Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marta Sánchez | -- | 1325 | 2022-09-16 12:02:13 | | | |
| 2 | Catherine Yang | -4 word(s) | 1321 | 2022-09-19 03:19:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sánchez, M.; Ruiz, I.; Soto, M. Removal of Emerging Contaminants from Municipal Wastewater. Encyclopedia. Available online: https://encyclopedia.pub/entry/27250 (accessed on 07 February 2026).
Sánchez M, Ruiz I, Soto M. Removal of Emerging Contaminants from Municipal Wastewater. Encyclopedia. Available at: https://encyclopedia.pub/entry/27250. Accessed February 07, 2026.
Sánchez, Marta, Isabel Ruiz, Manuel Soto. "Removal of Emerging Contaminants from Municipal Wastewater" Encyclopedia, https://encyclopedia.pub/entry/27250 (accessed February 07, 2026).
Sánchez, M., Ruiz, I., & Soto, M. (2022, September 16). Removal of Emerging Contaminants from Municipal Wastewater. In Encyclopedia. https://encyclopedia.pub/entry/27250
Sánchez, Marta, et al. "Removal of Emerging Contaminants from Municipal Wastewater." Encyclopedia. Web. 16 September, 2022.
Copy Citation
The presence of emerging organic contaminants (EOCs) in the environment is increasing and requires the development of technologies for their effective removal. EOCs can be classified into a wide variety of groups depending on their chemical structures and end uses. A popular group of EOCs are pharmaceutically active compounds (PhACs). The growing use of PhACs is leading to persistence and prolonged exposure of these compounds in the environment, which may eventually affect the enzymatic and metabolic mechanisms of living organisms.
emerging organic contaminants
constructed wetlands
anaerobic digesters
1. EOC Removal Technologies for Municipal Wastewater
Depending on the processes that may be involved, EOC removal technologies have been classified into three categories: physical, biological, and/or chemical processes (Figure 1). Over the last three decades, the main trends in PhACs removal were reported by Taoufik [1] through a systematic mapping study. Taoufik [1] concluded that adsorption was the most frequent process of PhACs’ removal from the water phase, followed by photodegradation and biodegradation. Meanwhile, these authors also found that systems based on chemical processes were the most studied [1]. Some of the most applied physical processes use sorbents, such as activated