elicit their activities in regulating transcription factors and signaling transduction cascades (e.g., PPAR, NF-κB, PI3K, PKC, and MAPK) are receiving interest in research to restore the architecture and function of the blood–brain barrier.
The protective effects of natural products against BBB breakdown are important mechanisms contributing to the clinical applications of some herbal medicines in the prevention and treatment of neuropsychiatric disorders. Their biological activities could be reflected by their chemical compositions, such as ginkgolide B from ginkgo, baicalein from skullcap, and tanshinone IIA from red sage, which are the most popular traditional medicinal products that exert neuroprotective effects [27]. Natural products possess several bioactive compounds that together have multicomponent, multitarget actions to address the intricacy of BBB breakdown. Panax ginseng, also known as ginseng, is a popular herbal medicine with neuroprotective potential. Its major terpenoid constituent, ginsenoide Rb1, has been reported as one of the major active constituents in ginseng. In rats, ginsenoside Rg1 has shown protective effects on BBB structural alteration and amended the severity of brain edema and BBB permeability [28]. Ginkgo biloba is a common complementary medicine that possesses anti-inflammatory, antioxidative, and vascular protective effects. Ginkgo is mainly known for its neuroprotective effects, and recent studies have verified its ability to protect the BBB [29]. Ginkgolide B, the major diterpenoid compound of the Ginkgo species, induced a protective effects on BBB permeability and brain edema in rats following hyperthermic brain injury.
2.2. Terpenoids Occupy the Receptor Protein Binding Sites to Exert their Agonist/Antagonist Effects
- (a)
-
MAO inhibition
The enzyme monoamine oxidase (MAO) metabolizes neurotransmitters (serotonin, dopamine, norepinephrine, tryptamine, etc.) and endogenous amines and xenobiotics. MAO is involved in catalyzing oxidative deamination of amines and neurotransmitters associated with oxidative stress and adverse pharmacological reactions (mood swing and depression) [30]. The two isoenzymes MAO-A and MAO-B have important roles both in the central nervous system and peripheral organs. MAO-A is associated with psychiatric conditions and depression, and MAO-B is involved in neurodegenerative diseases [31][32]. Oxidation of biogenic amines and neurotransmitters induced by MAO enzymes generates hydrogen peroxide (H2O2), oxygen radicals, and aldehydes. These phenomena increase the risk of cell oxidative injury. Therefore, inhibition of MAO may protect against oxidative stress and neurotoxins [33][34]. The inhibition of metabolizing enzymes may increase brain concentrations of their substrates, and thus, reduce disease symptoms of psychiatric disorders. Inhibitors of MAOs are considered to be an effective therapeutic intervention with neuroprotective and antidepressant effects. The expected therapeutic strategy in the treatment of depression and mood disorders is primarily pharmacological modulation of the monoamine system [34]. Because neurotransmitters (dopamine, norepinephrine, and serotonin) are metabolized by monoamine oxidase (MAO), inhibition of the enzyme may attenuate disease symptoms by balancing the concentration of neurotransmitters in the brain. Although synthetic MAO-A inhibitors are widely used as antidepressants, their prolonged use triggers adverse reactions (hypertension) [33]. Therefore, natural MAO inhibitors might be new alternatives for depression-related disorders.
Ixeris dentate Nakai (compositae) is a perennial herb that has been used for different purposes as a folk medicine in Korea. Sesquiterpene lactone, glycosides, and flavone are the primary bioactive compounds isolated from this herb. In 2003, one preclinical study first showed that two flavone compounds, luteolin and cymaroside, exhibited MAO-B inhibition activity in adult Sprague–Dawley rats [35].
Hypericum perforatum L. (Guttiferae), commonly known as St. John’s Wort, is used as an alternative treatment for mild and moderate depression [36]. Phytochemical characterization has reported that hyperforin (a prenylated phloroglucinol) and hypericin (a naphthodianthrone) were the primary chemicals responsible for effects on health, although other biologically active constituents, for example, flavonoids, tannins, and different terpenes, have also been reported to exert antidepressant effects [37].
