
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronny Priefer | -- | 1848 | 2022-09-07 13:46:51 | | | |
| 2 | Amina Yu | Meta information modification | 1848 | 2022-09-08 03:06:43 | | |
Video Upload Options
One of the most recognized diseases worldwide is diabetes. There are currently almost half a billion individuals globally with this disease and this is expected to crest three quarters of a billion by the end of the decade. Traditionally, diabetes is broken into three categories: Type 1 (previously referred to as juvenile); Type 2 (occasion defined as adult onset), and gestational diabetes. Gestational diabetes occurs in ~2–10% of pregnant women with roughly 50% of these cases leading to the mother developing T2D after giving birth. Generally, 5–10% of cases of diabetes are of the T1D form with the remaining 90–95% having T2D. The need to monitor blood glucose, whether for T1D or T2D, is vital for the health and welfare of those afflicted with these diseases. Equally, if not more important, is the need to administer the necessary drug once the knowledge of one’s blood glucose is determined. It is this key second part that has led to significant efforts and ultimate successes in bringing closed-loop systems for diabetes management to market.
1. Urine-Glucose Testing
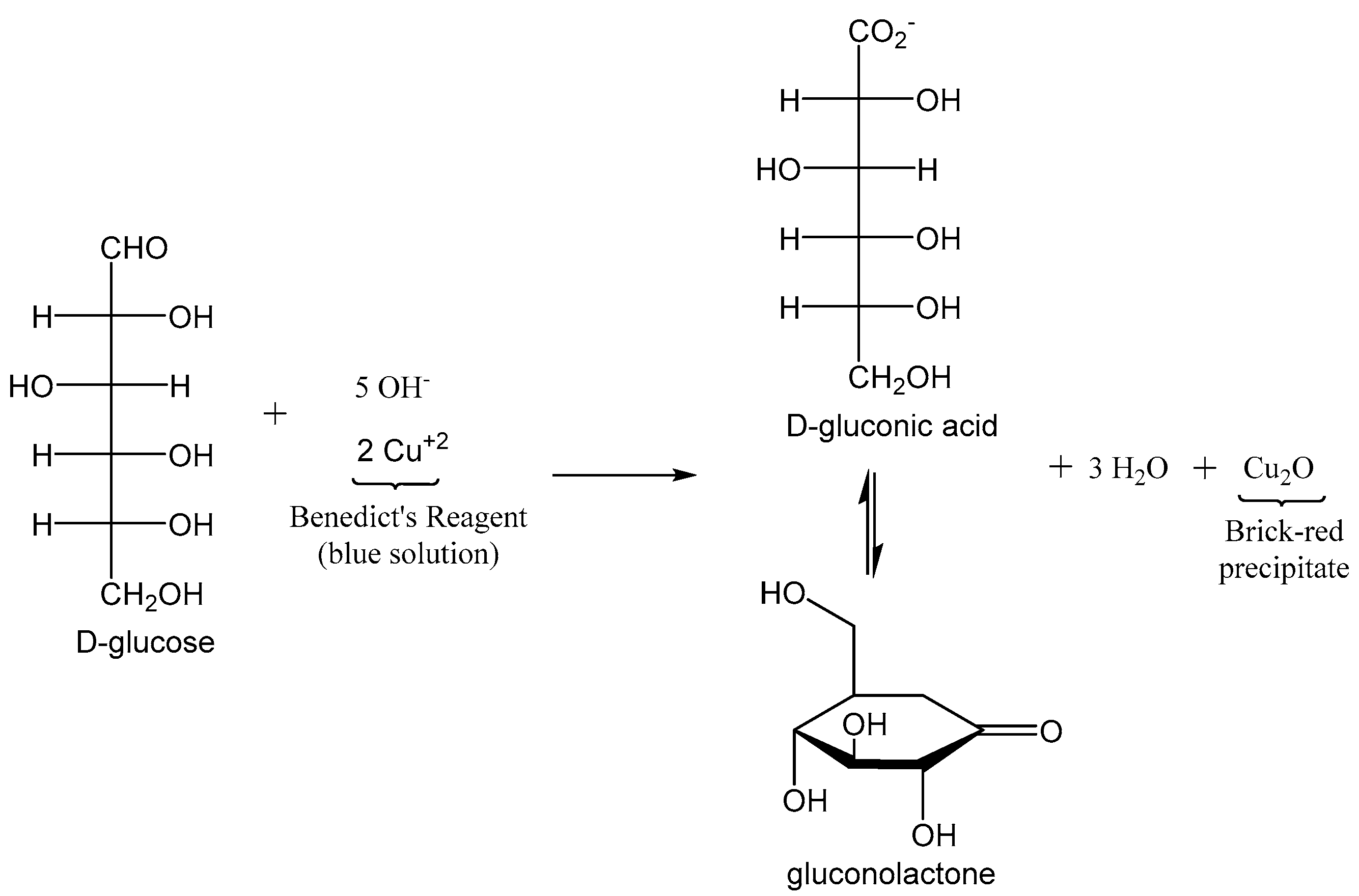
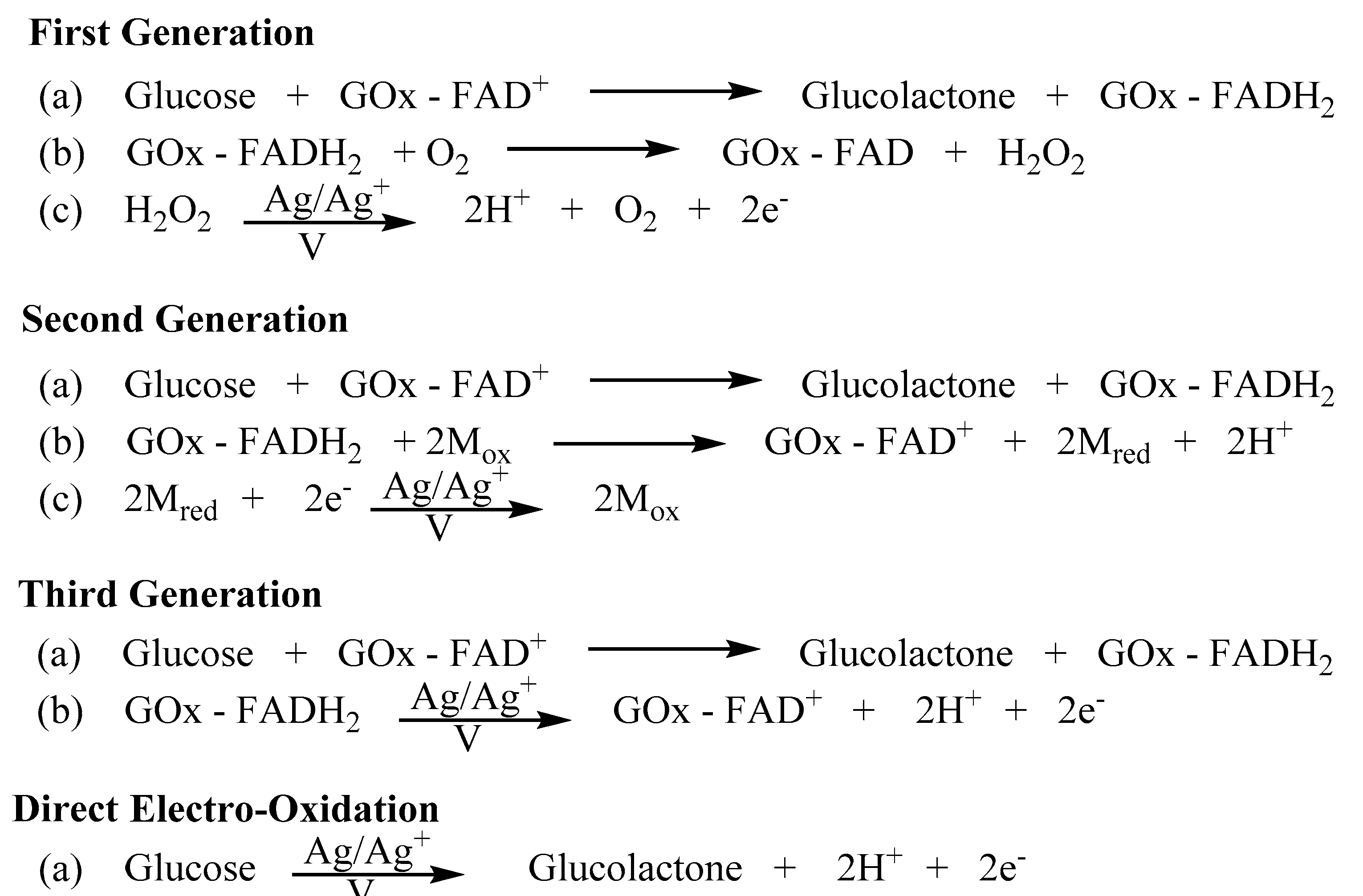
2. Blood Glucose Testing
3. Non-Invasive Glucose Testing
References
- Swidorski, D. Diabetes History. 2014. Available online: https://www.defeatdiabetes.org/diabetes-history/ (accessed on 3 May 2022).
