Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aija Maca-Kaleja | -- | 2329 | 2022-09-15 20:05:02 | | | |
| 2 | Catherine Yang | Meta information modification | 2329 | 2022-09-16 02:50:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vidusa, L.; Kalejs, O.; Maca-Kaleja, A.; Strumfa, I. Viral Myocarditis. Encyclopedia. Available online: https://encyclopedia.pub/entry/27226 (accessed on 07 February 2026).
Vidusa L, Kalejs O, Maca-Kaleja A, Strumfa I. Viral Myocarditis. Encyclopedia. Available at: https://encyclopedia.pub/entry/27226. Accessed February 07, 2026.
Vidusa, Liga, Oskars Kalejs, Aija Maca-Kaleja, Ilze Strumfa. "Viral Myocarditis" Encyclopedia, https://encyclopedia.pub/entry/27226 (accessed February 07, 2026).
Vidusa, L., Kalejs, O., Maca-Kaleja, A., & Strumfa, I. (2022, September 15). Viral Myocarditis. In Encyclopedia. https://encyclopedia.pub/entry/27226
Vidusa, Liga, et al. "Viral Myocarditis." Encyclopedia. Web. 15 September, 2022.
Copy Citation
Viral infections are the most frequently reported etiological factors of acute myocarditis, presumed even in cases where the viral pathogen cannot be detected, or when it could act as a trigger for a secondary reaction. No consensus exists regarding the necessary investigations to confirm infectious causes. Coronary artery disease and congenital or acquired structural deformities are routinely investigated, but dysfunction induced by toxic substances, collagen vascular diseases, postpartum cardiomyopathy and myocardial infarction with non-obstructed coronary arteries are just a few of the examples that occasionally can cause misdiagnosis, especially in patients having a coincidental history of a recent viral illness. The COVID-19 pandemic brought attention to viral myocarditis and problems with its diagnostics.
myocarditis
inflammatory cardiomyopathy
endomyocardial biopsy
1. Viral Myocarditis of Unspecified Etiology
There are some general patterns in the pathogenesis of viral myocarditis that have been identified in biopsy and autopsy studies. The early damage classically is caused by direct viral activity and replication inducing myocyte necrosis, release of intracellular contents and a maladaptive immune response, which includes enhanced synthesis of interleukin-6 and other cytokines, autoantibody production or direct inflammatory myocardial infiltration. In other cases, molecular mimicry between cardiomyocyte surface receptors and pathogens has been described. Natural killer cells and macrophages are known as first responders, followed by T-lymphocyte infiltration, causing inflammation that lasts weeks or even months and may result in either full remission or residual structural changes. Cardioprotection can be achieved with knowledge of these processes and reduction of their effects on the inflammatory stage, which can be detected early on, using a non-invasive approach, e.g., EchoCG and CMR. Still, in order to gain knowledge of cellular reactions, tissue sample acquiree and analysis is needed [1][2].
Not all viruses follow the same pattern of infection. Enteroviruses, adenoviruses and Zika virus are able to infect cardiomyocytes directly, parvovirus B19 can cause latent endothelial infection but Dengue virus presents with acute endothelial dysfunction. Influenza and coronaviruses can induce an immediate immune-mediated response, but human herpesvirus type 6, cytomegalovirus and Epstein–Barr virus are found in the majority of the population in latent forms and can reactivate in immunocompromised patients [3][4][5][6]. Therefore, the need for use of unified terminology has been actualized, distinguishing between virus-mediated and virus-triggered myocarditis and EMB with an adjunct PCR can help to distinguish between them [7].
Viral serology and positive peripheral blood PCR findings have not shown to be useful in precisely detecting the causative pathogen. However, the presence or absence of the pathogen in EMB and peripheral blood can help to evaluate the activity of a systemic infection or possibility of an endogenous reactivation [7][8][9]. Frequently, multiple infectious agents of unknown significance are found by PCR of EMB samples. It is believed that findings of a low active pathogen concentration in biopsy materials could be caused by delayed EMB acquiree within the chronic phase, inaccurate choice of ventricle, low sample quantity and sampling errors. In cases of low pathogen presence, a high value of perforin-positive infiltrates (>2.95 cells/mm2) has been proposed as a predictor of expectant deterioration of left ventricular ejection fraction [10][11][12].
To differentiate viral myocarditis from immune-mediated myocarditis in absence of the detected viral pathogen within EMB, several cardiac autoantibodies (e.g., anti-myosin, anti-fibrillary, anti-intercalated disk antibodies) have been described as possible serum markers for an immune-mediated process [3][10][12][13][14]. Circulating microRNAs have also been proposed as differential diagnostic markers (e.g., hsa-miR-Chr8:96), still needing further research for confirmation [15][16].