- (b)
-
Effects on GABAergic systems
GABA is an important inhibitory neurotransmitter that plays a pivotal role in the central nervous system. GABA-induced activation of ionotropic GABA receptors that are widely expressed in the brain exert a major inhibitory function. In the GABAergic terminal, GABA is formed from glutamate through an enzymatic reaction mediated by glutamic acid decarboxylase (GAD) and cofactor pyridoxal phosphate [38]. GABAergic dysfunction is crucial for the pathophysiological changes that occur in neuropsychiatric disorders. Therefore, targeting the GABAergic system is one of the mechanisms of antidepressants and mood stabilizers. These drugs affect several neurotransmitter systems, such as serotonergic, monoaminergic, and GABAergic systems [39][40]. Therefore, several lines of evidence support that low GABAergic function plays a key role in the pathophysiology of psychotic disorders, which are related to the dysfunction of multiple neurotransmitter systems [39]. Current antipsychotic treatments increase short-term levels of neurotransmitters in the brain, primarily affecting selective serotonin reuptake inhibitors (SSRIs), serotonin (5-HT) and noradrenaline (NE) reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors (MAOIs) [41]. The consequence of long-term use of these drugs is the desensitization of some receptors, such as 5-HT1A autoreceptors. The prevention of this inhibitory mechanism of 5-HT1A receptor antagonists augments the neurochemical and behavioral effects of SSRIs [42][43][44]. Therefore, conventional antidepressants and anxiolytics, such as benzodiazepine (a GABA receptor agonist) and SSRIs, induce significant side effects, such as nausea and insomnia, fatigue, sedation, sexual dysfunction, headaches, and weight gain [41]. Hence, the increasing demand for alternative medicines that affect the GABAergic system is becoming important for alleviating the symptoms of these psychiatric disorders. Several alkaloids, flavonoids, terpenoids, and essential oils extracted from herbs, flowers, and other plant parts exert dynamic effects on GABAergic systems and have been widely used in traditional medicine as antidepressants and anxiolytics for a long time. The hydroxyl group of terpenoids has been primarily reported to exert effects on the GABAergic system [43][44].
Different essential oils are rich in terpenoids and phenylpropanoid derivatives. Essential oils (EOs) are extracted from herbs, flowers, and other plant parts and are comprised of volatile aromatic compounds. These volatile oils are concentrated hydrophobic liquids of natural products. The chemical components of essential oils could exert their biological actions via regulating the GABAergic system and inhibition of Na+ channels [44]. Dysfunction or deficiency of the GABAergic system has been implicated in neuropsychiatric disorders. The most common terpenoids found in essential oils are monoterpenes and sesquiterpenes [45]. The essential oils of Anthemis nobilis (chamomile), Salvia sclarea (clary), Rosmarinus officinalis (rosemary), Lavandula angustifolia (lavender), and Rosa damascene (rose) have been used as popular anxiolytic essential oils in Europe for years [44]. The antidepressant effects of EOs of chamomile, clary, rosemary, and lavender were assessed using a forced swim test (FST) in rats [46]. In this study, the authors reported that among the tested essential oils, clary oil exhibited the strongest antistressor properties in a FST. The fruits, leaves, and roots of Piper guineense, a popular medicinal herb, have diverse therapeutic uses for treating convulsion, rheumatism, and respiratory disease in African traditional medicine [47]. Inhalation of essential oils of P. guineense exert significant sedative and anxiolytic effects. The primary compounds of P. guineense EO are linalool and 3,5-dimethoxytoluene. Linalool might be the major compound that exhibits sedative effects partially via the GABAergic receptor system [47]. Asarum heterotropoides var. mandshuricum, from the Aristolochiaceae family, is a traditional Chinese medicine called Xixin or wild ginger which is used for pain and inflammation. The essential oils extracted from A. heterotropoides effectively attenuated depression-like behavior and increased brain expression of serotonin (5-HT) under forced swimming or immobilization stress [48]. Melissa officinalis L (lemon balm) is a traditional herbal medicine native to the Eastern Mediterranean region, Western Asia, and tropical countries, such as Brazil. This herb is widely used as a mild sedative, spasmolytic and antibacterial agent. The essential oils of Melissa officinalis are commonly used for sedative effect, improvement of cognitive functions, and other physiological actions [49]. Abuhamdah et al. showed that EO of M. officinalis reversibly inhibited GABA-induced currents in a concentration-dependent manner. Lavender (Lavandula angustifolia) is cultivated worldwide for its essential oils. These essential oils are used in perfumes, in modern aroma therapy, cosmetics, or in food processing [50]. Lavender inhalation has been used in folk medicine for the treatment of anxiety [44]. Another study reported that the EO of this lavender plant likely exerts its anxiolytic effect through serotonergic but not GABAergic neurotransmitters [44][51]. Linalool and linalyl acetate are the main bioactive components of the Lavender species that exerts therapeutics effects [52]. Thymoquinone is a major constituent of the essential oil of Nigella sativa seeds that have exhibited anti-anxiety activity in mice. In 2011, Gilhotra and Dhingra [53] demonstrated the anti-anxiety effect of thymoquinone by GABAergic and nitriergic modulation. Thymoquinone at 20 mg/kg exhibited anxiolytic effects by decreasing plasma nitrite level and reversed the decreased brain GABA materials in stressed mice.
