Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Inkar Castellanos | -- | 1633 | 2022-09-20 00:48:18 | | | |
| 2 | Dean Liu | Meta information modification | 1633 | 2022-09-21 03:23:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Castellanos-Huerta, I.; Gómez-Verduzco, G.; Tellez-Isaias, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V.M.; Velázquez-Juárez, G.; Garcia-Casillas, L.A.; Fernández-Siurob, I. Dunaliella salina. Encyclopedia. Available online: https://encyclopedia.pub/entry/27322 (accessed on 07 February 2026).
Castellanos-Huerta I, Gómez-Verduzco G, Tellez-Isaias G, Ayora-Talavera G, Bañuelos-Hernández B, Petrone-García VM, et al. Dunaliella salina. Encyclopedia. Available at: https://encyclopedia.pub/entry/27322. Accessed February 07, 2026.
Castellanos-Huerta, Inkar, Gabriela Gómez-Verduzco, Guillermo Tellez-Isaias, Guadalupe Ayora-Talavera, Bernardo Bañuelos-Hernández, Víctor Manuel Petrone-García, Gilberto Velázquez-Juárez, Luis Alberto Garcia-Casillas, Isidro Fernández-Siurob. "Dunaliella salina" Encyclopedia, https://encyclopedia.pub/entry/27322 (accessed February 07, 2026).
Castellanos-Huerta, I., Gómez-Verduzco, G., Tellez-Isaias, G., Ayora-Talavera, G., Bañuelos-Hernández, B., Petrone-García, V.M., Velázquez-Juárez, G., Garcia-Casillas, L.A., & Fernández-Siurob, I. (2022, September 19). Dunaliella salina. In Encyclopedia. https://encyclopedia.pub/entry/27322
Castellanos-Huerta, Inkar, et al. "Dunaliella salina." Encyclopedia. Web. 19 September, 2022.
Copy Citation
Dunaliella sp. is a unicellular, halophilic, biflagellate, naked green alga Phylum Chlorophyta, Class Chlorophyceae, order Volvocales, family Polyblepharidaceae with a total of 29 species, as well as several varieties and forms.
Dunaliella salina
vaccines
recombinant protein
1. General Features
Dunaliella sp. is a unicellular, halophilic, biflagellate, naked green alga Phylum Chlorophyta, Class Chlorophyceae, order Volvocales, family Polyblepharidaceae with a total of 29 species, as well as several varieties and forms [1][2]. D. salina was first described in 1905 [3]. The genus Dunaliella sp., named in honor of Michel Felix Dunal [4] is the richest natural source of βcarotene, violaxanthin, neoxanthin, zeaxanthin, and lutein with the function of photoprotective to the high irradiance [5][6] and vitamins, antioxidants, polyunsaturated fatty acids, minerals, and enzymes [7]. Recently, the study of this species raised interest in its protein content, which ranges from 50 to 80% (dry weight), also for the content of essential amino acids, which is higher than recommended by the Food and Agriculture Organization of the United Nations (FAO) [8]. Dunaliella sp. presents different forms (spherical, pyriform, fusiform, ellipsoid), sizes from 5 to 25 μm in length and from 3 to 13 μm in width, also contain a single chloroplast, chlorophylls a and b, and organelles observed in green algae: membrane-bound nucleus, mitochondria, vacuoles, Golgi apparatus, and an eyespot and elastic plasma membrane covered by a mucus surface coat with the capacity of shrinks or swells according with the hypertonic and hypotonic conditions [7][9]. D. salina, similar to other microalgae, undergoes a complex life cycle, cellular divisions by lengthwise division in the motile state (vegetative cells), but also presents sexual reproduction (sexual zygote formation) [1].
Several species of Dunaliella sp. are observed in high salt concentrations, classifying them as halophilic organisms. However, some species thrive in freshwater [10][11] as well as over a wide pH range, from pH1 (D. acidophila) to pH11 (D. salina) [12]. The high capacity to adapt to different concentrations of salinity (3 to 31%) and temperature range (<0 °C to >38 °C) make Dunaliella sp. a unique and highly resistant eukaryotic organism [13]. Because of these characteristics, various species of Dunaliella sp. have been isolated in diverse ecosystems over the world [14]. Because of all high-value features, Dunaliella sp. can be considered a promisor recombinant expression system [9][15][16][17]; between these features are included: the capacity to grow in a wide range of salt concentrations which can prevent contamination of the culture [12], transcriptional modifications [18][19][20], and lacking a rigid cell wall, facilitating genetic transformation procedures as well as the extraction during downstream processing [9].