Understanding of pathophysiological mechanisms, medical history data, infection markers and viral load measurements could alleviate or even exclude the need for invasive detection of pathogen presence within the myocardium for treatment choice. Reviews of immunosuppressive therapy in myocarditis noted that previous trials showed a neutral effect upon outcomes in patients with myocarditis of unspecified etiology and a positive effect upon patients with biopsy-proven myocarditis with virus-negative PCR findings, coinciding with recommendations in the expert consensus from the European Society of Cardiology that empirical treatment can be used when deemed necessary [8][17][18].
As majority of pediatric cases are caused by viral infections, EMB has been used as the basis for treatment corrections—the addition of antiviral therapy or restrictions of immunosuppression. Population studies showed that children with virus-positive PCR findings and limited immunosuppression had fewer adverse events than those that were treated with immunosuppressive therapy [19][20]. Conflicting results on immunosuppression benefit have been reported in studies of clinically suspected myocarditis in children, with evidence of overall treatment benefit without the knowledge of viral particle presence [21][22]. In contrast, PCR findings did not change prognosis in the adult population and virus-negative patients had better results from conventional heart failure treatment alone [23].
A high overall rate (60–70%) of complete recovery after viral myocarditis has been reported, with healed myocardium in control biopsies and a significant reduction of residual fibrosis or calcification from use of conventional heart failure therapy. However, severe and persistent infections, especially by enteroviruses, represent an exception, causing a 30% mortality rate in neonates and 25% 2-year mortality in adults. In such cases, EMB would be initially indicated because of the severe clinical course and can bring clinical benefit due to the addition of antiviral agents against enteroviruses [11][24][25].
Previous studies have confirmed the lack of correlation between the degree of inflammation and risk of arrhythmias or sudden cardiac arrest. The risk of rhythm disturbances is elevated in physically active young adults, due to exercise-caused aggravation of subclinical inflammation. This correlation emphasizes the need for activity restrictions and screening of athletes or otherwise highly active patients after viral infections that are known to have high cardiac tropism. ECG, EchoCG, Holter monitoring and CMR should be used for screening. Suggestive EchoCG findings include increased wall thickness, global left ventricular dysfunction, localized wall motion abnormalities and pericardial effusion. CMR can detect capillary hyperemia, myocardial edema and signs of myocyte injury. Exercise intolerance after cardiotropic infections, with tests performed under medical surveillance, could be used to select patients for further non-invasive investigations and EMB for ambiguous cases [26][27][28].
2. COVID-19-Associated Myocarditis
The epidemiological importance of COVID-19 cannot be overestimated. Nevertheless, SARS-CoV-2 infection is notable also for the controversial issues that have been highlighted regarding the diagnostic criteria of myocarditis.