carbon, or membrane technologies, such as nanofiltration, ultrafiltration, or reverse osmosis [2][3][4]. Chemical processes include AOPs such as photolysis, ozonation, Fenton or photocatalysis, among others, and chemical precipitation. However, the use of these physical and chemical technologies involves significant electricity consumption, investment, and operational costs.
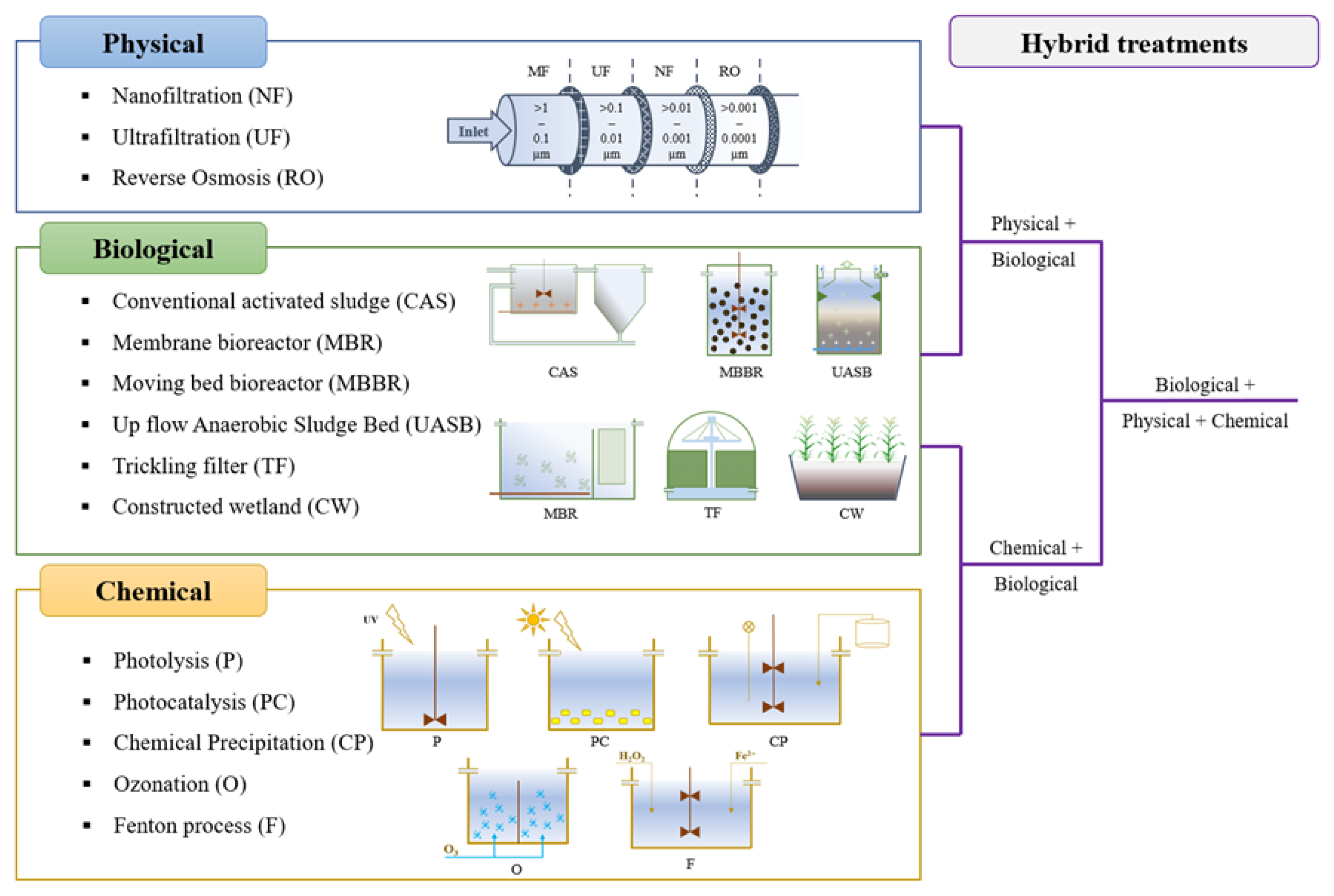
Figure 1. Main treatment methods for EOC removal from municipal wastewater.
Biological systems, on the other hand, were gaining popularity as several studies demonstrated their effectiveness in the degradation of many PhACs [5]. In addition, the use of biological technologies implies lower operating costs. Biological treatment of MW is due to the coexistence of different microenvironments that allow physical, chemical, and biological processes to take place. In terms of EOC removal, several studies support the effectiveness of biodegradation through systems such as: conventional activated sludge (CAS) in WWTPs, membrane bioreactors (MBR), sequencing batch reactors, trickling filters, or CW [6] (Figure 1). However, the complete removal of EOCs presented in MW by the above-mentioned technologies operating individually remains a challenge. Therefore, the combination of different technologies (e.g., hybrid systems based on biological+chemical, biological+physical, or biological+chemical+physical processes, Figure 1) emerged as the most effective approach to try to overcome the shortcomings of each specific treatment and attempt a global removal [2].
The ranges of EOC removal efficiencies in WWTPs based on the reviewed literature. EOC removal efficiencies in conventional WWTPs typically range from 20–50% during primary treatment, 30–70% in systems that include primary and secondary treatment, and over 90% in systems that reach tertiary treatment [7]. EOCs that exhibit hydrophilicity (i.e., log Dow < 1) were generally not well removed during primary treatment. Nevertheless, many hydrophobic EOCs (i.e., log Dow > 3) showed a tendency to adsorb strongly onto primary sludge and were therefore partially removed from the dissolved phase after primary treatment [8][9][10]. In fact, Martín [8] observed that only 3 of the 16 studied EOCs were not detected in the sludge, while 11 of them were identified even in the final compost from sewage sludge.