2. Production Aspects
D. salina culture media have wide ranges in salts and pH (6 to 23 % of NaCl, and pH 6 to 9) [1][21]. Optimal grown conditions are between 2 and 8% salt; a high salt concentration affects the growth rate in some cases. Under the best conditions for growth, the division rate can go from 0.5 to 1.22 divisions per 24 h [1]. Based on several studies, an average concentration of salt in the culture media of D. salina (12%) and D. viridis (6%) are the optimal salt concentration for growing [22][23]. However, other strains present different growth conditions [24]. In general, the culture conditions are a temperature of 25 ± 2 °C under the white fluorescent light of 52.84 μmol photons m−2 s−1 without aeration in stirring at 110 rpm/min in the orbital shaker [13][25]. The efforts focus on developing an efficient condition for growing under laboratory and industrial requirements [5][26][27][28]. Media for growth of D. salina suggested include: modified Johnson’s medium, Erdschreiber’s medium, Guillard’s F/2 medium, modified ASP medium, and enriched seawater [25][29].
3. Culture Systems of D. salina
Mass culture of microalgae is reported in systems such as open ponds, circular ponds, raceway ponds, cascade ponds, large bags, tanks, heterotrophic fermenters, and several kinds of closed PBR [30][31]. In the case of D. salina, it can be grown under controlled conditions in selective media and biological contamination-free [32]. Currently, PBR implementation for Dunaliella’s intensive culture is widely reported [9][33]. PBR has several advantages compared with other culture systems, such as higher yield, cleaner product, and concentration of secondary metabolites. In general, there are three types of PBR: flat plate bioreactors, tubular PBR, and ultrathin immobilized configurations [30][31][34]. The use of PBR for Dunaliella sp. culture has been focused on secondary metabolite production; however, their possible use as a PBR system for recombinant protein production is also feasible [35][36][37][38].
4. Genetic Engineering Tools Applied to D. salina
Among the genetic manipulation techniques reported for Dunaliella sp. include electroporation [39][40], particle bombardment [41], glass beads [42], lithium acetate/polyethylene glycol (PEG)-mediated method [43], and Agrobacterium-mediated method (agroinfiltration) [44]. In general, all techniques present a range of advantages and disadvantages for their use in microalgae [18]. Expression-efficacy depends on codon optimization, protease activity, protein toxicity, and transformation-associated genotypic modification [45]. In the case of D. salina, some of the technical approaches reported for nuclear transformation include LiAc/PEG-mediated method, glass bead method, and agroinfiltration protocol. In the case of chloroplast transformation, the most recommended method is particle bombardment. The possible use of other techniques, ultrasonic delivery [46], ultraviolet laser microbeam [47], and aerosol gene delivery [48], allows the opportunity to explore new approaches to achieve the best form of genetic manipulation in Dunaliella sp. These methods present a relatively low level of transformation and differences in their practicality and repeatability; however, most of these are focused on the expression of reporters, selecting genes, therapeutic application, and production of viral proteins. Viral antigens, including hepatitis B surface antigen (HBsAg), yielding 3.11 ng/mg of total soluble protein by transforming electroporation protocol, white spot syndrome virus (WSSV) VP28, yielding 3.04 ng/mg of soluble protein by gene glass beads transformation [18], and hemagglutinin influenza virus yielding 255.5 µg/2 g wet weight by agroinfiltration protocol [44]. Despite the low expression levels [49], these assays are focused on determining the ability of this system to express viral proteins, so yields require other approaches.
One of the most promising systems for expressing recombinant proteins in D. salina is the agroinfiltration protocol mediated by Agrobacterium tumefaciens [44][50][51]. This protocol is based on the ability of A. tumefaciens, an indirect method, to transfer exogenous desoxyribonucleic acid (DNA) to plant cells through a bacterial conjugation system (Type IV secretion system (T4SS) and protein-DNA complexes) [52]. Plants are naturally affected by A. tumefaciens, including angiosperms and gymnosperms [53]. Briefly, A. tumefaciens, a bacterium present in the soil, moves towards the wound upon detecting phenolic compounds from a wounded plant, adheres, and begins to transform plant cells by inducing the transcription of virulence genes present in a plasmid called Tumor-inducer (Ti-DNA). Ti-DNA, together with the bacterial virulence proteins (VirD1, VirD2, VirE2), induces the transcription, processing of transfer DNA (T-DNA), and integration into the plant genome. Transferential DNA with A. tumefaciens requires the insertion of a gene of interest in T-DNA present in Ti-DNA for its insertion into the genome of the nucleus of the cells [53][54]. The random insertion observed in this method suggests a non-homologous recombination mechanism [55]. Since the first experiments for the elaboration of transgenic plants using A. tumefaciens in 1983 [53], significant advances in understanding the T-DNA insertion process, protocols, and experimentation in model plants, including in D. salina have been achieved.