SARS-CoV-2 infection can cause serious and irreversible damage to multiple organs due to diffuse expression of angiotensin converting enzyme 2 (ACE2) receptor to which the virus binds, inducing direct cellular damage, dysregulation of the renin-angiotensin-aldosterone system and microangiopathy (Figure 1). The resulting tissue damage is caused by a systemic inflammatory response and demand–supply mismatch [29][30].
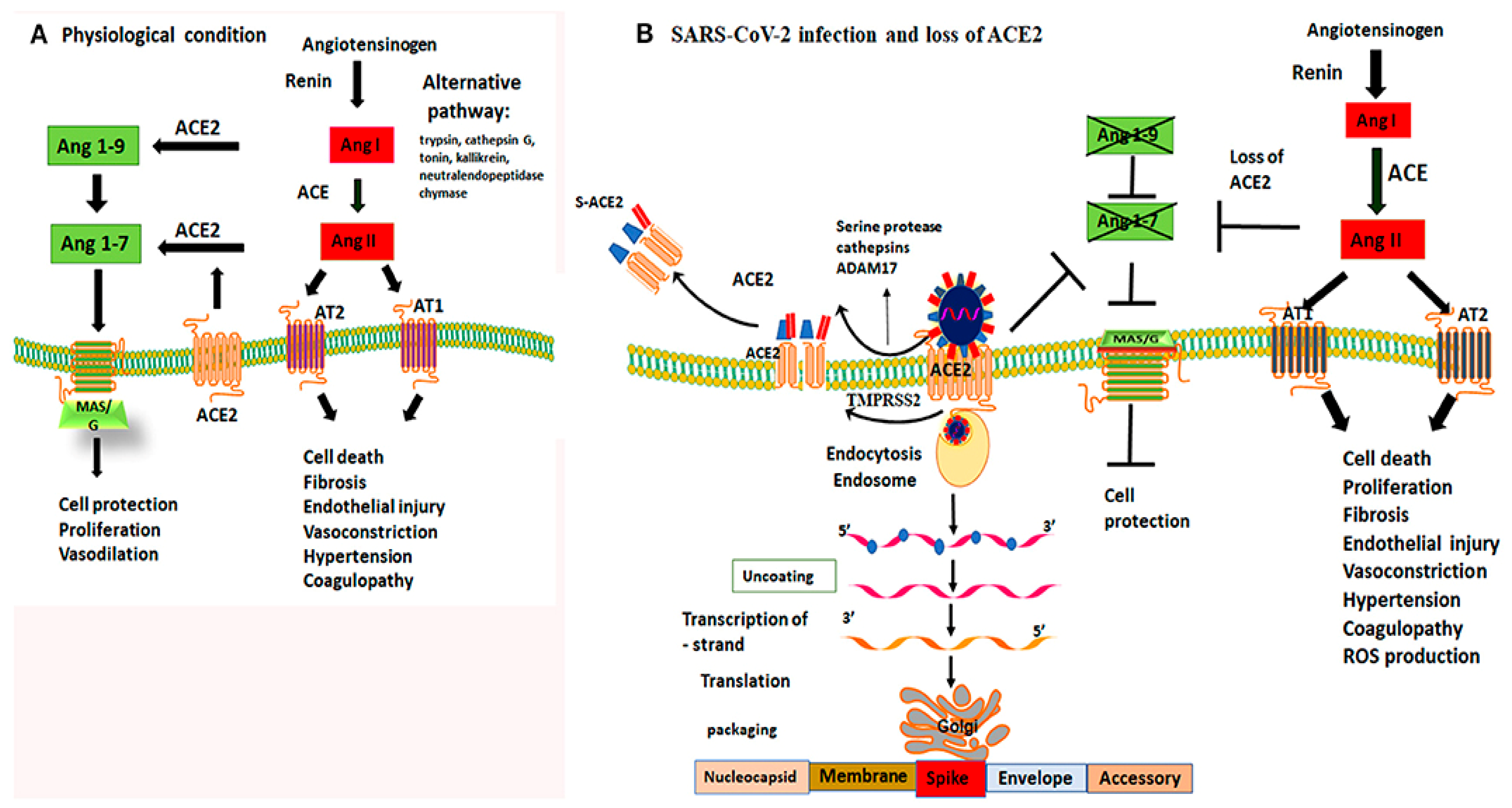
Figure 1. Mechanism of ACE2 receptor within physiological processes and COVID-19 infection. Figure replicated from [31] under Creative Commons license, provided at https://creativecommons.org/licenses/by-nc/4.0/ (accessed on 1 February 2022). Changes made: Figure legend. (A) Physiological conditions permit a balanced regulation of renin–angiotensin–aldosterone-mediated effects by the activity of both angiotensin-converting enzymes; (B) By binding to ACE2 receptors, the virus not only gains a point of entry, but is able to disrupt their protective capabilities, causing unbalanced activity of angiotensin I.
Clinically, symptoms of cardiac injury were seen in about a third of SARS-CoV-2 infected patients, correlating with higher mortality rates [29][32][33]. According to post-mortem assessment, the infection can induce myocyte ferroptosis and inflammatory infiltrates, edema in stroma and vascular walls, atrophy of cardiac muscle fibers, cardiac dilatation and focal necrosis or fibrosis, thus causing acute heart failure, reported as the second most frequent cause of mortality in SARS-CoV-2 infected patients. Although acute heart failure in some COVID-19 patients is attributable to direct cardiac injury, more frequently it occurred secondary to pulmonary overload, diffuse alveolar damage being the dominant pathological finding [34][35][36]. Initial screening for direct cardiac injury includes myocardial biomarkers, ECG and EchoCG. Interpretation of findings is difficult due to frequent cardiovascular comorbidities and the severe general condition of patients exhibiting cardiac symptoms. In autopsy samples, cardiac involvement was considered direct on the basis of specific histological findings, including infiltration of CD68+ macrophages and CD3+, CD4+ or CD8+ lymphocytes. The inflammatory infiltrate likely developed as a response to high levels of circulatory cytokines, such as interleukin-6 and tumor necrosis factor α [29][32][34].
Comparably lower susceptibility to COVID-19-associated myocarditis has been seen in children. The age-related pathogenetic differences confirm the importance of ACE2 receptor density, which is higher in adults and in patients with cardiovascular comorbidities. The immune response also differs by age—cytokine production intensity is lower and innate response adaptability is higher in children [35].
In a significant fraction (up to 48%) of SARS-CoV-2 infection-associated myocarditis, the cardiac diagnosis was confirmed only by EchoCG. Adjunct CMR was performed in only up to a half of those cases [37][38][39][40]. In patients who underwent CMR, non-ischemic LGE was found in less than a half (43%) of them, suggesting a reasonable likelihood of diagnostic errors [41]. An isolated rise in cardiac troponin levels could be assessed as a criterion for patient selection for CMR and EMB if elevated troponin levels are found in absence of other parameters, suggesting a severe course of the infection. This approach would be practical because cardiac troponins are routinely tested in COVID-19 patients as one of the predictors of outcome [42][43][44][45].