Through biological processes (i.e., anaerobic and/or aerobic systems), certain EOCs can be degraded to a greater or lesser extent, leading to complete or partial mineralization and potentially generating by-products. In addition to sorption and biodegradation, volatilization may also contribute to the removal of volatile EOCs from the water phase.
2. EOC Removal by Sorption
The main mechanisms involved in the biological degradation of EOCs are sorption on sludge particles and aerobic or anaerobic biodegradation [11].
Sorption on sludge particles can occur through two mechanisms: absorption and adsorption. When the hydrophobic EOCs pass from the aqueous phase into the lipophilic cell membrane of the biomass, this is referred to as absorption. If the positively charged EOCs are retained by electrostatic interactions on the negatively charged surface of the sludge particle, adsorption takes place [12][13]. In general, sorption on EOCs is related to their physicochemical properties (log Kow, log Dow, pKa, and kd). Tiwari [11] previously estimated the sorption of pharmaceutical compounds in sludge from their log Kow values and concluded that log Kow and pKa determine the affinity of EOCs to undergo sorption, so that EOCs with log Kow > 3.5 (i.e., lipophilic compounds) are prone to sorption onto sediments. For example, BPA with log Kow = 3.64 (Table 1) would have a high affinity for sorption.
The most relevant factors in terms of EOC sorption in sewage sludge are biomass composition and concentration, hydrodynamic parameters, and the use of sorbents [12]. Compared to granular biomass versus flocculent biomass, Alvarino [14] found that EOCs were absorbed more efficiently in flocculent biomass than in granular biomass due to a higher specific surface area available and lower mass transfer resistance. However, sorption was more important in UASB digesters than in CAS because of a higher concentration of accumulated biomass (7–30 g VSS/L in UASB vs. 1–2 g VSS/L in CAS).
In addition, EOCs with a low affinity to be retained in sludge may have a high affinity to interact with some adsorbents, such as activated carbon. For example, Alvarino [3] obtained 0% and 30% removal for DCL and CBZ, respectively, under biological treatment. It should be noted that DCL and CBZ (with 1 > log Dow < 3, Table 1) usually behave as recalcitrant to the biodegradation process. However, these authors improved the removals of DCL and CBZ to more than 80% and 90%, respectively, by adding activated carbon in a MBR. In contrast, these authors highlighted the progressive saturation of the active carbon, requiring new doses.
Table 1. Physicochemical properties of EOCs under review according to the references consulted on biological wastewater treatment processes a.
| Name Acronym Chemical Composition |
CAS MW b Formula |
pKa a | log Kow b | log Dow c | kbio (L/gSS·d) d |
kd (L/kgSS) d |
|---|---|---|---|---|---|---|
| Acetaminophen ACE 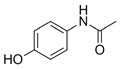 |
103-90-2 151.17 C8H9NO2 |
9.5 | 0.46 | 0.9 | 58–240 | 1.5–1160 |
| Ofloxacin OFL 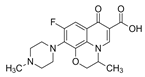 |
82419-36-1 361.37 C18H20FN3O4 |
5.97 9.28 |
−0.39 | - | 0.01–0.0933 | 12,000–22,100 |
| Caffeine CAF 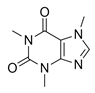 |
58-08-2 194.19 C8H10N4O2 |
10.4 | −0.07 | −0.55 | 0.48–156.24 | <30–140 |
| Carbamazepine CBZ 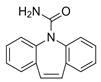 |
298-46-4 236.27 C15H12N2O |
13.9 15.96 |
2.45 | 2.77 | 0.005–0.389 | <8–314 |
| Ketoprofen KET 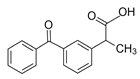 |
22071-15-4 254.28 C16H14O3 |
4.45 | 3.12 | 0.39 | 0.24–3.36 | 0.24–226 |
| Ibuprofen IBU 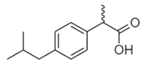 |
15687-27-1 206.28 C13H18O2 |