- (c)
-
Dopamine D1 and D2 receptors
The dopaminergic system is involved in delayed maturation of the brain and plays an important role in stabilizing and integrating functions on neural circuits. Excessive neurotransmission of dopamine is associated with the pathophysiological alterations of many psychotic disorders and is a clinical hallmark of schizophrenia [54]. Dopamine receptors (DRs), which are G-protein coupled receptors, are becoming important as primary targets for developing drugs to treat neuropsychological disorders. DRs usually transfer signals into cells through guanine nucleotide-binding regulatory G-proteins [55]. DRs can be classified into two major subfamilies, D1 and D2 receptors. Antipsychotic drugs and dopamine both act on the same binding sites, but antipsychotic drugs do not bind or activate the G-protein [56]. It blocks the binding site of dopamine and prevents sodium ions from entering postsynaptic cells [57]. Antipsychotics known as neuroleptics are a class of compounds with a high affinity for several subtypes of dopamine receptors [58]. The chemical composition and structural variation of the antipsychotics allows them to bind to different subtypes of dopamine receptors without triggering the postsynaptic response that is exerted by dopamine under psychotic conditions. Neuroleptics can block dopamine receptors without triggering the ion channels to be opened or set off an action potential. Therefore neuroleptics are being administered to schizophrenic patients to aid in reducing excess levels of dopamine and this mechanism of action is very effective to alleviate the positive symptoms of this disorder [59]. The most commonly used “typical antipsychotic drugs” including phenotiazines, thioanthenes, butyrophenones, diphenylbutyl piperidines, and dihydroindolones, have a high binding affinity for the dopamine receptors and exert their therapeutic action. D2 dopamine receptors are present on both presynaptic cells and the post-synaptic membrane. Commonly used antipsychotic compounds can interfere with dopaminergic neurotransmission at different sites in both pre- and postsynaptic cells. Usually typical antipsychotics inhibit the dopaminergic neurotransmission in the limbic system and in the cerebral cortex [60]. These areas are important for controlling the motivational and emotional behaviors and thoughts. These mechanisms of action of antipsychotics are useful for alleviating the positive symptoms of schizophrenia and many psychotic conditions.
Typical antipsychotics block all D2 receptors together with the other receptors involved in fine tuning of cognitive and motor association especially in the basal ganglia and cerebellum. Consequently, the inhibition of dopamine transmission causes exceedingly undesirable side effects such as tremors and akinesia [59]; additionally, it also inhibits the regular endocrine function, and elicits the anticholinergic, antiadrenergic, antihistaminic and antiserotonergic actions [57]. Another type of neuroleptic, known as “atypical antipsychotics,” exert very similar therapeutic effects as typical antipsychotics, but do not produce extensive level of side effects. The atypical antipsychotics demonstrate less affinity for D2 receptors than D3 and D4 dopamine receptors [60]. As the appearance of D3 and D4 receptors are limited to the neurons of the limbic system and cortex, therefore, new nature-based therapeutic interventions are needed to act on the vast region of the brain with safer effects.
Limonene is a common terpene found in citrus fruits. This monoterpene is widely used as a flavor and fragrance. Limonene has been shown to exert anxiolytic effects, regulatory effects on neurotransmitters, and antinociceptive effects [61]. Previously it has been shown that limonene increased the metabolic conversion of dopamine and serotonin in the hippocampus and prefrontal cortex and striatum, respectively, suggesting that anxiolytic and antidepressant-like effects can include suppression of dopamine activity associated with increased serotonergic neurons through 5-HT1A [62]. To date, a very dynamic bioactive compound named (−)-stepholidine has been isolated from the Chinese herb Stephania, which is used as a drug and exhibits dual effects on D1 receptor agonists and D2 receptor antagonists. In addition, another preclinical study has shown that SPD has superior antipsychotic effects as compafed with conventional perphenazine [55].