Overall, in reports of COVID-19-associated myocarditis, confirmation by EMB is neither frequent nor yields classical morphology. For example, in a single review, EMB was performed only in 20% of reported cases. The use of the procedure was likely limited by its invasive nature, general condition of the patient, unclear impact on further therapeutic decisions, concerns about infection control and shortage of medical resources during the pandemic. Regarding EMB findings, Dallas criteria were met only in a few of those cases. There was a high number of borderline findings with T-lymphocyte and macrophage infiltration and limited necrosis, highlighting the hypothesis of a virus-triggered hyperinflammatory response. Autopsy findings revealed diffuse inflammatory cell infiltration and varying signs of necrosis or ferroptosis; causing difficulties in distinguishing between direct involvement versus cardiac injury as a component of multiorgan failure in a severe course of the infection [42][46][47]. Cardiac inflammation was confirmed in 17.6% of overall samples (33.4% of biopsy specimens and 66.6% of autopsy materials) and only 11.4% of cases, showing inflammation, met the criteria for myocarditis. Dominant findings included thromboembolic events and endothelial inflammation with microvascular thrombosis. Thus, hypercoagulation and a hyperinflammatory response have a higher prevalence [48][49][50][51][52][53]. Other reviews mentioned signs of cardiac injury caused by exacerbations of preexisting conditions resulting in myocardial ischemia [49][51]. The role of hypoperfusion-related cardiac injury was supported by a review of coronary artery calcium score impact on mortality rates in COVID-19 patients, finding a two-fold increase in patients with a higher score [54]. Pediatric reviews showed a high incidence of clinically suspected myocarditis in children presenting with multisystem inflammatory syndrome [55][56][57][58]. There were only a few reported pediatric cases supported by CMR findings or confirmation by biopsy or autopsy [35][59][60]. The COVID-19 pandemic has also highlighted the need for EMB and autopsy sample collection to determine the correct underlying process and possible treatment directions, when facing novel pathogens [61][62].
Viral presence of SARS-Cov2 in EMB or myocardium autopsy samples tested with PCR was found in about a third (33%) of affected patients. Difficulties to determine the significance of these findings and distinguish between subcellular structures and viral particles were noted. It has been presumed that phagocyte migration from lung tissue could be a possible reason for a non-specific presence of viral particles [48][49]. In several cases, patients tested negative with nasopharyngeal swabs, but viral particles were found in postmortem cardiac tissue samples, suggesting that cardiac involvement could persist long after respiratory symptoms have resolved [42]. Even when high viral replication was reported (47% of autopsy samples), lymphocytic infiltration was scarce (approximately 10%), and myocarditis meeting Dallas criteria was confirmed only in 1.5% of cases. Detected viral presence was contributing to a combination of non-specific myocardial edema and endothelial inflammation, with dominant pathological changes in autopsy materials being cardiac dilatation, ischemia, intracardiac thrombi and pericardial effusion. These findings confirmed that cardiac injury mainly developed as a part of respiratory overload or multiorgan damage caused by hypoperfusion and hypercoagulation. Predisposition to this type of damage was observed in patients with preexisting cardiac conditions, such as myocardial hypertrophy or fibrosis [46][49].
CMR follow-up studies have shown that nonspecific myocardial edema as a muted response to direct viral activity tends to persist, possibly causing delayed or long-term implications [26]. CMR follow-up after 6 months yielded high frequency (46.5%) of pathological findings, with predominantly (87.9%) non-ischemic LGE pattern and T2 abnormalities, found in approximately a fifth of cases (20.7%). Persistent CMR abnormalities have been reported even in patients having normal cardiac biomarker levels on follow-up and lacking medical history of cardiac disease. LGE with T2-based criteria indicates myocarditis in the inflammatory phase. Non-ischemic LGE without T2 abnormalities indicates residual myocardial scarring and is associated with development of heart failure and arrhythmias after recovery. Thus, myocardial inflammation can persist long after clinical recovery from the infection. Prolonged follow-up studies and more frequent EMB acquirees are desired to determine the specifics of this persistent inflammation and its possible consequences. The extent of LGE can diminish significantly, as previous studies have shown at 12 months of follow-up [41][63][64][65][66][67].
COVID-19 vaccine safety reviews in children and adolescents disclosed myopericarditis at an estimated incidence of 0.01% in the age group between 12–15 years and 0.008% in 16–17 years old. Most of these cases exhibited a mild course [68]. In accordance with the latest population-based studies, young males were affected more frequently, usually a few days following their second dose, and the presentation was mainly self-limiting. It should be noted that CMR and EMB were rarely carried out, determining whether this reaction to vaccines meets any myocarditis criteria [15][69][70][71][72]. Autopsies have been performed in rarely occurring (38) fatal cases in whom the causality relationship was described as possible. The histological picture in these cases differed from viral or immune-mediated myocarditis: neutrophils and histiocytes predominated in the infiltrates [73].