4.85 | 3.5 3.97 |
- | 3.24–38.7 | 6–103 |
| Diclofenac DCL 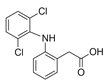 |
15307-86-5 296.15 C14H11Cl2NO2 |
4.2 | 4.98 | 2.26 0.86 |
0.02–8 | 1.9–321 |
| Clofibric Acid ACB 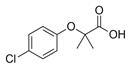 |
882-09-7 214.64 C10H11ClO3 |
−4.9 3.2 |
2.57 | -0.42 | 0.03–1 | 7–87.5 |
| Bisphenol A BPA 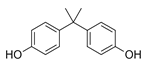 |
80-05-7 228.29 C15H16O2 |
9.6 | 3.32 | 4.05 | 0.24–16.56 | 314–505 |
| Sotalol SOT 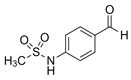 |
3930-20-9 272.37 C12H20N2O3S |
8.3 | 0.85 | - | - | - |
3. Biodegradation
Biodegradation is influenced by the concentration and chemical structure of EOCs. In addition, the type of metabolism would be determined by the concentration of the compound, while the degree and rate of biodegradation would be determined by the activity of the biomass [6][12]. Co-metabolism is the predominant mechanism in the degradation of EOCs contained in wastewater, due to the relatively low concentration of EOCs and the high concentrations of other substrates, such as easily biodegradable organic matter, acetate, ammonium, or nitrate. During co-metabolism, persistent EOCs are transformed into biodegradable intermediates within the overall metabolic pathways. A well-known example of co-metabolism is the nitrification process [19]. Ammonium oxidizing bacteria contain the enzyme ammonium monooxygenase, which is responsible for the degradation of certain EOCs [20]. For instance, an improvement in the degradation of recalcitrant ACB, CBZ, and DCL compounds was obtained by Tran [21] by increasing the ammonium concentration (from 20 mg to 200 mg NH4+-N/L) in a nitrifying activated sludge reactor compared to a CAS. Meanwhile, these authors found a high removal of IBU in the presence of allylthiourea (an ammonium monooxygenase inhibitor) and concluded that heterotrophic bacteria were also able to degrade IBU.
Aerobic metabolism and co-metabolism, on the other hand, appeared to be preferable to anaerobic or anoxic routes [22]. The redox potential and chemical structure of EOCs could also determine the metabolic pathway of degradation. According to Alvarino [12], most of the EOCs were transformed under aerobic conditions (such as IBU), and globally these authors, observed that aerobic systems were more efficient for a broad group of EOCs. However, due to their chemical structure, certain EOCs, such as naproxen or sulfamethoxazole, show higher degradation under anaerobic conditions [12]. These authors also highlighted that the combination of different redox conditions could be a feasible alternative to improve EOC removal efficiencies.
References
- Taoufik, N.; Boumya, W.; Janani, F.Z.; Elhalil, A.; Mahjoubi, F.Z.; Barka, N. Removal of Emerging Pharmaceutical Pollutants: A Systematic Mapping Study Review. J. Environ. Chem. Eng. 2020, 8, 104251.
- Dhangar, K.; Kumar, M. Tricks and Tracks in Removal of Emerging Contaminants from the Wastewater through Hybrid Treatment Systems: A Review. Sci. Total Environ. 2020, 738, 140320.
- Alvarino, T.; Torregrosa, N.; Omil, F.; Lema, J.M.; Suarez, S. Assessing the Feasibility of Two Hybrid MBR Systems Using PAC for Removing Macro and Micropollutants. J. Environ. Manage. 2017, 203, 831–837.
- Schäfer, A.I.; Akanyeti, I.; Semião, A.J.C. Micropollutant Sorption to Membrane Polymers: A Review of Mechanisms for Estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117.
- Park, J.; Yamashita, N.; Park, C.; Shimono, T.; Takeuchi, D.M.; Tanaka, H. Removal Characteristics of Pharmaceuticals and Personal Care Products: Comparison between Membrane Bioreactor and Various Biological Treatment Processes. Chemosphere 2017, 179, 347–358.
