Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sonya E.L. Craig | -- | 1128 | 2022-09-06 19:49:00 | | | |
| 2 | Sirius Huang | Meta information modification | 1128 | 2022-09-09 02:44:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, H.; Craig, S.E.L.; Molchanov, V.; Floramo, J.S.; Zhao, Y.; Yang, T. SUMOylation in Skeletal Development and Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/26968 (accessed on 08 February 2026).
Liu H, Craig SEL, Molchanov V, Floramo JS, Zhao Y, Yang T. SUMOylation in Skeletal Development and Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/26968. Accessed February 08, 2026.
Liu, Huadie, Sonya E. L. Craig, Vladimir Molchanov, Joseph S. Floramo, Yaguang Zhao, Tao Yang. "SUMOylation in Skeletal Development and Disease" Encyclopedia, https://encyclopedia.pub/entry/26968 (accessed February 08, 2026).
Liu, H., Craig, S.E.L., Molchanov, V., Floramo, J.S., Zhao, Y., & Yang, T. (2022, September 07). SUMOylation in Skeletal Development and Disease. In Encyclopedia. https://encyclopedia.pub/entry/26968
Liu, Huadie, et al. "SUMOylation in Skeletal Development and Disease." Encyclopedia. Web. 07 September, 2022.
Copy Citation
The modification of proteins by small ubiquitin-related modifier (SUMO) molecules, SUMOylation, is a key post-translational modification involved in a variety of biological processes, such as chromosome organization, DNA replication and repair, transcription, nuclear transport, and cell signaling transduction. Emerging evidence has shown that SUMOylation regulates the development and homeostasis of the skeletal system.
SUMO
MSC
osteoblast
chondrocyte
osteoclast
signaling pathway
arthritis
osteosarcoma
developmental disorders
post-translational modification
1. Introduction
The emergence of a skeletal system was a leap forward in evolution, for it created a strong framework for the body, protecting vital organs, facilitating movement, establishing niches for hematopoiesis, and serving as a mineral reservoir.
The skeletal system develops from mesenchymal cells originating from the ectoderm and mesoderm through one of two types of ossifications processes: intramembranous or endochondral ossification. In intramembranous ossification, mesenchymal cells directly differentiate into osteoblasts to generate the flat bones of the skull and lateral clavicles [1]. Endochondral ossification, which gives rise to the bones at the base of the skull and the long bones, starts from mesenchymal cell condensation followed by primary and secondary ossification [2]. Condensed mesenchymal cells first undergo chondrogenic differentiation to form cartilage templates [2][3][4]; next, chondrocytes in the center of the cartilage templates mature and differentiate into hypertrophic chondrocytes that secrete factors to promote vascular invasion [2][3][4][5]. This brings in hematopoietic cells from the blood and osteogenic progenitors from the perichondrium [2][3][4][5]. Next, osteoblasts, derived from either osteogenic progenitors or hypertrophic chondrocytes, produce bone matrix to replace the cartilage templates generated by the apoptotic hypertrophic chondrocytes [2][3][4][5][6][7]. At the same time, bone-absorbing osteoclasts derived from the hematopoietic lineage remodel the bone and form the bone marrow cavity [8]. Secondary ossification areas form at the center of the cartilage at both ends of long bones in a process similar to primary ossification [8][9], dividing cartilage into two parts: the growth plate, which contains growth plate chondrocytes (GPCs); and articular cartilage, which consists of articular cartilage chondrocytes (ACCs). The finely controlled, directional chondrocyte proliferation and differentiation in the growth plate propels bone elongation. The coupling between osteoblast-mediated bone formation and osteoclast-mediated bone resorption continues throughout life to maintain bone tissue homeostasis [10][11].
The development and homeostasis of the skeletal system require diverse and responsive signaling and cell–cell communication, which heavily rely on dynamic posttranslational modification (PTM) systems. PTMs expand the proteome size without needing de novo protein synthesis, allowing cells to regulate complex cellular processes dynamically and efficiently. PTMs participate in every aspect of cell homeostasis, and their dysregulation often leads to disease [12]. PTM pathways are common drug targets for disease treatments, for they are reversible and dependent on enzymatic activity. SUMOylation is a branch of ubiquitination-like (Ubl) PTMs that conjugate SUMO (an ~100 aa protein tag) to target proteins, with a strong connection to stress responses and aging.