Overall, literature on viral myocarditis suggests that knowledge of pathophysiological patterns holds high value over clinical choices. Medical history, infection markers and early detection of myocardial inflammation via non-invasive investigations, such as EchoCG and CMR, can guide the treatment plan, but knowledge of the inflammation pattern can increase its effectivity and prevent unnecessary actions. Without a sufficient number of tissue samples confirming similarities or specific nuances of different etiology viral myocarditis, this knowledge cannot be obtained [2][17][18][61][62].
References
- Zheng, Q.; Zhuang, Z.; Wang, Z.H.; Deng, L.H.; Jin, W.J.; Huang, Z.J.; Zheng, G.Q.; Wang, Y. Clinical and preclinical systematic review of Astragalus membranaceus for viral myocarditis. Oxidative Med. Cell. Longev. 2020, 2020, 1560353.
- Shafi, A.M.A.; Shaikh, S.A.; Shirke, M.M.; Iddawela, S.; Harky, A. Cardiac manifestations in COVID-19 patients—A systematic review. J. Card. Surg. 2020, 35, 1988–2008.
- Schultheiss, H.P.; Baumeier, C.; Aleshcheva, G.; Bock, C.T.; Escher, F. Viral myocarditis—From pathophysiology to treatment. J. Clin. Med. 2021, 10, 5240.
- Scatularo, C.E.; Ballesteros, O.A.; Saldarriaga, C.; Mendoza, I.; Wyss, F.; Liprandi, A.S.; Munera, A.; Liendro, M.C.; Baranchuk, A.; Neglected Tropical Diseases and other Infectious Diseases affecting the Heart (NET-Heart project). Zika & heart: A systematic review. Trends Cardiovasc. Med. 2022, 32, 52–58.
- Lippi, G.; Sanchis-Gomar, F. Cardiac troponin elevation in patients with influenza virus infections. Biomed. J. 2021, 44, 183–189.
- Araiza-Garaygordobil, D.; García-Martínez, C.E.; Burgos, L.M.; Saldarriaga, C.; Liblik, K.; Mendoza, I.; Martinez-Selles, M.; Scatularo, C.E.; Farina, J.M.; Baranchuk, A.; et al. Dengue and the heart. Cardiovasc. J. Afr. 2021, 32, 276–283.
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: An expert consensus document. Circ. Heart Fail. 2020, 13, e007405.
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648.
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364.
- Dominguez, F.; Kühl, U.; Pieske, B.; Garcia-Pavia, P.; Tschöpe, C. Update on myocarditis and inflammatory cardiomyopathy: Reemergence of endomyocardial biopsy. Rev. Esp. Cardiol. (Engl. Ed.) 2016, 69, 178–187.
- Robinson, J.; Harting, L.; Vandermeer, B.; Sebastianski, M.; Klassen, T.P. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst. Rev. 2020, 8, CD004370.
- Rigopoulos, A.G.; Klutt, B.; Matiakis, M.; Apostolou, A.; Mavrogeni, S.; Noutsias, M. Systematic review of PCR proof of parvovirus B19 genomes in endomyocardial biopsies of patients presenting with myocarditis or dilated cardiomyopathy. Viruses 2019, 11, 566.
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Cir. Res. 2016, 119, 159–176.
- Tschope, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hubner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193.
- Tymińska, A.; Ozierański, K.; Skwarek, A.; Kapłon-Cieślicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L. Personalized management of myocarditis and inflammatory cardiomyopathy in clinical practice. J. Pers. Med. 2022, 12, 183.
- Fung, G.; Luo, H.; Qiu, Y.; Yang, D.; McManus, B. Myocarditis. Circ. Res. 2016, 118, 496–514.
- Cheng, C.Y.; Cheng, G.Y.; Shan, Z.G.; Baritussio, A.; Lorenzoni, G.; Tyminska, A.; Ozieranski, K.; Iliceto, S.; Marcolongo, R.; Gregori, D.; et al. Efficacy of immunosuppressive therapy in myocarditis: A 30-year systematic review and meta-analysis. Autoimmun. Rev. 2021, 20, 102710.
- Winter, M.P.; Sulzgruber, P.; Koller, L.; Bartko, P.; Goliasch, G.; Niessner, A. Immunomodulatory treatment for lymphocytic myocarditis—A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 573–581.
- Pilati, M.; Rebonato, M.; Formigari, R.; Butera, G. Endomyocardial biopsy in pediatric myocarditis and dilated cardiomyopathy: A tool in search for a role. J. Cardiovasc. Dev. Dis. 2022, 9, 24.
