Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jingya Jane PU | -- | 2426 | 2022-08-26 11:43:52 | | | |
| 2 | Conner Chen | -4 word(s) | 2422 | 2022-08-29 04:12:44 | | | | |
| 3 | Conner Chen | -5 word(s) | 2417 | 2022-08-29 04:46:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pu, J.J.; Hakim, S.G.; Melville, J.C.; Su, Y. Reconstruction and Rehabilitation of Jaw following Ablative Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/26542 (accessed on 07 February 2026).
Pu JJ, Hakim SG, Melville JC, Su Y. Reconstruction and Rehabilitation of Jaw following Ablative Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/26542. Accessed February 07, 2026.
Pu, Jane J., Samer G. Hakim, James C. Melville, Yu-Xiong Su. "Reconstruction and Rehabilitation of Jaw following Ablative Surgery" Encyclopedia, https://encyclopedia.pub/entry/26542 (accessed February 07, 2026).
Pu, J.J., Hakim, S.G., Melville, J.C., & Su, Y. (2022, August 26). Reconstruction and Rehabilitation of Jaw following Ablative Surgery. In Encyclopedia. https://encyclopedia.pub/entry/26542
Pu, Jane J., et al. "Reconstruction and Rehabilitation of Jaw following Ablative Surgery." Encyclopedia. Web. 26 August, 2022.
Copy Citation
The reconstruction and rehabilitation of jaws following ablative surgery have been transformed by the development of computer-assisted surgery and virtual surgical planning. With strides made in computer-assisted surgery and patient-specific implants, the individual functional reconstruction of the jaw is evolving rapidly and the prompt rehabilitation of both the masticatory function and aesthetics after jaw resection has been made possible.
jaw reconstruction
microvascular reconstruction
free flaps
1. Computer-Assisted Surgery (CAS) and Virtual Surgical Planning
Computer-assisted surgery has revolutionized jaw reconstructive surgeries. Through virtual surgical planning, more predictable and accurate jaw reconstructions can be achieved. In addition, a significant amount of operative time can be saved, thus enabling complex functional jaw reconstruction.
1.1. Procedures of Computer-Assisted Surgery
Computer-assisted surgery normally starts from the clinical history-taking and physical examination. The history of previous surgeries and radiation therapy to the head and neck is crucial for the selection of suitable recipient vessels. A clinical examination is performed to determine the nature and extent of the lesion and formulate a tentative surgical plan. An intraoral scan is obtained to register the occlusion and the intraoral extension of the lesion. A CT scan of the head & neck and a CT angiogram of the donor site are crucial for assessing the vessel’s condition and the suitability of the free flap donor site.
Three-dimensional models of the donor and recipient sites are built based on the CT and intraoral scans. Virtual resections and reconstructions may be performed for a better understanding of the intraoperative condition. Based on the virtual planning, surgical positioning, cutting guides, and/or patient-specific implants are designed. (Figure 1).
