
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Davide Cervia | -- | 2473 | 2022-09-27 15:31:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 2473 | 2022-09-28 02:59:29 | | |
Video Upload Options
Unlike in vitro cell cultures that cannot mimic tissue homeostasis and physiology, 3D retinal organoids are relatively cheap models and have an undeniable complexity rate. However, they are challenging to isolate and maintain long enough to investigate complex processes such as inflammation and neovascularization. These disadvantages are exacerbated considering the retina, which is mainly due to the global complexity of this tissue. Several classic diagnostic techniques could be applied to retinal organ cultures, such as optical coherence tomography, which explores the morphological aspect of the retinal architecture, electroretinograms that record the electrical response of retinal cells, and microelectrode array recording, which stimulates and records the electrical activity of RGC. Several mammalian retinal organ cultures as alternative models are currently available and well established, including those derived from mice, rats, rabbits, cats, dogs, non-human primates, bovines, and pigs. They are excellent samples for the preliminary phase before the in vivo step and for therapy tests, although organ cultures for the study of complex retinal neurodegenerative pathologies such as diabetic retinopathy (DR), retinitis pigmentosa (RP), age-related macular degeneration, and glaucoma are not entirely reproducing the human condition. Although all the events occurring during the various steps of retinal neurodegenerative diseases, including the clinical progression, are not fully mimicked by a single animal, preclinical in vivo models provide important information on the molecular and cellular mechanisms at the basis of the neuronal impairment. Thus, multiple organisms, including non-mammalian ones, are crucial for validating the mechanisms involved in retinal pathologies and developing new therapeutic options.
1. Introduction
2. Alternative Organism Models for Retina Neuroregeneration
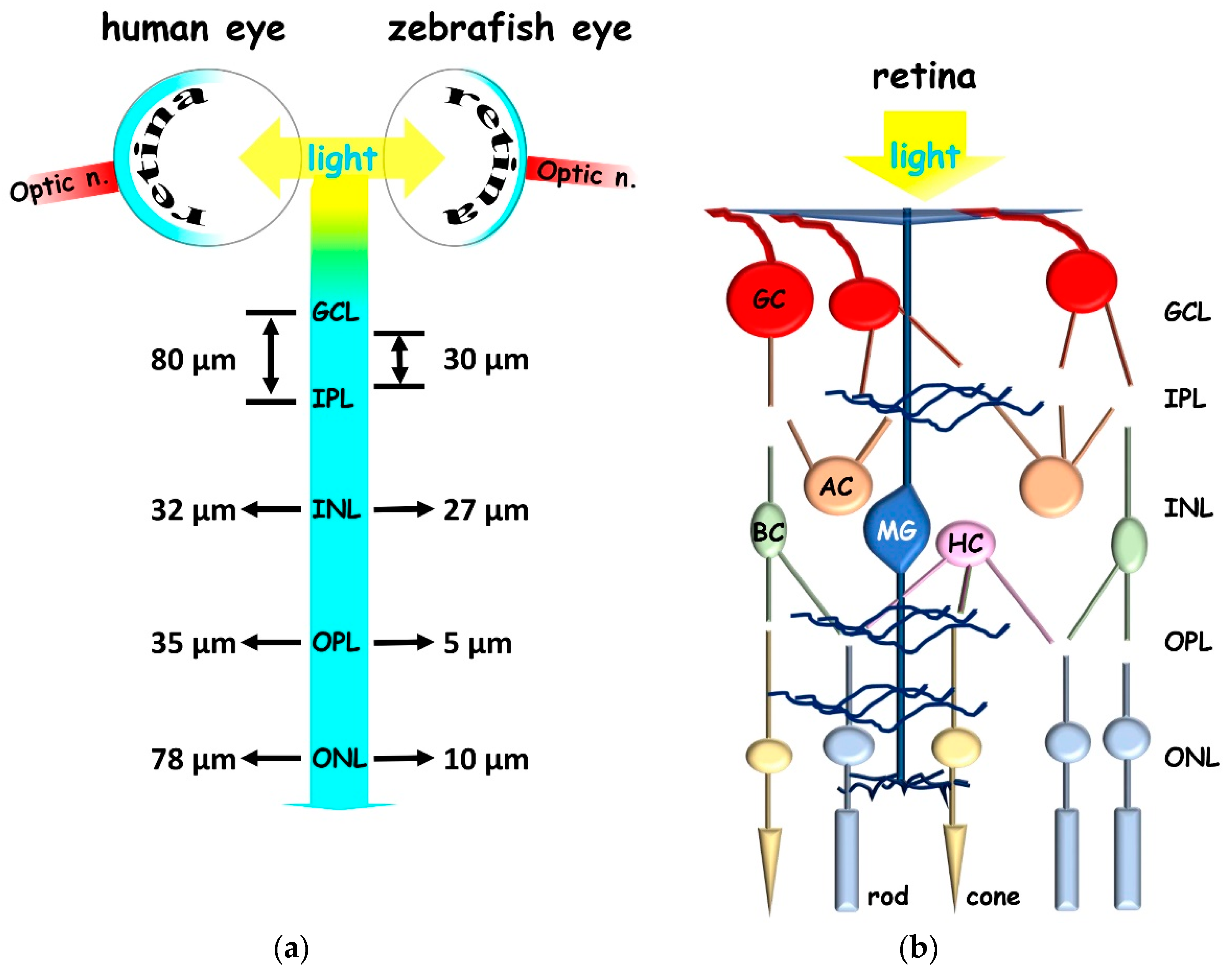
2.1. Zebrafish to Gain Insight in Vertebrate Retina
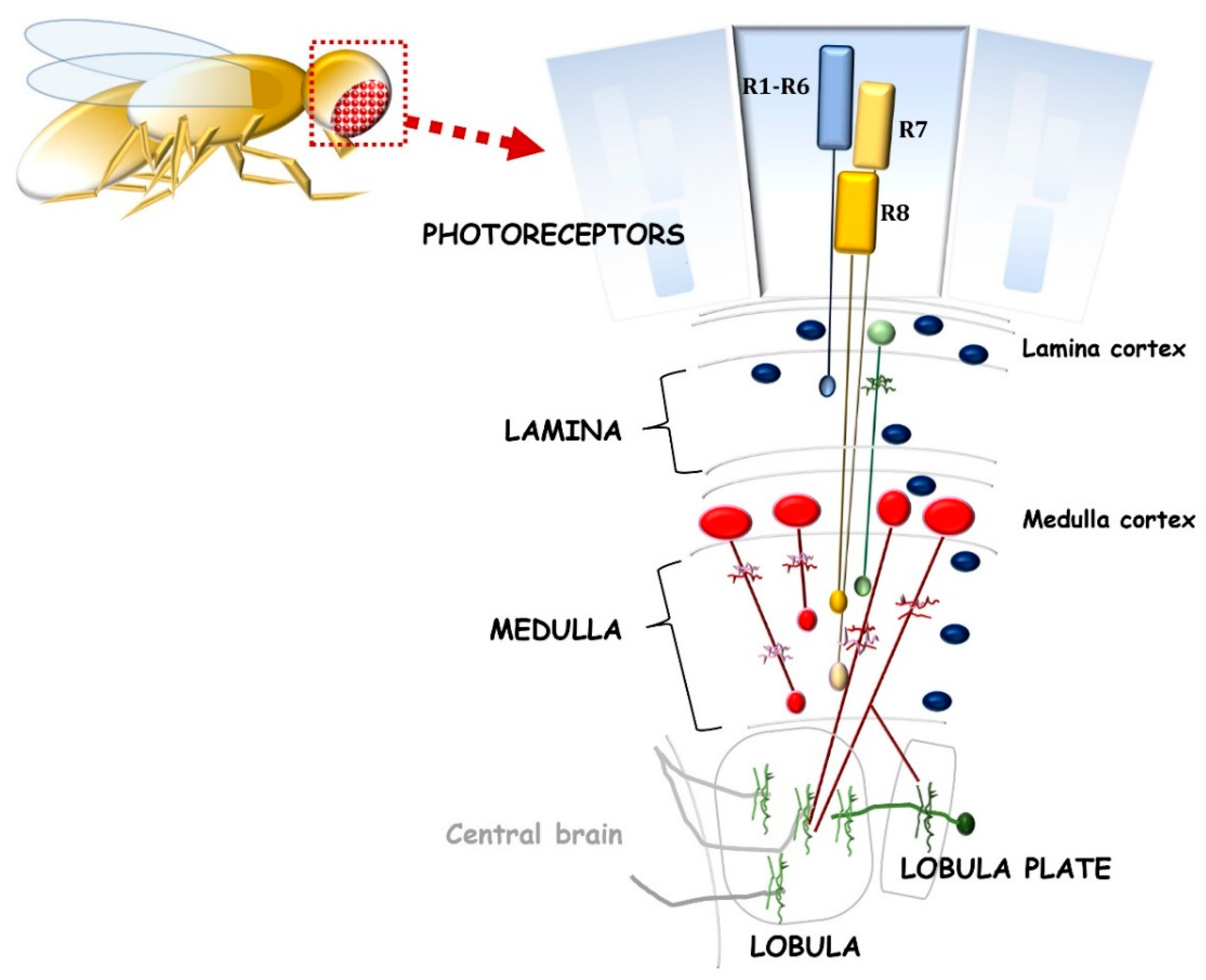
2.2. The Opportunity of D. melanogaster for Neuroregenerative Strategies
3. Conclusions
References
- Maneu, V.; Lax, P.; Cuenca, N. Current and future therapeutic strategies for the treatment of retinal neurodegenerative diseases. Neural Regen. Res. 2022, 17, 103–104.
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23.
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53.