- Yen, C.Y.; Hung, M.C.; Wong, Y.C.; Chang, C.Y.; Lai, C.C.; Wu, K.G. Role of intravenous immunoglobulin therapy in the survival rate of pediatric patients with acute myocarditis: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10459.
- He, B.; Li, X.; Li, D. Immunosuppressive treatment for myocarditis in the pediatric population: A meta-analysis. Front. Pediatr. 2019, 7, 430.
- Li, Y.; Yu, Y.; Chen, S.; Liao, Y.; Du, J. Corticosteroids and intravenous immunoglobulin in pediatric myocarditis: A meta-analysis. Front. Pediatr. 2019, 7, 342.
- Chen, W.H.; Guo, Y.S.; Zhang, D.H.; Zhang, H.J. Long-term prognosis of suspected myocarditis and cardiomyopathy associated with viral infection of the myocardial tissue: A meta-analysis of cohort studies. Cardiovasc. Ther. 2019, 2019, 9342792.
- Silverblatt, J.A.; Ziff, O.J.; Dancy, L.; Daniel, A.; Carter, B.; Scott, P.; Sado, D.M.; Shah, A.; Bromage, D.I. Therapies to limit myocardial injury in animal models of myocarditis: A systematic review and meta-analysis. Basic Res. Cardiol. 2019, 114, 48.
- Zhang, M.; Wang, H.; Tang, J.; He, Y.; Xiong, T.; Li, W.; Qu, Y.; Mu, D. Clinical characteristics of severe neonatal enterovirus infection: A systematic review. BMC Pediatr. 2021, 21, 127.
- Raukar, N.P.; Cooper, L.T. Implications of SARS-CoV-2-associated myocarditis in the medical evaluation of athletes. Sports Health 2021, 13, 145–148.
- Van Hattum, J.C.; Spies, J.L.; Verwijs, S.M.; Verwoert, G.C.; Planken, R.N.; Boekholdt, S.M.; Groenink, M.; Malekzadeh, A.; Pinto, Y.M.; Wilde, A.A.M.; et al. Cardiac abnormalities in athletes after SARS-CoV-2 infection: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e001164.
- Davey, M.S.; Davey, M.G.; Hurley, R.; Hurley, E.T.; Pauzenberger, L. Return to play following COVID-19 infection—A systematic review of current evidence. J. Sport Rehabil. 2022, 31, 218–223.
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial pathology in COVID-19-associated cardiac injury: A systematic review. Diagnostics 2021, 11, 1647.
- Dou, Q.; Wei, X.; Zhou, K.; Yang, S.; Jia, P. Cardiovascular manifestations and mechanisms in patients with COVID-19. Trend Endocrinol. Metab. 2020, 31, 893–904.
- Samavati, L.; Uhal, B.D. ACE2, much more than just a receptor for SARS-CoV-2. Front. Cell. Infect. Microbiol. 2020, 10, 371.
- Diaz-Arocutipa, C.; Torres-Valencia, J.; Saucedo-Chinchay, J.; Cuevas, C. ST-segment elevation in patients with COVID-19: A systematic review. J. Thromb. Thrombolysis 2021, 52, 738–745.
- Li, J.W.; Han, T.W.; Woodward, M.; Anderson, C.S.; Zhou, H.; Chen, Y.D.; Neal, B. The impact of 2019 novel coronavirus on heart injury: A systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2020, 63, 518–524.
- Shaw, P.; Senguttuvan, N.B.; Raymond, G.; Sankar, S.; Mukherjee, A.G.; Kunale, M.; Kodiveri Muthukaliannan, G.; Baxi, S.; Mani, R.R.; Rajagopal, M.; et al. COVID-19 outcomes in patients hospitalised with acute myocardial infarction (AMI): A protocol for systematic review and meta-analysis. COVID 2022, 2, 138–147.
- Rodriguez-Gonzalez, M.; Castellano-Martinez, A.; Cascales-Poyatos, H.M.; Perez-Reviriego, A.A. Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World J. Clin. Cases 2020, 8, 5250–5283.
- Kang, Y.; Chen, T.; Mui, D.; Ferrari, V.; Jagasia, D.; Scherrer-Crosbie, M.; Chen, Y.; Han, Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020, 106, 1132–1141.
- Rathore, S.S.; Rojas, G.A.; Sondhi, M.; Pothuru, S.; Pydi, R.; Kancherla, N.; Singh, R.; Ahmed, N.K.; Shah, J.; Tousif, S.; et al. Myocarditis associated with Covid-19 disease: A systematic review of published case reports and case series. Int. J. Clin. Pract. 2021, 75, e14470.