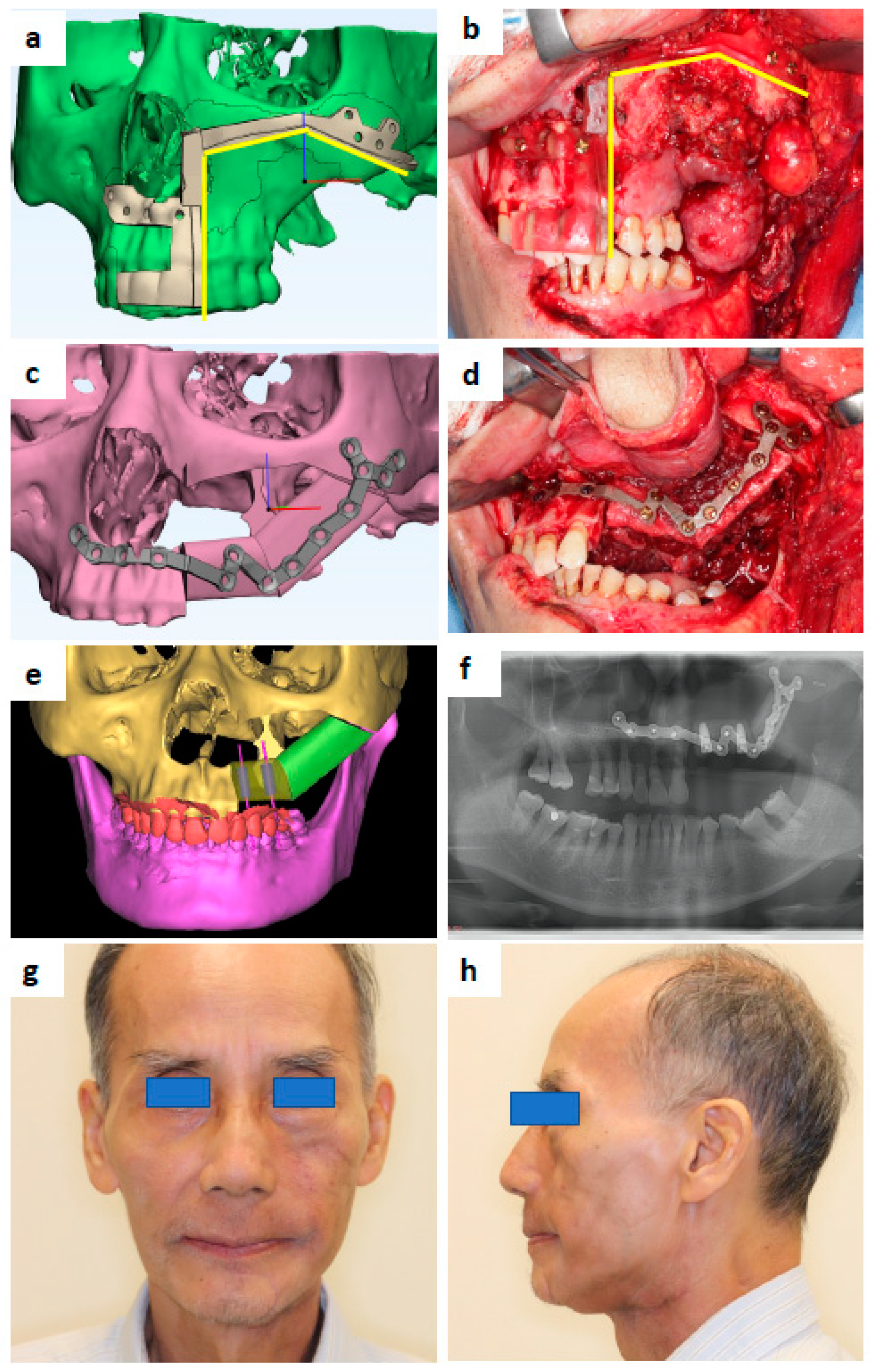
Figure 1. A 69-year-old male that presented with carcinoma ex pleomorphic adenoma at the left maxilla. (a) Maxilla resection guide design. (b) 3D-printed maxilla resection guide fitted intraoperatively. (c) Patient-specific Titanium plate design. (d) 3D-printed Ti plate fitted intraoperatively. (e) Design showing the location of simultaneous dental implants to be inserted during fibula free flap harvest. (f) Post-operative orthopantomography. (g) Postoperative 7 months and post-radiation 4 months—frontal view. (h) Profile view.
A post-operative evaluation that compares the virtual surgical plan and the final surgical outcome is crucial for the assessment of the accuracy of the computer-assisted surgery and potential future improvements [1].
1.2. Advantages of Computer-Assisted Surgery
Since the surgical procedure is well planned before the operation, the efficiency of surgery significantly improves with CAS. The systematic review and meta-analysis by Powchareon et al. demonstrated a reduced ischemic time to free flaps, reconstruction time, total operative time, and postoperative hospital stay [2]. The application of CAS also reduces the burden of decision-making intraoperatively, which helps to achieve adequate tumor resection margins [3]. By performing virtual surgical planning, the accuracy of the reconstruction is increased [4] and inter-segmental bone gaps can be reduced to a minimum [5]. This could lead to a more predictable reconstructive contour and bone healing. A comprehensive approach was also developed to systematically assess the spatial deviation of the reconstructed mandible in a computer-assisted reconstruction [6]. With the increased efficiency and accuracy of jaw reconstruction, more complicated functional jaw reconstructions with dental and neurosensory rehabilitation have increased in popularity.