- American Diabetes Association. Severe Hypoglycemia Predicts Mortality in Diabetes. Available online: https://care.diabetesjournals.org/content/35/9/1814 (accessed on 3 May 2022).
- Storey, H.L.; van Pelt, M.H.; Bun, S.; Daily, F.; Neogi, T.; Thompson, M.; McGuire, H.; Weigl, B.H. Diagnostic accuracy of self-administered urine glucose test strips as a diabetes screening tool in a low-resource setting in Cambodia. BMJ Open 2018, 8, e019924.
- Patel, B.; Priefer, R. Infections associated with diabetic-care devices. Diabetes Metab. Syndr. 2021, 15, 519–524.
- Vaddiraju, S.; Burgess, D.J.; Tomazos, I.; Jain, F.C.; Papadimitrakopoulos, F. Technologies for continuous glucose monitoring: Current problems and future promises. JDST 2010, 4, 1540–1562.
- Vaddiraju, S.; Burgess, D.; Jain, F.; Papadimitrakopoulos, F. The role of H2O2 outer diffusion on the performance of implantable glucose sensors. Biosens. Bioelectron. 2009, 24, 1557–1562.
- Vaddiraju, S.; Singh, H.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Enhanced glucose sensor linearity using poly (vinyl alcohol) hydrogels. JDST 2009, 3, 863–874.
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal electrodes. I. Electron transfer from glucose oxidase to metal electrodes via electron relays, bound covalently to the enzyme. J. Phys. Chem. 1987, 91, 1285–1289.
- Pishko, M.V.; Katakis, I.; Lindquist, S.E.; Ye, L.; Gregg, B.A.; Heller, A. Direct electrical communication between graphite electrodes and surface adsorbed glucose oxidase/redox polymer complexes. Angew. Chem. Int. Ed. Engl. 1990, 29, 82–84.
- Dong, S.; Wang, B.; Liu, B. Amperometric glucose sensor with ferrocene as an electron transfer mediator. Biosens. Bioelectron. 1992, 7, 215–222.
- Joshi, P.P.; Merchant, S.A.; Wang, Y.; Schmidtke, D.W. Amperometric biosensors based on redox polymer− carbon nanotube− enzyme composites. Anal. Chem. 2005, 77, 3183–3188.
- Vaddiraju, S.; Tomazos, I.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Emerging synergy between nanotechnology and implantable biosensors: A review. Biosens. Bioelectron. 2010, 25, 1553–1565.
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis 2001, 13, 983–988.
- Gorton, L.; Bremle, G.; Csöregi, E.; Jönsson-Pettersson, G.; Persson, B. Amperometric glucose sensors based on immobilized glucose-oxidizing enzymes and chemically modified electrodes. Anal. Chim. Acta 1991, 249, 43–54.