5. Advances in Dunaliella Transformation for Recombinant Biopharmaceutical Production
In general, expression in nucleus D. salina cells is focused mainly on reporter genes such as β-glucuronidase gene [19], enhanced green fluorescent protein [56], and selection markers such as phosphinothricin acetyltransferase under promoter DCA1 [57], chloramphenicol acetyltransferase [12], and zeocin resistance protein [58]. However, the expression of commercial value proteins is reduced [59][60], including immunogens [19]. Although several results [18][39][44][61], none of these proteins has led to the generation of products at the industrial level. According to findings, the Dunaliella system can be used in an approach for industrial applications, in particular in antigen production. The chloroplast is also an attractive expression protein system in microalgae due to advantages such as directed integration of genes via homologous recombination [62], high-level expression, organization of transgenes into operons, and no epigenetic interference [63][64], as previously reported [65][66]. Although there are few reports of expression in the chloroplast of D. salina [67], other systems such as Chlamydomonas [68], demonstrated that the use of chloroplast for the expression of recombinant proteins could be a proposal for proteins of commercial value. The purpose of new promoters and construction of expression vectors for D. salina chloroplast transformation is the following step [18] (Figure 1).
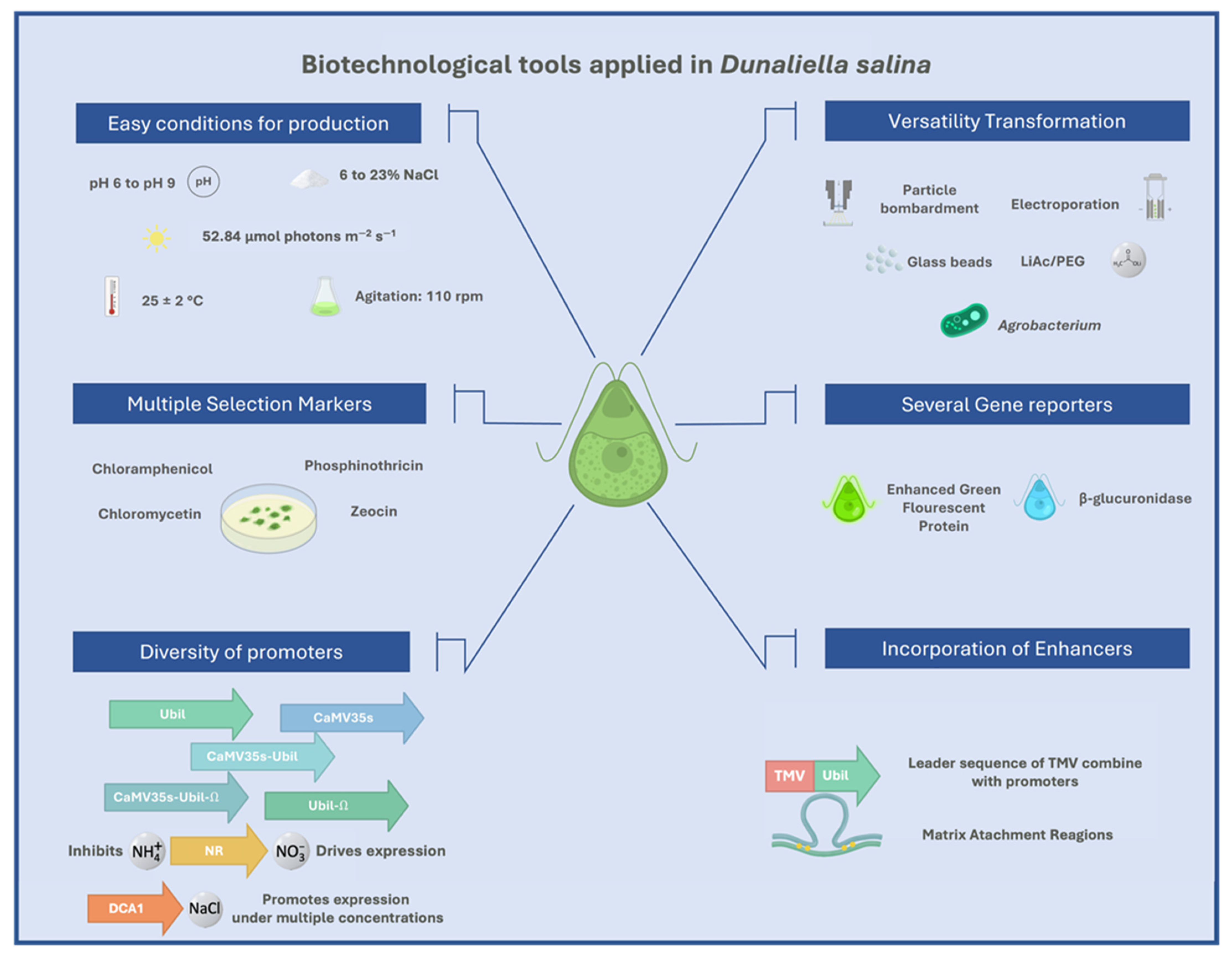
Figure 1. Biotechnological tools applied in Dunaliella salina.