Another advantage of CAS lies in its value for medical education and surgical training. Compared to free hand surgery, junior and less experienced surgeons can achieve more consistently favorable tumor resections and reconstruction outcomes, which is important for teaching hospitals [3].
1.3. Concerns about Computer-Assisted Surgery
While providing a high predictability and reproducibility, CAS is also challenged for its rigidity and difficult improvisation during the surgery as most of the planning is performed before the operation.
The first and the most significant concern is for the oncological safety of the predetermination of the surgical margins, especially in malignant jaw pathologies where positive and close margins can lead to compromised local-regional control of disease and patient survival [3]. In CAS, virtual resections are performed with reference to the clinical examination, types of pathology, and preoperative imaging, which might cause uncertainties during the surgery [7]. Positive intraoperative frozen sections are possible and preoperative resection and reconstruction plans will have to be adjusted accordingly [8]. A study on ameloblastoma patients also yielded a comparable margin status with or without CAS. Furthermore, the possible additional advantage of decreasing the occurrence of close or positive margins was reported with CAS when applied to the resection of benign intraosseous lesions [9]. Compared to benign jaw lesions, CAS for malignant tumors poses more challenges in terms of the fast progress of disease during the period of virtual surgical planning and soft tissue margin determination. As reported by Pu et al., compared to the determination of the bone margin purely from a CT scan, the integration of careful clinical examination, intraoral optical scanning, and MRI was warranted for a reliable soft tissue margin during virtual surgical planning [3]. The timing of the surgery also plays a crucial role whereby surgeon dominated planning and in-house printing are very helpful. With the above measures, the study showed no significant difference in the margin status and survival performance of patients suffering from oral cancer with or without CAS after the adjustment of other significant risk factors [3].
Another concern is the low adherence to the CAS planning when the unexpected change of surgical plans arises intraoperatively. Wilde et al. [10] and Ma et al. [11] reported the need for an intraoperative change of plans in 19% and 17.6% of cases, respectively. However, a significantly lower rate of 5.1% was reported by Pu et al. [1] Different teams have different planning protocols which can lead to the significantly different performance of CAS [12]. Preoperative patient assessment and case selection play important parts as margin determination might be more difficult from imaging in certain cases, such as osteonecrosis of the jaw and tumors with a perineural invasion tendency. For oncological patients, timely surgery and confirmation of the surgical plan by the responsible surgeon before proceeding to surgery are crucial. A postoperative evaluation by comparing the preoperative plan to the final surgical outcomes with the feedback from the surgical team facilitates the rapid improvement in CAS skills.
The steep learning curve of CAS has also been regarded as a challenge when popularizing the technique. A cumulative sum analysis revealed a three-stage learning curve of CAS, including initial learning, plateau, and overlearning, and surgical proficiency was obtained after 23 cases [13]. This can guide the teaching and training of CAS.
Currently, CAS is used mostly for bony reconstruction. The computer-assisted reconstruction of soft tissue defects after the resection of malignant tumors is still under investigation.
2. Patient-Specific Implants (PSI)
A significant improvement in bone fixation plates has been observed in the past decades. The traditional mass-produced fixation plates come with universal shapes. Bending and adaptation to the specific defect may be technique sensitive and time-consuming, especially for complex cases [14]. The repetitive bending may also decrease the fatigue and corrosion resistance of the plates leading to a higher risk of plate fracture, screw loosening, and bone resorption [15]. These complications led to the development of patient-specific implants which are prefabricated to fit the specific shape of the ideal reconstructed jaw.