- Mizutani, F.; Yabuki, S.; Katsura, T. Amperometric enzyme electrode with the use of dehydrogenase and NAD (P) H oxidase. Sens. Actuators B Chem. 1993, 14, 574–575.
- Skoog, M.; Johansson, G. Internal supply of coenzyme to an amperometric glucose biosensor based on a chemically modified electrode. Biosens. Bioelectron. 1991, 6, 407–412.
- Laurinavicius, V.; Kurtinaitiene, B.; Liauksminas, V.; Ramanavicius, A.; Meskys, R.; Rudomanskis, R.; Skotheim, T.; Boguslavsky, L. Oxygen insensitive glucose biosensor based on PQQ-dependent glucose dehydrogenase. Anal. Lett. 1999, 32, 299–316.
- Loew, N.; Scheller, F.W.; Wollenberger, U. Characterization of Self-Assembling of Glucose Dehydrogenase in Mono-and Multilayers on Gold Electrodes. Electroanalysis 2004, 16, 1149–1154.
- HabermuÈller, K.; Ramanavicius, A.; Laurinavicius, V.; Schuhmann, W. An oxygen-insensitive reagentless glucose biosensor based on osmium-complex modified polypyrrole. Electroanalysis 2000, 12, 1383–1389.
- Wang, J.; Thomas, D.F.; Chen, A. Nonenzymatic electrochemical glucose sensor based on nanoporous PtPb networks. Anal. Chem. 2008, 80, 997–1004.
- Zhou, Y.-G.; Yang, S.; Qian, Q.-Y.; Xia, X.-H. Gold nanoparticles integrated in a nanotube array for electrochemical detection of glucose. Electrochem. Commun. 2009, 11, 216–219.
- Yuan, J.; Wang, K.; Xia, X. Highly ordered platinum-nanotubule arrays for amperometric glucose sensing. Adv. Funct. Mater. 2005, 15, 803–809.
- Myung, Y.; Jang, D.M.; Cho, Y.J.; Kim, H.S.; Park, J.; Kim, J.-U.; Choi, Y.; Lee, C.J. Nonenzymatic amperometric glucose sensing of platinum, copper sulfide, and tin oxide nanoparticle-carbon nanotube hybrid nanostructures. J. Phys. Chem. C. 2009, 113, 1251–1259.
- Clarke, S.; Foster, J. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93.
- Olczuk, D.; Priefer, R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab. Syndr. 2018, 12, 181–187.
- Bolla, A.S.; Priefer, R. Blood glucose monitoring-an overview of current and future non-invasive devices. Diabetes Metab. Syndr. 2020, 14, 739–751.
- Zhang, R.; Liu, S.; Jin, H.; Luo, Y.; Zheng, Z.; Gao, F.; Zheng, Y. Noninvasive Electromagnetic Wave Sensing of Glucose. Sensors 2019, 19, 1151.
- Boatemaa, M.A.; Doss, S. Non-invasive glucose estimation based on near infrared laser diode spectroscopy. Asian J. Biomed. Pharmaceut. Sci. 2017, 1, 22–28.
- Pleitez, M.A.; Lieblein, T.; Bauer, A.; Hertzberg, O.; von Lilienfeld-Toal, H.; Mäntele, W. In vivo noninvasive monitoring of glucose concentration in human epidermis by mid-infrared pulsed photoacoustic spectroscopy. Anal. Chem. 2013, 85, 1013–1020.
- Yeh, Y.-C.; Yang, S.; Zhao, F.; Schmidt, D.J. Noninvasive Glucose Monitoring by Mid-infrared Self-emission Method. In Proceedings of the 7th International Conference on Biomedical Electronics and Devices (BIODEVICES 2014), Eeseo, France, 3–6 March 2014; pp. 107–111.
- Abd Salam, N.A.B.; bin Mohd Saad, W.H.; Manap, Z.B.; Salehuddin, F. The evolution of non-invasive blood glucose monitoring system for personal application. JTEC 2016, 8, 59–65.