2. SUMO and SUMOylation
SUMOylation is a highly dynamic and reversible PTM that attaches SUMO proteins onto target proteins. Five SUMO paralogues (SUMO1, 2, 3, 4, and 5) have been identified in mammals, each exhibiting unique expression patterns and levels of homology [13][14][15][16]. SUMO1-3 are ubiquitously expressed in all tissues, whereas SUMO4 is mainly found in kidney, spleen, and lymph nodes. SUMO5 has more restricted expression, with exceptionally high levels in testes and peripheral blood leukocytes [14][15][16][17]. In humans, SUMO2 shares 97%, 86%, 50%, and 48% amino acid sequence homology with SUMO3, 4, 5, and 1, respectively [14][15][18]. SUMO5 is 88% identical to SUMO1 [14].
SUMO modifications are attached to a single or multiple lysine residue(s) of target proteins (mono-SUMOylation and multi-SUMOylation, respectively). SUMO2 and 3 contain several lysine residues that are themselves SUMOylated, allowing for polymeric and branched SUMO chain formation (polySUMOylation) [14][19][20][21]. Generally, SUMO1 modifications tend to occur under normal physiological conditions, while SUMO2 and 3 conjugations are more prominent in response to stress [22], with some exceptions [23][24][25][26][27]. SUMO4 and 5 are not well characterized, and their functions remain unknown.
SUMOylation involves a series of enzymatic reactions with E1, E2, and E3 ligases [28] (Figure 1). First, the SUMO precursor protein is cleaved by sentrin-specific proteases (SENPs), a family of SUMO-specific C-terminal hydrolases, to expose its C-terminal di-glycine (GG) motif. This mature SUMO is then activated by the E1 complex, which consists of SUMO activating enzyme subunit 1 (SAE1) and SAE2 (UBA1), by forming a thioester bond at the cysteine of SAE2 via an ATP-dependent reaction [29]. Next, the activated SUMO group is transferred to the sole SUMO E2 enzyme, UBC9 (SUMO ubiquitin-conjugating enzyme 9). Finally, UBC9, with or without the help of SUMO E3 ligases, conjugates the SUMO group to the epsilon-NH2 of a lysine in the target protein. SUMOylation substrate specificity is determined by UBC9 or SUMO E3 ligases. UBC9 recognizes consensus motifs, typically ψKxE (ψ represents a hydrophobic amino acid; K, lysine; x, any amino acid; and E, glutamic acid) [28][30]. SUMO E3 ligases facilitate the transfer of the SUMO molecule from UBC9 to the substrate proteins [28][29][31]. Unlike the ubiquitylation system, where hundreds of distinct E3 ligases have been identified, there are only a few known SUMO E3 ligases, including TRIM28, PC2, and members of the protein inhibitor of STAT (PIAS) [32][33][34][35][36].