6. Immunological Aspects in Mucosal Vaccination with D. salina
Vaccination is one of the leading practices in medicine to control and prevent the vast majority of infectious-contagious diseases [69], based on the correct presentation of an antigen to the immune system. For this, it is necessary to determine the route of application, components of the formulation (adjuvant), type of immune responses, the dose required, and type of vaccine, either first group: (i) live attenuated vaccines (e.g., smallpox, yellow fever, measles, mumps, rubella, and chicken pox), or second group: (i) subunit vaccines (e.g., a vaccine against recombinant hepatitis B), (ii) toxoid vaccines (e.g., vaccines against diphtheria and tetanus), (iii) carbohydrate vaccines (e.g., vaccines against pneumococcus), and (iv) conjugate vaccines (e.g., vaccines against Haemophilus influenzae type B) [70].
Vaccination protocol and the immune system play a decisive role in correct immune response [71], particularly the immune system on the mucosal surface [72]. In general, the mucosal immune system presents highly specialized MALT, responsible for antigen presentation for the generation of an efficient mucosal immune response [73]. Due to the presence of these specialized tissues, mucosal administration of antigens demonstrated efficiency in wide pathologies [74], including influenza virus [75].
The expression of subunit vaccines in microalgae presents a convenient mucosal administration option with advantages including minimum processing before application [76] relatively low cost (<$1mg protein) in contrast to synthetic peptide antigen range between $35 and $95/mg peptide [76], algal cell wall appears sufficient to reduce antigen degradation by digestive system (bio-encapsulated) [49], subsequently broken down by digestive enzymes and commensal bacteria. Consequently, the recombinant proteins are released to be in contact with MALT [77][78]. Therefore, these drawbacks in consideration for oral vaccination are overcome by this expression model, as previously reported [44][61]. Considering that more than ten milligrams of an average subunit vaccine are required for oral administration (1000 times more than is necessary for an injected route) [79], it is estimated that hundreds of grams of recombinant tissue are needed to stimulate an immune response. Nevertheless, increasing the concentration of the antigen by freeze-dried microalgae without losing antigenic capacity [79], antimicrobial activity [80], and immunomodulatory compounds naturally present in certain species of microalgae (Dunaliella sp.) exert synergistic effects with the vaccine formulation [81][82].
References
- Oren, A. A Hundred Years of Dunaliella Research: 1905–2005. Saline Syst. 2005, 1, 1–14.
- Massyuk, N. Morphology, Taxonomy, Ecology and Geographic Distribution of the Genus Dunaliella Teod. and Prospects for Its Potential Utilization; Naukova Dumka. Massyuk: Kiev, Ukraine, 1973; Volume 312.
- Colin, C.D. Organisation et Développement Du Dunaliella, Nouveau Genre de Volvocacée-Polyblépharidée. Beih. Zum Bot. Cent. Syst. Pflanzengeogr. Angew. Bot. Etc. Abt. B 1905, 18, 215.
- Dunal, M. Note Sur Les Algues Qui Colourent En Rouge Certaines Eaux Des Marais Salants Méditerranéens. Compte Rendu Hebd. Des Seances De I’Academie Des Sci. 1837, 15, 585–587.
- Lamers, P.P.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Exploring and Exploiting Carotenoid Accumulation in Dunaliella Salina for Cell-Factory Applications. Trends Biotechnol. 2008, 26, 631–638.
- Ben-Amotz, A. Glycerol Production in the Alga Dunaliella. In Biochemical and Photosynthetic Aspects of Energy Production; Elsevier: Amsterdam, Netherlands, 1980; pp. 191–208.
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella Biotechnology: Methods and Applications. J. Appl. Microbiol. 2009, 107, 14–35.