- Jaiswal, V.; Sarfraz, Z.; Sarfraz, A.; Mukherjee, D.; Batra, N.; Hitawala, G.; Yaqoob, S.; Patel, A.; Agarwala, P.; Ruchika; et al. COVID-19 infection and myocarditis: A state-of-the-art systematic review. J. Prim. Care Community Health 2021, 12, 21501327211056800.
- Haussner, W.; DeRosa, A.P.; Haussner, D.; Tran, J.; Torres-Lavoro, J.; Kamler, J.; Shah, K. COVID-19 associated myocarditis: A systematic review. Am. J. Emerg. Med. 2022, 51, 150–155.
- Sawalha, K.; Abozenah, M.; Kadado, A.J.; Battisha, A.; Al-Akchar, M.; Salerno, C.; Hernandez-Montfort, J.; Islam, A.M. Systematic review of COVID-19 related myocarditis: Insights on management and outcome. Cardiovasc. Revasc. Med. 2021, 23, 107–113.
- Willi, S.; Lüthold, R.; Hunt, A.; Hänggi, N.V.; Sejdiu, D.; Scaff, C.; Bender, N.; Staub, K.; Schlagenhauf, P. COVID-19 sequelae in adults aged less than 50 years: A systematic review. Travel Med. Infect. Dis. 2021, 40, 101995.
- Castiello, T.; Georgiopoulos, G.; Finocchiaro, G.; Claudia, M.; Gianatti, A.; Delialis, D.; Aimo, A.; Prasad, S. COVID-19 and myocarditis: A systematic review and overview of current challenges. Heart Fail. Rev. 2022, 27, 251–261.
- Thakkar, S.; Arora, S.; Kumar, A.; Jaswaney, R.; Faisaluddin, M.; Ammad Ud Din, M.; Shariff, M.; Barssoum, K.; Patel, H.P.; Nirav, A.; et al. A systematic review of the cardiovascular manifestations and outcomes in the setting of coronavirus-19 disease. Clin. Med. Insights Cardiol. 2020, 14, 1179546820977196.
- Mahenthiran, A.K.; Mahenthiran, A.K.; Mahenthiran, J. Cardiovascular system and COVID-19: Manifestations and therapeutics. Rev. Cardiovasc. Med. 2020, 21, 399–409.
- Imazio, M.; Klingel, K.; Kindermann, I.; Brucato, A.; De Rosa, F.G.; Adler, Y.; De Ferrari, G.M. COVID-19 pandemic and troponin: Indirect myocardial injury, myocardial inflammation or myocarditis? Heart 2020, 106, 1127–1131.
- Roshdy, A.; Zaher, S.; Fayed, H.; Coghlan, J.G. COVID-19 and the heart: A systematic review of cardiac autopsies. Front. Cardiovasc. Med. 2021, 7, 626975.
- Guzik, T.J.; Mohiddin, S.A.; Dimarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszevski, M.; Maffia, P.; D’Acquisto, F.D.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687.
- Peiris, S.; Mesa, H.; Aysola, A.; Manivel, J.; Toledo, J.; Borges-Sa, M.; Aldighieri, S.; Reveiz, L. Pathological findings in organs and tissues of patients with COVID-19: A systematic review. PLoS ONE 2021, 16, e0250708.
- Saed Aldien, A.; Ganesan, G.S.; Wahbeh, F.; Al-Nassr, N.; Altarawneh, H.; Al Theyab, L.; Saed Aldien, S.; Tomerak, S.; Naveed, H.; Elshazly, M.B.; et al. Systemic inflammation may induce cardiac injury in COVID-19 patients including children and adolescents without underlying cardiovascular diseases: A systematic review. Cardiovasc. Revasc. Med. 2022, 35, 169–178.
- Vasquez-Bonilla, W.O.; Orozco, R.; Argueta, V.; Sierra, M.; Zambrano, L.I.; Muñoz-Lara, F.; López-Molina, D.S.; Arteaga-Livias, K.; Grimes, Z.; Bryce, C.; et al. A review of the main histopathological findings in coronavirus disease 2019. Hum. Pathol. 2020, 105, 74–83.
- Kamarullah, W.; Nurcahyani; Josephine, C.M.; Multazam, B.R.; Nawing, A.G.; Dharma, S. Corticosteroid therapy in management of myocarditis associated with COVID-19; a systematic review of current evidence. Arch. Acad. Emerg. Med. 2021, 9, e32.
- Lang, J.P.; Wang, X.; Moura, F.A.; Siddiqi, H.A.; Morrow, D.A.; Bohula, E.A. A current review of COVID-19 for the cardiovascular specialist. Am. Heart J. 2020, 226, 29–44.
- Mitrani, R.D.; Dabas, N.; Goldberg, J.J. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm 2020, 17, 1984–1990.
- Lee, K.K.; Rahimi, O.; Lee, C.K.; Shafi, A.; Hawwass, D. A meta-analysis: Coronary artery calcium score and COVID-19 prognosis. Med. Sci. 2022, 10, 5.