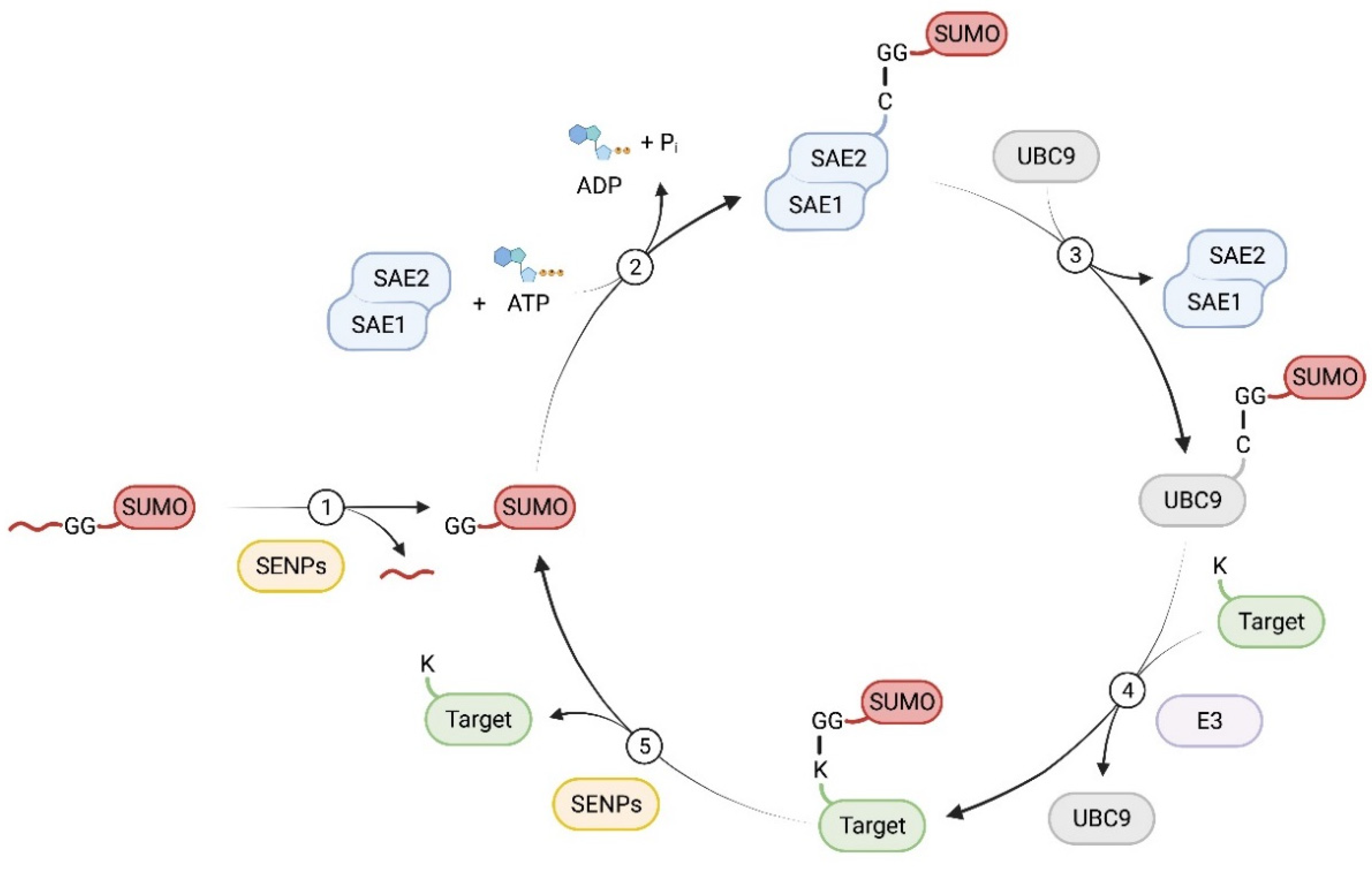
Figure 1. The enzymatic process of protein SUMOylation and deSUMOylation. (1) The nascent SUMO precursor protein is proteolytically cleaved to expose its C-terminal Gly-Gly motif by SENPs. (2) Mature SUMO is then activated by a heterodimer consistent of SAE1 and SAE2, the E1 complex, in an ATP dependent reaction, resulting in the formation of a thioester bond between SUMO and SAE2. (3) The activated SUMO is transferred to the E2 enzyme, UBC9. (4) With or without the help of an E3 ligase, UBC9 conjugates the SUMO group to the substrate protein by forming an isopeptide bond on a Lys residue. (5) SUMO modifications are removed from the target protein by SENPs.
In addition to proteolyzing the SUMO precursor, SENPs can also remove SUMO proteins from their targets, a process known as deSUMOylation [31]. Seven SENP proteins have been identified in humans (SENP1-3, SENP5-7, and SENP8 [19]). SENP1, 2, 3, and 5 catalyze both SUMO maturation and deconjugation, whereas SENP6 and 7 do not catalyze SUMO maturation but instead have a poly-SUMO chain-editing function [28][37][38]. Besides the SENP family, three additional SUMO proteases have been identified in humans: desumoylating isopeptidase 1 and 2 (DeSI1 and DeSI2) [39] and ubiquitin-specific protease-like 1 (USPL1) [40]. These desumoylases share little sequence homology with the SENP proteases, and their functions are less well characterized [41].
The effects of SUMO modifications on their target proteins are diverse and are mainly classified into three categories [13]: first, the attachment of the SUMO group can mask binding sites of the target protein, thus impairing its interaction with other molecules [13][42]; second, SUMOylation can introduce novel binding sites within the target protein, thus conferring novel molecular interactions [13][42]; finally, SUMO can change the structure of the target protein, thereby affecting its function [13][42]. The SUMOylation/deSUMOylation equilibrium regulates many cellular processes, including DNA damage response, mitochondrial dynamics, cell growth, proliferation, senescence, and apoptosis. Disruption of this balance is associated with many diseases, including cancer, neurodegenerative diseases, heart disease, and skeletal diseases, such as osteoarthritis (OA) and rheumatoid arthritis (RA) [29][43][44][45].