- Sui, Y.; Vlaeminck, S.E. Dunaliella Microalgae for Nutritional Protein: An Undervalued Asset. Trends Biotechnol. 2020, 38, 10–12.
- Ben-Amotz, A.; Avron, M. Dunaliella: Physiology, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 1992.
- MELKONIAN, M. The Eyespot Apparatus of Flagellated Green Algae: A Critical Review. Prog. Phycol. Res. 1984, 3, 193–268.
- Borowitzka, M.A.; Siva, C.J. The Taxonomy of the Genus Dunaliella (Chlorophyta, Dunaliellales) with Emphasis on the Marine and Halophilic Species. J. Appl. Phycol. 2007, 19, 567–590.
- Gimmler, H.; Weis, U.; Weiss, C.; Kugel, H.; Treffny, B. Dunaliella Acidophila (Kalina) Masyuk-an Alga with a Positive Membrane Potential. New Phytol. 1989, 113, 175–184.
- Ginzburg, M. Dunaliella: A Green Alga Adapted to Salt. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 1988; Volume 14, pp. 93–183.
- Borowitzka, M.A.; Borowitzka, L.J. Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988.
- Rasala, B.A.; Lee, P.A.; Shen, Z.; Briggs, S.P.; Mendez, M.; Mayfield, S.P. Robust Expression and Secretion of Xylanase1 in Chlamydomonas Reinhardtii by Fusion to a Selection Gene and Processing with the FMDV 2A Peptide. PLoS ONE 2012, 7, e43349.
- Andersen, D.C.; Krummen, L. Recombinant Protein Expression for Therapeutic Applications. Curr. Opin. Biotechnol. 2002, 13, 117–123.
- Butinar, L.; Zalar, P.; Frisvad, J.C.; Gunde-Cimerman, N. The Genus Eurotium–Members of Indigenous Fungal Community in Hypersaline Waters of Salterns. FEMS Microbiol. Ecol. 2005, 51, 155–166.
- Feng, S.; Li, X.; Xu, Z.; Qi, J. Dunaliella Salina as a Novel Host for the Production of Recombinant Proteins. Appl. Microbiol. Biotechnol. 2014, 98, 4293–4300.
- Akbari, F.; Eskandani, M.; Khosroushahi, A.Y. The Potential of Transgenic Green Microalgae; a Robust Photobioreactor to Produce Recombinant Therapeutic Proteins. World J. Microbiol. Biotechnol. 2014, 30, 2783–2796.
- Wang, T.; Xue, L.; Hou, W.; Yang, B.; Chai, Y.; Ji, X.; Wang, Y. Increased Expression of Transgene in Stably Transformed Cells of Dunaliella Salina by Matrix Attachment Regions. Appl. Microbiol. Biotechnol. 2007, 76, 651–657.
- Baas-Becking, L. Salt Effects on Swarmers of Dunaliella Viridis Teod. J. Gen. Physiol. 1931, 14, 765.
- McLachlan, J. The Growth of Unicellular Algae in Artificial and Enriched Sea Water Media. Can. J. Microbiol. 1959, 5, 9–15.
- Borowitzka, L.J. The Microflora. In Salt lakes; Springer: Berlin/Heidelberg, Germany, 1981; pp. 33–46.
- Brock, T. Salinity and the Ecology of Dunaliella from Great Salt Lake. Microbiology 1975, 89, 285–292.
- Sathasivam, R.; Juntawong, N. Others Modified Medium for Enhanced Growth of Dunaliella Strains. Int. J. Curr. Sci. 2013, 5, 67–73.
- Fu, W.; Paglia, G.; Magnúsdóttir, M.; Steinarsdóttir, E.A.; Gudmundsson, S.; Palsson, B.Ø.; Andrésson, Ó.S.; Brynjólfsson, S. Effects of Abiotic Stressors on Lutein Production in the Green Microalga Dunaliella Salina. Microb. Cell Factories 2014, 13, 3.
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.S.; Salley, S.O. Effect of Nutrients on Growth and Lipid Accumulation in the Green Algae Dunaliella Tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655.
- Fu, W.; Guðmundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, Ó.S.; Palsson, B.Ø.; Brynjólfsson, S. Enhancement of Carotenoid Biosynthesis in the Green Microalga Dunaliella Salina with Light-Emitting Diodes and Adaptive Laboratory Evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403.
- Johnson, M.K.; Johnson, E.J.; MacElroy, R.D.; Speer, H.L.; Bruff, B.S. Effects of Salts on the Halophilic Alga Dunaliella Viridis. J. Bacteriol. 1968, 95, 1461–1468.