- Bustos, R.B.; Jaramillo-Bustamante, J.C.; Vasquez-Hoyos, P.; Cruces, P.; Díaz, F. Pediatric inflammatory multisystem syndrome associated with SARS-CoV-2: A case series quantitative systematic review. Pediatr. Emerg. Care 2021, 37, 44–47.
- Wang, J.G.; Zhong, Z.J.; Li, M.; Fu, J.; Su, Y.H.; Ping, Y.M.; Xu, Z.J.; Li, H.; Chen, Y.H.; Huang, Y.L. Coronavirus disease 2019-related multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Biochem. Res. Int. 2021, 2021, 5596727.
- Dhar, D.; Dey, T.; Samim, M.M.; Padmanabha, H.; Chatterjee, A.; Naznin, P.; Chandra, S.R.; Mallesh, K.; Shah, R.; Siddiqui, S.; et al. Systemic inflammatory syndrome in COVID-19-SISCoV study: Systematic review and meta-analysis. Pediatr. Res. 2022, 91, 1334–1349.
- Sood, M.; Sharma, S.; Sood, I.; Sharma, K.; Kaushik, A. Emerging evidence on multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection: A systematic review with meta-analysis. SN Compr. Clin. Med. 2021, 3, 38–47.
- Henrina, J.; Putra, I.C.S.; Lawrensia, S.; Marta, D.S.; Wijaya, E.; Saboe, A.; Cool, C.J.; Suciadi, L.P. Cardiac manifestations, treatment characteristics, and outcomes of paediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2: A systematic review. Prog. Pediatr. Cardiol. 2021, 63, 101365.
- Sanna, G.; Serrau, G.; Bassero, P.P.; Neroni, P.; Fanos, V.; Marcialis, M.A. Children’s heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur. J. Pediatr. 2020, 179, 1079–1087.
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Satangeli, P.; Khanji, M.Y.; Cooper, L.T.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471.
- Tomasoni, D.; Italia, L.; Adamo, M.; Inciardi, R.M.; Lombardi, C.M.; Solomon, S.D.; Metra, M. COVID-19 and heart failure: From infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur. J. Heart Fail. 2020, 22, 957–966.
- Kim, J.Y.; Han, K.; Suh, Y.J. Prevalence of abnormal cardiovascular magnetic resonance findings in recovered patients from COVID-19: A systematic review and meta-analysis. J. Cardiovasc. Magn. Reason. 2021, 23, 100.
- Ahmad, M.S.; Shaik, R.A.; Ahmad, R.K.; Yusuf, M.; Khan, M.; Almutairi, A.B.; Alghuyaythat, W.K.Z.; Almutairi, S.B. “LONG COVID”: An insight. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5561–5577.
- Phelan, D.; Kim, J.H.; Elliott, M.D.; Wasfy, M.M.; Cremer, P.; Johri, A.M.; Emery, M.S.; Sengupta, P.P.; Sharma, S.; Martinez, M.W.; et al. Screening of Potential Cardiac Involvement in Competitive Athletes Recovering from COVID-19: An Expert Consensus Statement. JACC Cardiovasc. Imaging 2020, 13, 2635–2652.
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 347, n1648.
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161.
- Lv, M.; Luo, X.; Shen, Q.; Lei, R.; Liu, X.; Liu, E.; Li, Q.; Chen, Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: A systematic review. Vaccines 2021, 9, 1102.
- Fazlollahi, A.; Zahmatyar, M.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Shekarriz-Foumani, R.; Kolahi, A.A.; Singh, K.; Safiri, S. Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series. Rev. Med. Virol. 2021, 32, e2318.
- Al-Ali, D.; Elshafeey, A.; Mushannen, M.; Kawas, H.; Shafiq, A.; Mhaimeed, N.; Mhaimeed, O.; Mhaimeed, N.; Zeghlache, R.; Salameh, M.; et al. Cardiovascular and haematological events post COVID-19 vaccination: A systematic review. J. Cell. Mol. Med. 2022, 26, 636–653.
- Matta, A.; Kunadharaju, R.; Osman, M.; Jesme, C.; McMiller, Z.; Johnson, E.M.; Matta, D.; Kallamadi, R.; Bande, D. Clinical presentation and outcomes of myocarditis post mRNA vaccination: A meta-analysis and systematic review. Cureus 2021, 13, e19240.
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484.
- Sessa, F.; Salerno, M.; Esposito, M.; Di Nunno, N.; Zamboni, P.; Pomara, C. Autopsy findings and causality relationship between death and COVID-19 vaccination: A systematic review. J. Clin. Med. 2021, 10, 5876.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
599
Revisions:
2 times
(View History)
Update Date:
16 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