References
- Percival, C.J.; Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 2013, 242, 909–922.
- Long, F.; Ornitz, D.M. Development of the endochondral skeleton. Cold Spring Harb. Perspect. Biol. 2013, 5, a008334.
- Ono, N.; Ono, W.; Nagasawa, T.; Kronenberg, H.M. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat. Cell Biol. 2014, 16, 1157–1167.
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336.
- Wan, C.; Shao, J.; Gilbert, S.R.; Riddle, R.C.; Long, F.; Johnson, R.S.; Schipani, E.; Clemens, T.L. Role of HIF-1alpha in skeletal development. Ann. N. Y. Acad. Sci. 2010, 1192, 322–326.
- Zhou, X.; von der Mark, K.; Henry, S.; Norton, W.; Adams, H.; de Crombrugghe, B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014, 10, e1004820.
- Yang, L.; Tsang, K.Y.; Tang, H.C.; Chan, D.; Cheah, K.S. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 12097–12102.
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62.
- Morini, S.; Continenza, M.A.; Ricciardi, G.; Gaudio, E.; Pannarale, L. Development of the microcirculation of the secondary ossification center in rat humeral head. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 278, 419–427.
- Novack, D.V.; Teitelbaum, S.L. The osteoclast: Friend or foe? Annu. Rev. Pathol. 2008, 3, 457–484.
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108.
- Liu, Y.; Molchanov, V.; Yang, T. Enzymatic Machinery of Ubiquitin and Ubiquitin-Like Modification Systems in Chondrocyte Homeostasis and Osteoarthritis. Curr. Rheumatol. Rep. 2021, 23, 62.
- Wilkinson, K.A.; Henley, J.M. Mechanisms, regulation and consequences of protein SUMOylation. Biochem. J. 2010, 428, 133–145.
- Liang, Y.C.; Lee, C.C.; Yao, Y.L.; Lai, C.C.; Schmitz, M.L.; Yang, W.M. SUMO5, a Novel Poly-SUMO Isoform, Regulates PML Nuclear Bodies. Sci. Rep. 2016, 6, 26509.
- Bohren, K.M.; Nadkarni, V.; Song, J.H.; Gabbay, K.H.; Owerbach, D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J. Biol. Chem. 2004, 279, 27233–27238.
- Guo, D.; Li, M.; Zhang, Y.; Yang, P.; Eckenrode, S.; Hopkins, D.; Zheng, W.; Purohit, S.; Podolsky, R.H.; Muir, A.; et al. A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat. Genet. 2004, 36, 837–841.
- Han, Z.J.; Feng, Y.H.; Gu, B.H.; Li, Y.M.; Chen, H. The post-translational modification, SUMOylation, and cancer (Review). Int. J. Oncol. 2018, 52, 1081–1094.
- Saitoh, H.; Hinchey, J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 2000, 275, 6252–6258.
- Kunz, K.; Piller, T.; Muller, S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J. Cell Sci. 2018, 131, jcs211904.
- Matic, I.; van Hagen, M.; Schimmel, J.; Macek, B.; Ogg, S.C.; Tatham, M.H.; Hay, R.T.; Lamond, A.I.; Mann, M.; Vertegaal, A.C.O. In vivo identification of human small ubiquitin-like modifier polymerization sites by high accuracy mass spectrometry and an in vitro to in vivo strategy. Mol. Cell Proteom. 2008, 7, 132–144.
- Hendriks, I.A.; D’Souza, R.C.; Yang, B.; Verlaan-de Vries, M.; Mann, M.; Vertegaal, A.C. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat. Struct. Mol. Biol. 2014, 21, 927–936.
- Pichler, A.; Fatouros, C.; Lee, H.; Eisenhardt, N. SUMO conjugation—A mechanistic view. Biomol. Concepts 2017, 8, 13–36.
- Bonne-Andrea, C.; Kahli, M.; Mechali, F.; Lemaitre, J.M.; Bossis, G.; Coux, O. SUMO2/3 modification of cyclin E contributes to the control of replication origin firing. Nat. Commun. 2013, 4, 1850.