- Borowitzka, M.A. Commercial Production of Microalgae: Ponds, Tanks, Tubes and Fermenters. J. Biotechnol. 1999, 70, 313–321.
- Pulz, O. Photobioreactors: Production Systems for Phototrophic Microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293.
- Borowitzka, L.J.; Borowitzka, M.A. Commercial Production of β-Carotene by Dunaliella Salina in Open Ponds. Bull. Mar. Sci. 1990, 47, 244–252.
- Su, W.W.; Li, J.; Xu, N.-S. State and Parameter Estimation of Microalgal Photobioreactor Cultures Based on Local Irradiance Measurement. J. Biotechnol. 2003, 105, 165–178.
- Tredici, M.R.; Zitelli, G. Cultivation of Spirulina (Arthrospira) Platensis in Flat Plate Reactors. Spirulina Platensis (Arthrospira): Physiol. Cell-Biol. Biotechnology. Taylor Fr. Lond. 1997, 117, 130.
- Zhu, Y.-H.; Jiang, J.-G. Continuous Cultivation of Dunaliella Salina in Photobioreactor for the Production of β-Carotene. Eur. Food Res. Technol. 2008, 227, 953–959.
- Hejazi, M.A.; De Lamarliere, C.; Rocha, J.; Vermue, M.; Tramper, J.; Wijffels, R. Selective Extraction of Carotenoids from the Microalga Dunaliella Salina with Retention of Viability. Biotechnol. Bioeng. 2002, 79, 29–36.
- León, R.; Vila, M.; Hernánz, D.; Vílchez, C. Production of Phytoene by Herbicide-Treated Microalgae Dunaliella Bardawil in Two-Phase Systems. Biotechnol. Bioeng. 2005, 92, 695–701.
- Joo, D.-S.; Cho, M.-G.; Lee, J.-S.; Park, J.-H.; Kwak, J.-K.; Han, Y.-H.; Bucholz, R. New Strategy for the Cultivation of Microalgae Using Microencapsulation. J. Microencapsul. 2001, 18, 567–576.
- Geng, D.; Wang, Y.; Wang, P.; Li, W.; Sun, Y. Stable Expression of Hepatitis B Surface Antigen Gene in Dunaliella Salina (Chlorophyta). J. Appl. Phycol. 2003, 15, 451–456.
- Sun, Y.; Yang, Z.; Gao, X.; Li, Q.; Zhang, Q.; Xu, Z. Expression of Foreign Genes in Dunaliella by Electroporation. Mol. Biotechnol. 2005, 30, 185–192.
- Tan, C.; Qin, S.; Zhang, Q.; Jiang, P.; Zhao, F. Establishment of a Micro-Particle Bombardment Transformation System for Dunaliella Salina. J. Microbiol. 2005, 43, 361–365.
- Feng, S.; Xue, L.; Liu, H.; Lu, P. Improvement of Efficiency of Genetic Transformation for Dunaliella Salina by Glass Beads Method. Mol. Biol. Rep. 2009, 36, 1433.
- Chai, X.-J.; Chen, H.-X.; Xu, W.-Q.; Xu, Y.-W. Expression of Soybean Kunitz Trypsin Inhibitor Gene SKTI in Dunaliella Salina. J. Appl. Phycol. 2013, 25, 139–144.
- Castellanos-Huerta, I.; Gómez-Verduzco, G.; Tellez-Isaias, G.; Ayora-Talavera, G.; Bañuelos-Hernández, B.; Petrone-García, V.M.; Velázquez-Juárez, G.; Fernández-Siurob, I. Transformation of Dunaliella Salina by Agrobacterium Tumefaciens for the Expression of the Hemagglutinin of Avian Influenza Virus H5. Microorganisms 2022, 10, 361.
- Surzycki, R.; Greenham, K.; Kitayama, K.; Dibal, F.; Wagner, R.; Rochaix, J.-D.; Ajam, T.; Surzycki, S. Factors Effecting Expression of Vaccines in Microalgae. Biologicals 2009, 37, 133–138.
- Daniell, H.; Lee, S.-B.; Panchal, T.; Wiebe, P.O. Expression of the Native Cholera Toxin B Subunit Gene and Assembly as Functional Oligomers in Transgenic Tobacco Chloroplasts. J. Mol. Biol. 2001, 311, 1001–1009.
- Badr, Y.; Kereim, M.; Yehia, M.; Fouad, O.; Bahieldin, A. Production of Fertile Transgenic Wheat Plants by Laser Micropuncture. Photochem. Photobiol. Sci. 2005, 4, 803–807.