Different types of patient specific implants have been fabricated so far, the most common being the patient-specific fixation plates. Other applications include various types of prostheses used for the reconstruction of the midface, mandible, and temporomandibular joint [16]. However, the application of these prostheses is currently limited to jaw defects after the ablation of benign lesions with a good soft tissue envelop. The problems of the fatigue and fracture of prostheses after long-term use and exposure though the skin after radiation therapy remain to be solved. In situations where implantable PSIs are not available, jaw models can be printed, and the surgeons can pre-bend the plates before the operation [17].
There are two main technologies for the fabrication of PSI: subtractive manufacturing (CAD/CAM) and additive manufacturing (3D printing) (Figure 1).
2.1. CAD-CAM Patient-Specific Implants
The development of PSIs started with the popularization of the technology of computer-assisted design and computer-assisted manufacturing (CAD-CAM).
The traditional CAD-CAM technique was developed in the 1960s and started to be used in medical care in the 2000s, which led to a paradigm shift in head and neck reconstruction [18]. It led the frontier of osseous jaw reconstruction in the 2010s with its precision and intraoperative efficiency [19][20].
However, traditional CAD-CAM fabrication by subtractive milling from a block of material by computer numerical control (CNC) causes material waste. Certain complicated shapes cannot possibly be manufactured by subtractive milling, which led to the introduction and development of additive manufacturing, i.e., 3D printing [21].
2.2. 3D-Printed Patient-Specific Implants
The three-dimensional (3D) printing technique, also known as additive manufacturing, experienced its significant development phase in the 2010s. Compared to CAD-CAM, 3D printing is a form of additive manufacturing that produces solid objects by adding materials layer by layer from base to top [22]. It offers a more versatile solution for complex structures and causes less material loss. However, some 3D printing technologies can be time-consuming, and the machines have a relatively high initial cost. Unlike objects made from a material block by CAD-CAM, 3D-printed objects cannot accommodate a high internal stress during manufacturing, which can lead to cracking and easy fracturing under functional stress [21].
Different types of 3D printing technologies have been developed. Each has its own advantages and disadvantages. The commonly used ones in the medical field include stereolithography (SLA) with liquid resin and selective laser melting (SLM) with powder materials [22]. In jaw reconstruction, the commonly used PSIs include reconstruction plates with Titanium, contour augmentation with porous polyethylene (e.g., Medpor), or polyetheretherketone (PEEK) [23].
2.3. Advantages and Disadvantages of PSI
Compared to commercial stock plates, surgeons can avoid the time-consuming procedure of bending plates intraoperatively and avoid the risk of fatigue-induced plate fracture from repeated reverse bending. In combination with patient-specific osteotomy guides, PSIs can save a significant amount of intraoperative time which was previously used to segmentalize and adjust the bone segments of a bone graft (e.g., fibula flap) to produce an appropriate jaw contour. Yang et al. and Rana et al. reported increased accuracy with the use of PSIs in computer-assisted surgery [23][24]. With the increased efficiency and accuracy, PSIs bring opportunities for functional reconstruction such as immediate dental rehabilitation by simultaneous insertion of dental implants.
However, at the moment, an implantable PSI is still relatively expensive, and the printing technology may not be available in some parts of the world. Careful post-printing treatments are needed to reduce the chance of infection due to the inborn rough surface and plate fracture under stress due to possible microcracks inside the PSI [25].
2.4. Guidelines and Regulations
With the popularization of 3D-printing technology, more 3D-printed medical devices are being adopted at the point of care (PoC). The recent systematic review by Murtezani et al. showed that 35% of studies were based on POC production methods while 12% were outsourced [26]. This has provided the timely production of devices suitable for specific clinical use. However, this also causes new challenges for the regulatory bodies’ s ability to ensure the safety and effectiveness of 3D-printed medical devices. A discussion paper was published in December 2021 by the U.S. Food & Drug Administration to seek advice from health care providers, facilities, medical device manufacturers, and other stakeholders in order to form guidelines and regulations for future use [27].