- Nawaz, A.; Øhlckers, P.; Sælid, S.; Jacobsen, M.; Nadeem Akram, M. Review: Non-invasive continuous blood glucose measurement techniques. J. Bioinform. Diabetes 2016, 1, 1–27.
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Naidis, E.; Mayzel, Y. Noninvasive glucose monitoring: Increasing accuracy by combination of multi-technology and multi-sensors. JDST 2010, 4, 583–595.
- Srivastava, A.; Chowdhury, M.K.; Sharma, S.; Sharma, N. Measurement of glucose by using modulating ultrasound with optical technique in normal and diabetic human blood serum. In Proceedings of the 2014 International Conference on Advances in Engineering & Technology Research (ICAETR-2014), Unnao, India, 1–2 August 2014; pp. 1–5.
- Kit, S.Y.H.; Kassim, N.M. Non-invasive blood glucose measurement using temperature-based approach. J. Teknol. 2013, 64.
- Cho, O.K.; Kim, Y.O.; Mitsumaki, H.; Kuwa, K. Noninvasive measurement of glucose by metabolic heat conformation method. Clin. Chem. 2004, 50, 1894–1898.
- Park, J.-H.; Kim, C.-S.; Choi, B.-C.; Ham, K.-Y. The correlation of the complex dielectric constant and blood glucose at low frequency. Biosens. Bioelectron. 2003, 19, 321–324.
- So, C.-F.; Choi, K.-S.; Wong, T.K.; Chung, J.W. Recent advances in noninvasive glucose monitoring. Med. Devices 2012, 5, 45.
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Murray, K.; Hughson, R. Glucose Levels Detection Using mm-Wave Radar. IEEE Sens. Lett. 2018, 2, 1–4.
- Cano-Garcia, H.; Saha, S.; Sotiriou, I.; Kosmas, P.; Gouzouasis, I.; Kallos, E. Millimeter-Wave Sensing of Diabetes-Relevant Glucose Concentration Changes in Pigs. J. Infrared. Millim Terahertz Waves 2018, 39, 761–772.
- Mohd Bahar, A.A.; Zakaria, Z.; Md Arshad, M.K.; Isa, A.A.M.; Dasril, Y.; Alahnomi, R.A. Real Time Microwave Biochemical Sensor Based on Circular SIW Approach for Aqueous Dielectric Detection. Sci. Rep. 2019, 9, 5467.
- Cameron, B.D.; Gorde, H.W.; Satheesan, B.; Cote, G.L. The use of polarized laser light through the eye for noninvasive glucose monitoring. Diabetes Technol. Ther. 1999, 1, 135–143.
- Purvinis, G.; Cameron, B.D.; Altrogge, D.M. Noninvasive polarimetric-based glucose monitoring: An in vivo study. JDST 2011, 5, 380–387.
- Woods, J.; Smith, J.; Rice, M.; Routt, W.; Messerschmidt, R.; Ou, J. Non-Invasive Measurement of Blood Glucose Using Retinal Imaging. U.S. Patent 20050267343A1, 3 May 2022.
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z.A. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuators B Chem. 2013, 177, 522–528.
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si: WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587.
- Shin, J.; Choi, S.J.; Lee, I.; Youn, D.Y.; Park, C.O.; Lee, J.H.; Tuller, H.L.; Kim, I.D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367.
- Choi, S.-J.; Lee, I.; Jang, B.-H.; Youn, D.-Y.; Ryu, W.-H.; Park, C.O.; Kim, I.-D. Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796.
- Ryabtsev, S.; Shaposhnick, A.; Lukin, A.; Domashevskaya, E. Application of semiconductor gas sensors for medical diagnostics. Sens. Actuators B Chem. 1999, 59, 26–29.
- Paldus, B.A.; Kachanov, A.A. An historical overview of cavity-enhanced methods. Can. J. Phys. 2005, 83, 975–999.
- Ronny Priefer; Rust, M. Breath Acetone Monitor and Method of Detecting Breath Acetone. Utility Patent US9921209B2, 3 May 2022.
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-responsive smart polymers and biomedical applications. JPhys 2019, 2, 032004.