- Minai-Tehrani, A.; Park, Y.-C.; Hwang, S.-K.; Kwon, J.-T.; Chang, S.-H.; Park, S.-J.; Yu, K.-N.; Kim, J.-E.; Shin, J.-Y.; Kim, J.-H.; et al. Aerosol Delivery of Kinase-Deficient Akt1 Attenuates Clara Cell Injury Induced by Naphthalene in the Lungs of Dual Luciferase Mice. J. Vet. Sci. 2011, 12, 309–317.
- Specht, E.A.; Mayfield, S.P. Algae-Based Oral Recombinant Vaccines. Front. Microbiol. 2014, 5, 60.
- Dehghani, J.; Movafeghi, A.; Barzegari, A.; Barar, J. Efficient and Stable Transformation of Dunaliella Pseudosalina by 3 Strains of Agrobacterium Tumefaciens. BioImpacts: BI 2017, 7, 247.
- Anila, N.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Establishment of Agrobacterium Tumefaciens-Mediated Genetic Transformation in Dunaliella Bardawil. Eur. J. Phycol. 2011, 46, 36–44.
- Voth, D.E.; Broederdorf, L.J.; Graham, J.G. Bacterial Type IV Secretion Systems: Versatile Virulence Machines. Future Microbiol. 2012, 7, 241–257.
- Hoekema, A.; Hirsch, P.R.; Hooykaas, P.J.; Schilperoort, R.A. A Binary Plant Vector Strategy Based on Separation of Vir-and T-Region of the Agrobacterium Tumefaciens Ti-Plasmid. Nature 1983, 303, 179–180.
- Komari, T.; Takakura, Y.; Ueki, J.; Kato, N.; Ishida, Y.; Hiei, Y. Binary Vectors and Super-Binary Vectors. In Agrobacterium Protocols; Humana Press: Totowa, NJ, USA, 2006; pp. 15–42.
- Valentine, L. Agrobacterium Tumefaciens and the Plant: The David and Goliath of Modern Genetics. Plant Physiol. 2003, 133, 948–955.
- Jia, Y.; Li, S.; Allen, G.; Feng, S.; Xue, L. A Novel Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Promoter for Expressing Transgenes in the Halotolerant Alga Dunaliella Salina. Curr. Microbiol. 2012, 64, 506–513.
- Peach, C.; Velten, J. Transgene Expression Variability (Position Effect) of CAT and GUS Reporter Genes Driven by Linked Divergent T-DNA Promoters. Plant Mol. Biol. 1991, 17, 49–60.
- Jiang, G.-Z.; Lü, Y.-M.; Niu, X.-L.; Xue, L.-X. The Actin Gene Promoter-Driven Bar as a Dominant Selectable Marker for Nuclear Transformation of Dunaliella Salina. Yi Chuan Xue Bao= Acta Genet. Sin. 2005, 32, 424–433.
- Li, J.; Lu, Y.; Xue, L.; Xie, H. A Structurally Novel Salt-Regulated Promoter of Duplicated Carbonic Anhydrase Gene 1 from Dunaliella Salina. Mol. Biol. Rep. 2010, 37, 1143–1154.
- Li, J.; Xue, L.; Yan, H.; Wang, L.; Liu, L.; Lu, Y.; Xie, H. The Nitrate Reductase Gene-Switch: A System for Regulated Expression in Transformed Cells of Dunaliella Salina. Gene 2007, 403, 132–142.
- Feng, S.; Feng, W.; Zhao, L.; Gu, H.; Li, Q.; Shi, K.; Guo, S.; Zhang, N. Preparation of Transgenic Dunaliella Salina for Immunization against White Spot Syndrome Virus in Crayfish. Arch. Virol. 2014, 159, 519–525.
- Doron, L.; Segal, N.; Shapira, M. Transgene Expression in Microalgae—from Tools to Applications. Front. Plant Sci. 2016, 7, 505.
- Bock, R. Plastid Biotechnology: Prospects for Herbicide and Insect Resistance, Metabolic Engineering and Molecular Farming. Curr. Opin. Biotechnol. 2007, 18, 100–106.
- Wang, H.-H.; Yin, W.-B.; Hu, Z.-M. Advances in Chloroplast Engineering. J. Genet. Genom. 2009, 36, 387–398.
- Chen, H.-C.; Melis, A. Marker-Free Genetic Engineering of the Chloroplast in the Green Microalga C Hlamydomonas Reinhardtii. Plant Biotechnol. J. 2013, 11, 818–828.