2.3. Alteration of Several Signaling Pathways for the Protection and Survival of Neuronal Cells
Several signaling pathways are associated with neuropsychiatric disorders. Inflammation is a typical pathological feature involved in the progression of neuropsychiatric disorders. Microglia play an important role in the central nervous system and host defense mechanisms. Acute or chronic inflammatory processes cause long-lasting and excessive tissue damage in the central nervous system. Damaging stimuli such as wounds, infection, pathogens, or other foreign substances associated with brain injuries produce proinflammatory and inflammatory cytokines, such as IL-1β, IL-16, TNFα, and TGF-β1, which can induce microglia-mediated inflammation involved in acute or chronic neuropsychiatric disorders [63]. Therefore, anti-inflammatory pathways or reduced production of proinflammatory cytokines are therapeutic targets for antipsychotic drugs. The synthetic compounds of antipsychotic drugs provoke side effects with long-term use. Several studies have reported that the vast groups of terpenoids exert anti-inflammatory and antioxidative effects by altering inflammatory and oxidative stress-related pathways. Several groups of terpenes (D-limonene, α-phellandrene, terpinolene, boreol, linalool, and triterpene glycosides) have been reported to reduce the expression of TNF-α, IL-1, and IL-6 in in vivo models, such as Swiss mice, Wistar rats, and albino mice (BALB/C) [64].
The potent compounds bacoside A, bacopaside I and II, and bacosaponin of Bacopa monnieri have been reported to inhibit neuronal death by preventing AChE activity in vitro and in vivo, exerted anti-inflammatory and antidepressant effects and improved memory dysfunction in animal models [65]. In addition, α-pinene, d-limonene, camphene, myrcene, p-cymene, terpinolene, camphor, linalool, humulene, and β-caryophyllene have been reported as the group of terpenes that modulate the inflammatory process and oxidative stress [66]. In a former study, the antioxidant, anti-inflammatory, and neuroprotective activities of Ginkgolide B are reported and ascribed to downregulation of the Toll-like-receptor 4/NF-κB pathway [67].
References
- Hu, D.; Gao, H.; Yao, X.S. Biosynthesis of Triterpenoid Natural Products. In Comprehensive Natural Products III; Liu, H.-W., Begley, T.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 577–612.
- Heras, B.D.L.; Rodriguez, B.; Bosca, L.; Villar, A.M. Terpenoids: Sources, Structure Elucidation and Therapeutic Potential in Inflammation. Curr. Top. Med. Chem. 2003, 3, 171–185.
- Alihosseini, F. Plant-based compounds for antimicrobial textiles. In Antimicrobial Textiles; Sun, G., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 155–195.
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Stud. Nat. Prod. Chem. 2017, 54, 21–86.
- Ruszkowski, P.; Bobkiewicz-Kozlowska, T. Natural Triterpenoids and their Derivatives with Pharmacological Activity Against Neurodegenerative Disorders. Mini. Rev. Org Chem. 2014, 11, 307–315.
- Semwal, D.K.; Semwal, R.B. Triterpenoids: An Important Bioactive Class of Natural Products; LAP GmbH: Saarbrucken, Germany, 2011; pp. 1–68.
- Connolly, J.D.; Hill, R.A. Triterpenoids. Nat. Prod. Rep. 2010, 27, 79–132.
- Parmar, S.K.; Sharma, T.P.; Airao, V.B.; Bhatt, R.; Aghra, R.; Chavda, S. Neuropharmacological effects of triterpenoids. Phytopharmacology 2013, 4, 354–372.
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98.
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflammation 2013, 10, 816.
- Kealy, J.; Greene, C.; Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2018, 726, 133664.
- Engelhardt, B.; Sorokin, L. The blood–brain and the blood–cerebrospinal fluid barriers: Function and dysfunction. In Seminars in Immunopathology; Springer Science & Business Media: Berlin, Germany, 2009; pp. 497–511.
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Chan, K.; Li, Y.; Li, G. The Protective Effects of Natural Products on Blood-Brain Barrier Breakdown. Curr. Med. Chem. 2012, 19, 1830–1845.
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192.
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25.
- Petty, M.A.; Lo, E.H. Junctional complexes of the blood–brain barrier: Permeability changes in neuroinflammation. Prog. Neurobiol. 2002, 68, 311–323.
- Basuroy, S.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am. J. Physiol. Physiol. 2009, 296, C422–C432.
- Guo, S.; Stins, M.; Ning, M.; Lo, E.H. Amelioration of Inflammation and Cytotoxicity by Dipyridamole in Brain Endothelial Cells. Cerebrovasc. Dis. 2010, 30, 290–296.