- Cubeñas-Potts, C.; Srikumar, T.; Lee, C.; Osula, O.; Subramonian, D.; Zhang, X.D.; Cotter, R.J.; Raught, B.; Matunis, M.J. Identification of SUMO-2/3-modified proteins associated with mitotic chromosomes. Proteomics 2015, 15, 763–772.
- Hong, Y.; Rogers, R.; Matunis, M.J.; Mayhew, C.N.; Goodson, M.L.; Park-Sarge, O.K.; Sarge, K.D. Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. J. Biol. Chem. 2001, 276, 40263–40267.
- Ritho, J.; Arold, S.T.; Yeh, E.T. A Critical SUMO1 Modification of LKB1 Regulates AMPK Activity during Energy Stress. Cell Rep. 2015, 12, 734–742.
- Zhang, X.D.; Goeres, J.; Zhang, H.; Yen, T.J.; Porter, A.C.; Matunis, M.J. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol. Cell 2008, 29, 729–741.
- Gareau, J.R.; Lima, C.D. The SUMO pathway: Emerging mechanisms that shape specificity, conjugation and recognition. Nat. Rev. Mol. Cell Biol. 2010, 11, 861–871.
- Sarge, K.D.; Park-Sarge, O.K. Sumoylation and human disease pathogenesis. Trends Biochem. Sci. 2009, 34, 200–205.
- Rodriguez, M.S.; Dargemont, C.; Hay, R.T. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem. 2001, 276, 12654–12659.
- Hay, R.T. SUMO: A history of modification. Mol. Cell 2005, 18, 1–12.
- Yunus, A.A.; Lima, C.D. Structure of the Siz/PIAS SUMO E3 ligase Siz1 and determinants required for SUMO modification of PCNA. Mol. Cell 2009, 35, 669–682.
- Werner, A.; Flotho, A.; Melchior, F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol. Cell 2012, 46, 287–298.
- Kagey, M.H.; Melhuish, T.A.; Wotton, D. The polycomb protein Pc2 is a SUMO E3. Cell 2003, 113, 127–137.
- Yang, Y.; Fiskus, W.; Yong, B.; Atadja, P.; Takahashi, Y.; Pandita, T.K.; Wang, H.G.; Bhalla, K.N. Acetylated hsp70 and KAP1-mediated Vps34 SUMOylation is required for autophagosome creation in autophagy. Proc. Natl. Acad. Sci. USA 2013, 110, 6841–6846.
- Rabellino, A.; Andreani, C.; Scaglioni, P.P. The Role of PIAS SUMO E3-Ligases in Cancer. Cancer Res. 2017, 77, 1542–1547.
- Yeh, E.T. SUMOylation and De-SUMOylation: Wrestling with life’s processes. J. Biol. Chem. 2009, 284, 8223–8227.
- Nayak, A.; Muller, S. SUMO-specific proteases/isopeptidases: SENPs and beyond. Genome Biol. 2014, 15, 422.
- Shin, E.J.; Shin, H.M.; Nam, E.; Kim, W.S.; Kim, J.H.; Oh, B.H.; Yun, Y. DeSUMOylating isopeptidase: A second class of SUMO protease. EMBO Rep. 2012, 13, 339–346.
- Schulz, S.; Chachami, G.; Kozaczkiewicz, L.; Winter, U.; Stankovic-Valentin, N.; Haas, P.; Hofmann, K.; Urlaub, H.; Ovaa, H.; Wittbrodt, J.; et al. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep. 2012, 13, 930–938.
- Hickey, C.M.; Wilson, N.R.; Hochstrasser, M. Function and regulation of SUMO proteases. Nat. Rev. Mol. Cell Biol. 2012, 13, 755–766.
- Seeler, J.S.; Dejean, A. Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell Biol. 2003, 4, 690–699.
- Flotho, A.; Melchior, F. Sumoylation: A regulatory protein modification in health and disease. Annu. Rev. Biochem. 2013, 82, 357–385.
- Yan, D.; Davis, F.J.; Sharrocks, A.D.; Im, H.J. Emerging roles of SUMO modification in arthritis. Gene 2010, 466, 1–15.
- Chang, H.M.; Yeh, E.T.H. SUMO: From Bench to Bedside. Physiol. Rev. 2020, 100, 1599–1619.
More
Information
Subjects:
Cell Biology; Developmental Biology; Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
692
Revisions:
2 times
(View History)
Update Date:
09 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