- Cui, C.; Song, F.; Tan, Y.; Zhou, X.; Zhao, W.; Ma, F.; Liu, Y.; Hussain, J.; Wang, Y.; Yang, G.; et al. Stable Chloroplast Transformation of Immature Scutella and Inflorescences in Wheat (Triticum Aestivum L.). Acta Biochim Biophys Sin 2011, 43, 284–291.
- Li, D.; Han, X.; Zuo, J.; Xie, L.; He, R.; Gao, J.; Chang, L.; Yuan, L.; Cao, M. Construction of Rice Site-Specific Chloroplast Transformation Vector and Transient Expression of EGFP Gene in Dunaliella Salina. J. Biomed. Nanotechnol. 2011, 7, 801–806.
- Rasala, B.A.; Muto, M.; Lee, P.A.; Jager, M.; Cardoso, R.M.; Behnke, C.A.; Kirk, P.; Hokanson, C.A.; Crea, R.; Mendez, M.; et al. Production of Therapeutic Proteins in Algae, Analysis of Expression of Seven Human Proteins in the Chloroplast of Chlamydomonas Reinhardtii. Plant Biotechnol. J. 2010, 8, 719–733.
- Dawson, A. Vaccination and the Prevention Problem. Bioethics 2004, 18, 515–530.
- Pulendran, B.; Ahmed, R. Immunological Mechanisms of Vaccination. Nat. Immunol. 2011, 12, 509–517.
- van den Berg, T.; Lambrecht, B.; Marché, S.; Steensels, M.; Van Borm, S.; Bublot, M. Influenza Vaccines and Vaccination Strategies in Birds. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 121–165.
- McGhee, J.R.; Fujihashi, K. Inside the Mucosal Immune System. PLoS Biol. 2012, 10, e1001397.
- Brandtzaeg, P. Overview of the Mucosal Immune System. In New Strategies for Oral Immunization; Springer: Berlin/Heidelberg, Germany, 1989; pp. 13–25.
- Ike, A.C.; Ononugbo, C.M.; Obi, O.J.; Onu, C.J.; Olovo, C.V.; Muo, S.O.; Chukwu, O.S.; Reward, E.E.; Omeke, O.P. Towards Improved Use of Vaccination in the Control of Infectious Bronchitis and Newcastle Disease in Poultry: Understanding the Immunological Mechanisms. Vaccines 2021, 9, 20.
- Wang, T.; Wei, F.; Liu, J. Emerging Role of Mucosal Vaccine in Preventing Infection with Avian Influenza a Viruses. Viruses 2020, 12, 862.
- Siripornadulsil, S.; Dabrowski, K.; Sayre, R. Microalgal Vaccines. In Transgenic Microalgae as Green Cell Factories; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 122–128.
- Kwon, K.-C.; Lamb, A.; Fox, D.; Jegathese, S.J.P. An Evaluation of Microalgae as a Recombinant Protein Oral Delivery Platform for Fish Using Green Fluorescent Protein (GFP). Fish Shellfish Immunol. 2019, 87, 414–420.
- Kiataramgul, A.; Maneenin, S.; Purton, S.; Areechon, N.; Hirono, I.; Brocklehurst, T.W.; Unajak, S. An Oral Delivery System for Controlling White Spot Syndrome Virus Infection in Shrimp Using Transgenic Microalgae. Aquaculture 2020, 521, 735022.
- Streatfield, S.J. Mucosal Immunization Using Recombinant Plant-Based Oral Vaccines. Methods 2006, 38, 150–157.
- Chang, T.; Ohta, S.; Ikegami, N.; Miyata, H.; Kashimoto, T.; Kondo, M. Antibiotic Substances Produced by a Marine Green Alga, Dunaliella Primolecta. Bioresour. Technol. 1993, 44, 149–153.
- Yang, D.-J.; Lin, J.-T.; Chen, Y.-C.; Liu, S.-C.; Lu, F.-J.; Chang, T.-J.; Wang, M.; Lin, H.-W.; Chang, Y.-Y. Suppressive Effect of Carotenoid Extract of Dunaliella Salina Alga on Production of LPS-Stimulated pro-Inflammatory Mediators in RAW264. 7 Cells via NF-ΚB and JNK Inactivation. J. Funct. Foods 2013, 5, 607–615.
- Lin, H.-W.; Chen, Y.-C.; Liu, C.-W.; Yang, D.-J.; Chen, S.-Y.; Chang, T.-J.; Chang, Y.-Y. Regulation of Virus-Induced Inflammatory Response by Dunaliella Salina Alga Extract in Macrophages. Food Chem. Toxicol. 2014, 71, 159–165.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
23 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