- Lv, S.; Song, H.-L.; Zhou, Y.; Li, L.-X.; Cui, W.; Wang, W.; Liu, P. Tumour necrosis factor-α affects blood-brain barrier permeability and tight junction-associated occludin in acute liver failure. Liver Int. 2010, 30, 1198–1210.
- Ohara, Y.; McCarron, R.M.; Hoffman, T.T.; Sugano, H.; Bembry, J.; Lenz, F.A.; Spatz, M. Adrenergic mediation of TNF alpha-stimulated ICAM-1 expression on human brain microvascular endothelial cells. Acta. Neurochir. Suppl. 2000, 76, 117–120.
- Deplanque, D.; Gelé, P.; Pétrault, O.; Six, I.; Furman, C.; Bouly, M.; Nion, S.; Dupuis, B.; Leys, D.; Fruchart, J.-C.; et al. Peroxisome Proliferator-Activated Receptor-α Activation as a Mechanism of Preventive Neuroprotection Induced by Chronic Fenofibrate Treatment. J. Neurosci. 2003, 23, 6264–6271.
- Huang, W.; Eum, S.Y.; András, I.E.; Hennig, B.; Toborek, M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 2009, 23, 1596–1606.
- Klotz, L.; Diehl, L.; Dani, I.; Neumann, H.; Endl, E.; Klockgether, T.; Engelhardt, B.; Knolle, P. Brain endothelial PPAR-gamma controls inflammation-induced CD4+ T cell adhesion and transmigration in vitro. J. Neuroimmunol. 2019, 190, 34–43.
- Pardridge, W.M. Why is the global CNS pharmaceutical market so under-penetrated? Drug Discov. Today 2002, 7, 5–7.
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972.
- O’Keeffe, E.; Campbell, M. Modulating the paracellular pathway at the blood–brain barrier: Current and future approaches for drug delivery to the CNS. Drug Discov. Today Technol. 2016, 20, 35–39.
- Adams, M.; Gmunder, F.; Hamburger, M. Plants traditionally used in age related brain-disorders- a survey of etthnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381.
- Zhai, K.; Duan, H.; Wang, W.; Zhao, S.; Khan, G.J.; Wang, M.; Zhang, Y.; Thakur, K.; Fang, X.; Wu, C.; et al. Ginsenoside Rg1 ameliorates blood–brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm. Sin. B 2021, 11, 3493–3507.
- Yan, F.-L.; Zheng, Y.; Zhao, F.-D. Effects of ginkgo biloba extract EGb761 on expression of RAGE and LRP-1 in cerebral microvascular endothelial cells under chronic hypoxia and hypoglycemia. Acta Neuropathol. 2008, 116, 529–535.
- Herraiz, T.; Guillén, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. BioMed Res. Int. 2018, 2018, 4810394.
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S287–S296.
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309.
- Cohen, G.; Kesler, N. Monoamine Oxidase and Mitochondrial Respiration. J. Neurochem. 2002, 73, 2310–2315.
- Herraiz, T.; Guillén, H. Inhibition of the bioactivation of the neurotoxin MPTP by antioxidants, redox agents and monoamine oxidase inhibitors. Food Chem. Toxicol. 2011, 49, 1773–1781.
- Sook, C.H. Inhibition of Monamine Oxidase by a Flavone and Its Glycoside from Ixeris dentata Nakai. Prev. Nutr. Food. Sci. 2003, 8, 141–144.
- Kasper, S.; Caraci, F.; Forti, B.; Drago, F.; Aguglia, E. Efficacy and tolerability of Hypericum extract for the treatment of mild to moderate depression. Eur. Neuropsychopharmacol. 2010, 20, 747–765.
- Russo, E.; Scicchitano, F.; Whalley, B.J.; Mazzitello, C.; Ciriaco, M.; Esposito, S.; De Sarro, G. Hypericum perforatum: Pharmacokinetics, mechanism of action, tolerability, and clinical drug-drug interactions. Phytother. Res. 2014, 28, 643–655.
- Peng, L.; Hertz, L.; Huang, R.; Sonnewald, U.; Petersen, S.B.; Westergaard, N.; Larsson, O.; Schousboe, A. Utilization of glutamine and of TCA cycle constituents as precursors for transmitter glutamate and GABA. Dev. Neurosci. 1993, 15, 367–377.
- Bonanno, G.; Raiteri, M. Coexistence of carriers for dopamine and GABA uptake on a same nerve terminal in the rat brain. Br. J. Pharmacol. 1987, 91, 237–243.