- Sharma, S.; You, Y. Editorial: Retinal Changes in Neurological Diseases. Front. Neurosci. 2021, 15, 813044.
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the move towards ophthalmological research. Eye 2014, 28, 367–380.
- Stella, S.L., Jr.; Geathers, J.S.; Weber, S.R.; Grillo, M.A.; Barber, A.J.; Sundstrom, J.M.; Grillo, S.L. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633.
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86.
- Baden, T.; Euler, T.; Berens, P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 2020, 21, 5–20.
- Gallina, D.; Todd, L.; Fischer, A.J. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp. Eye Res. 2014, 123, 121–130.
- Kaur, G.; Singh, N.K. The Role of Inflammation in Retinal Neurodegeneration and Degenerative Diseases. Int. J. Mol. Sci. 2021, 23, 386.
- Gao, H.; Luoden, A.; Huang, X.; Chen, X.; Xu, H. Muller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361.
- Powell, C.; Grant, A.R.; Cornblath, E.; Goldman, D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 19814–19819.
- Van Dyck, A.; Bollaerts, I.; Beckers, A.; Vanhunsel, S.; Glorian, N.; van Houcke, J.; van Ham, T.J.; De Groef, L.; Andries, L.; Moons, L. Muller glia-myeloid cell crosstalk accelerates optic nerve regeneration in the adult zebrafish. Glia 2021, 69, 1444–1463.
- Martins, R.R.; Zamzam, M.; Tracey-White, D.; Moosajee, M.; Thummel, R.; Henriques, C.M.; MacDonald, R.B. Muller Glia maintain their regenerative potential despite degeneration in the aged zebrafish retina. Aging Cell 2022, 21, e13597.
- Yang, S.G.; Wang, X.W.; Qian, C.; Zhou, F.Q. Reprogramming neurons for regeneration: The fountain of youth. Prog. Neurobiol. 2022, 214, 102284.
- Wang, F.; Cheng, L.; Zhang, X. Reprogramming Glial Cells into Functional Neurons for Neuro-regeneration: Challenges and Promise. Neurosci. Bull. 2021, 37, 1625–1636.
- Neriec, N.; Desplan, C. From the Eye to the Brain: Development of the Drosophila Visual System. Curr. Top. Dev. Biol. 2016, 116, 247–271.
- Malin, J.; Desplan, C. Neural specification, targeting, and circuit formation during visual system assembly. Proc. Natl. Acad. Sci. USA 2021, 118, e2101823118.
- Fox, D.T.; Cohen, E.; Smith-Bolton, R. Model systems for regeneration: Drosophila. Development 2020, 147, dev173781.
- Catalani, E.; Silvestri, F.; Cervia, D. A Drosophila perspective on retina functions and dysfunctions. Neural Regen. Res. 2022, 17, 341–343.
- Kremer, M.C.; Jung, C.; Batelli, S.; Rubin, G.M.; Gaul, U. The glia of the adult Drosophila nervous system. Glia 2017, 65, 606–638.
- Chotard, C.; Salecker, I. Glial cell development and function in the Drosophila visual system. Neuron Glia Biol. 2007, 3, 17–25.
- Charlton-Perkins, M.A.; Sendler, E.D.; Buschbeck, E.K.; Cook, T.A. Multifunctional glial support by Semper cells in the Drosophila retina. PLoS Genet. 2017, 13, e1006782.
- Ahmed-de-Prado, S.; Baonza, A. Drosophila as a Model System to Study Cell Signaling in Organ Regeneration. Biomed Res. Int. 2018, 2018, 7359267.
- Crocker, K.L.; Marischuk, K.; Rimkus, S.A.; Zhou, H.; Yin, J.C.P.; Boekhoff-Falk, G. Neurogenesis in the adult Drosophila brain. Genetics 2021, 219, iyab092.
- Crocker, K.L.; Ahern-Djamali, S.; Boekhoff-Falk, G. Stimulating and Analyzing Adult Neurogenesis in the Drosophila Central Brain. J. Vis. Exp. 2021, 176, e63182.
- Fernandez-Hernandez, I.; Rhiner, C.; Moreno, E. Adult neurogenesis in Drosophila. Cell Rep. 2013, 3, 1857–1865.
- Janovjak, H.; Kleinlogel, S. Optogenetic neuroregeneration. Neural Regen. Res. 2022, 17, 1468–1470.
- Wang, Q.; Fan, H.; Li, F.; Skeeters, S.S.; Krishnamurthy, V.V.; Song, Y.; Zhang, K. Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila. eLife 2020, 9, e57395.
- Ingles-Prieto, A.; Furthmann, N.; Crossman, S.H.; Tichy, A.M.; Hoyer, N.; Petersen, M.; Zheden, V.; Biebl, J.; Reichhart, E.; Gyoergy, A.; et al. Optogenetic delivery of trophic signals in a genetic model of Parkinson’s disease. PLoS Genet. 2021, 17, e1009479.