- Saidulu, D.; Gupta, B.; Gupta, A.K.; Ghosal, P.S. A Review on Occurrences, Eco-Toxic Effects, and Remediation of Emerging Contaminants from Wastewater: Special Emphasis on Biological Treatment Based Hybrid Systems. J. Environ. Chem. Eng. 2021, 9, 105282.
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990.
- Martín, J.; Camacho-Muñoz, M.D.; Santos, J.L.; Aparicio, I.; Alonso, E. Distribution and Temporal Evolution of Pharmaceutically Active Compounds alongside Sewage Sludge Treatment. Risk Assessment of Sludge Application onto Soils. J. Environ. Manage. 2012, 102, 18–25.
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and Distribution of Pharmaceuticals in Wastewater and Sewage Sludge of the Conventional Activated Sludge (CAS) and Advanced Membrane Bioreactor (MBR) Treatment. Water Res. 2009, 43, 831–841.
- Reyes-Contreras, C.; Neumann, P.; Barriga, F.; Venegas, M.; Domínguez, C.; Bayona, J.M.; Vidal, G. Organic Micropollutants in Sewage Sludge: Influence of Thermal and Ultrasound Hydrolysis Processes Prior to Anaerobic Stabilization. Environ. Technol. 2020, 41, 1358–1365.
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater Using Biological Approach. Bioresour. Technol. 2017, 224, 1–12.
- Alvarino, T.; Suarez, S.; Lema, J.; Omil, F. Understanding the Sorption and Biotransformation of Organic Micropollutants in Innovative Biological Wastewater Treatment Technologies. Sci. Total Environ. 2018, 615, 297–306.
- Sophia, A.C.; Lima, E.C. Removal of Emerging Contaminants from the Environment by Adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17.
- Alvarino, T.; Suarez, S.; Lema, J.M.; Omil, F. Understanding the Removal Mechanisms of PPCPs and the Influence of Main Technological Parameters in Anaerobic UASB and Aerobic CAS Reactors. J. Hazard. Mater. 2014, 278, 506–513.
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically Active Compounds in Aqueous Environment: A Status, Toxicity and Insights of Remediation. Environ. Res. 2019, 176, 108542.
- Tran, N.H.; Gin, K.Y.H. Occurrence and Removal of Pharmaceuticals, Hormones, Personal Care Products, and Endocrine Disrupters in a Full-Scale Water Reclamation Plant. Sci. Total Environ. 2017, 599–600, 1503–1516.
- Faria, C.V.; Moreira, G.C.; Araújo, A.P.B.; Marques, L.E.; Oliveira, L.P.; Ricci, B.C.; Amaral, M.C.S.; Fonseca, F.V. Integration of Ozonation and an Anaerobic Expanded Granular Sludge Bed Reactor for Micropollutant Removal from Sewage. Environ. Sci. Pollut. Res. 2021, 28, 23778–23790.
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Plants from Different Geographical Regions-a Review. Water Res. 2018, 133, 182–207.
- Men, Y.; Achermann, S.; Helbling, D.E.; Johnson, D.R.; Fenner, K. Relative Contribution of Ammonia Oxidizing Bacteria and Other Members of Nitrifying Activated Sludge Communities to Micropollutant Biotransformation. Water Res. 2017, 109, 217–226.
- Yi, T.; Harper, W.F.; Holbrook, R.D.; Love, N.G. Role of Particle Size and Ammonium Oxidation in Removal of 17α-Ethinyl Estradiol in Bioreactors. J. Environ. Eng. 2006, 132, 1527–1529.
- Tran, N.H.; Urase, T.; Kusakabe, O. The Characteristics of Enriched Nitrifier Culture in the Degradation of Selected Pharmaceutically Active Compounds. J. Hazard. Mater. 2009, 171, 1051–1057.
- Pomiès, M.; Choubert, J.M.; Wisniewski, C.; Miège, C.; Budzinski, H.; Coquery, M. Lab-Scale Experimental Strategy for Determining Micropollutant Partition Coefficient and Biodegradation Constants in Activated Sludge. Environ. Sci. Pollut. Res. 2015, 22, 4383–4395.
More
Information
Subjects:
Water Resources
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
19 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