- Brambilla, P.; Perez, J.; Barale, F.; Schettini, G.; Soares, J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry 2003, 8, 721–737.
- Rakofsky, J.J.; Holtzheimer, P.E.; Nemeroff, C.B. Emerging targets for antidepressant therapies. Curr. Opin. Chem. Biol. 2009, 13, 291–302.
- Hervás, I.; Vilaró, M.T.; Romero, L.; Scorza, M.C.; Mengod, G.; Artigas, F. Desensitization of 5-HT1A Autoreceptors by a Low Chronic Fluoxetine Dose Effect of the Concurrent Administration of WAY-100635. Neuropsychopharmacology 2001, 24, 11–20.
- Kessler, A.; Sahin-Nadeem, H.; Lummis, S.C.; Weigel, I.; Pischetsrieder, M.; Buettner, A.; Villmann, C. GABAA receptor modulation by terpenoids from Sideritis extracts. Mol. Nutr. Food. Res. 2014, 58, 851–862.
- Wang, Z.-J.; Heinbockel, T. Essential Oils and Their Constituents Targeting the GABAergic System and Sodium Channels as Treatment of Neurological Diseases. Molecules 2018, 23, 1061.
- Chizzola, R. Regular monoterpenes and sesquiterpenes (Essential Oils). In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2973–3008.
- Seol, G.H.; Shim, H.S.; Kim, P.-J.; Moon, H.K.; Lee, K.H.; Shim, I.; Suh, S.H.; Min, S.S. Antidepressant-like effect of Salvia sclarea is explained by modulation of dopamine activities in rats. J. Ethnopharmacol. 2010, 130, 187–190.
- Tankam, J.M.; Ito, M. Inhalation of the Essential Oil of Piper guineense from Cameroon Shows Sedative and Anxiolytic-Like Effects in Mice. Biol. Pharm. Bull. 2013, 36, 1608–1614.
- Park, H.-J.; Lim, E.-J.; Zhao, R.J.; Oh, S.R.; Jung, J.W.; Ahn, E.-M.; Lee, E.S.; Koo, J.S.; Kim, H.Y.; Chang, S.; et al. Effect of the fragrance inhalation of essential oil from Asarum heterotropoides on depression-like behaviors in mice. BMC Complement. Altern. Med. 2015, 15, 43.
- Abuhamdah, S.; Huang, L.; Elliott, M.S.J.; Howes, M.R.; Ballard, C.; Holmes, C.; Burns, A.; Perry, E.K.; Francis, P.T.; Lees, G.; et al. Pharmacological profile of an essential oil derived from Melissa officinalis with anti-agitation properties: Focus on ligand-gated channels. J. Pharm. Pharmacol. 2008, 60, 377–384.
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11.
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280.
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681.
- Gilhotra, N.; Dhingra, D. Thymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levels. Pharmacol. Rep. 2011, 63, 660–669.
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532.
- Jin, G.-Z.; Zhu, Z.-T.; Fu, Y. (−)-Stepholidine: A potential novel antipsychotic drug with dual D1 receptor agonist and D2 receptor antagonist actions. Trends Pharmacol. Sci. 2002, 23, 4–7.
- Li, P.; Snyder, G.L.; Vanover, K.E. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403.
- Miller, R. Mechanisms of Action of Antipsychotic Drugs of Different Classes, Refractoriness to Therapeutic Effects of Classical Neuroleptics, and Individual Variation in Sensitivity to their Actions: PART I. Curr. Neuropharmacol. 2009, 7, 302–314.
- Martel, J.C.; McArthur, S.G. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003.
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391.
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087.
- Gu, S.M.; Kim, S.Y.; Lamichhane, S.; Hong, J.T.; Yun, J. Limonene Inhibits Methamphetamine-Induced Sensitizations via the Regulation of Dopamine Receptor Supersensitivity. Biomol. Ther. 2019, 27, 357–362.
- Komiya, M.; Takeuchi, T.; Harada, E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006, 172, 240–249.
- Allan, S.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744.
- Ramalho, T.R.; Oliveira, M.T.; Lima, A.L.; Bezerra-Santos, C.R.; Piuvezam, M.R. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254.
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H.K. A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900.
- González-Burgos, E.; Gómez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341.
- Gu, J.H.; Ge, J.B.; Li, M.; Wu, F.; Zhang, W.; Qin, Z.H. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur. J. Pharm. Sci. 2012, 47, 652–660.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
28 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