3. Dental Rehabilitation
Traditionally, dental rehabilitation was performed as a secondary procedure after the primary reconstruction of the jaw was completed, at least 3 to 6 months after the primary surgery. Besides the long waiting time until the patient can have a functional occlusion, this technique has several other disadvantages. If dental rehabilitation was not taken into consideration when designing the osseous flaps, the bony segments could be placed at an unfavorable position and angle, making further dental rehabilitation difficult, if not impossible. Moreover, when patients have undergone radiation therapy after the resection and reconstruction of the jaw, the placement of osseointegrated dental implants always carries the risk of osteonecrosis of the jaw and a loss of bone flap in the long term.
With the development of CAS and PSI, the accuracy and efficiency of jaw reconstruction have been significantly increased [28]. On top of restoring facial aesthetics and maintaining the airway, the timely and predictable restoration of the mastication function has become the new aim of functional jaw reconstruction. Different techniques of dental rehabilitation with osseointegrated dental implants in the reconstructed jaw have been reported.
Schepers et al. described a technique comprising the secondary reconstruction of the jaw using prefabricated fibula grafts with pre-placed dental implants [29]. In the first stage of surgery, dental implants were placed into the fibula with prefabricated guides and left in situ for osseointegration. A CT scan of the fibula was performed, and the reconstruction of the jaw was planned virtually with reference to the implants placed in the fibula. A second surgery was performed for the reconstruction of the jaw with the delivery of a dental prosthesis. This technique has the advantage of reducing the effect of errors in the placement of dental implants in the fibula. However, it requires multiple operations and is thus not popularized in most centers.
Levine et al. proposed the concept of “Jaw-in-a-day”, where dental implants and dental prostheses were placed at the same stage of the primary reconstruction of the jaw [30]. As the preliminary report, it proved the effectiveness of performing immediate dental rehabilitation at the same stage of tumor resection and reconstruction. The detailed total virtual workflow was further described by Zweifel et al. in 2018 [31].
When the immediate delivery of a dental prosthesis is planned, a higher accuracy for the jaw reconstruction and implant placement is required. Multiple attempts have been made to improve the accuracy of simultaneous dental implants. A tooth-borne or plate-borne implant position verification guide was developed by Zweifel et al. [32] However, this technique cannot be applied when the patient’s preexisting or remaining teeth are less than ideal. Schepers et al. used an occlusal splint to locate the implant-borne prosthesis when fixing the reconstruction segments [33]. To use this technique, an accurate jaw relationship registration is important, but it is often difficult, especially in oncological patients where preoperative occlusion is deranged due to the pathology. A “three-in-one” patient-specific surgical guide was reported by Zhu et al. to serve the purpose of fibula harvesting, segmentation, and simultaneous dental implant placement [34]. To overcome the sliding and rotating errors caused by the placement of fibula cutting guides, Pu et al. developed a novel malleolus cap for fibula flap harvesting. With the use of a malleolus cap, the simultaneous dental implants in the fibula approached a similar level of accuracy compared to the guided implant placement in the native maxilla and mandible, which further proved the reliability of the jaw-in-a-day technique with simultaneous dental rehabilitation [35].
References
- Pu, J.J.; Choi, W.S.; Yang, W.F.; Zhu, W.Y.; Su, Y.X. Unexpected Change of Surgical Plans and Contingency Strategies in Computer-Assisted Free Flap Jaw Reconstruction: Lessons Learned from 98 Consecutive Cases. Front. Oncol. 2022, 12, 746952.
- Powcharoen, W.; Yang, W.F.; Li, K.Y.; Zhu, W.; Su, Y.X. Computer-Assisted Versus Conventional Freehand Mandibular Reconstruction with Fibula Free Flap: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2019, 144, 1417–1428.
- Pu, J.J.; Choi, W.S.; Yu, P.; Wong, M.C.M.; Lo, A.W.I.; Su, Y.X. Do predetermined surgical margins compromise oncological safety in computer-assisted head and neck reconstruction? Oral Oncol. 2020, 111, 104914.
- Pucci, R.; Weyh, A.; Smotherman, C.; Valentini, V.; Bunnell, A.; Fernandes, R. Accuracy of Virtual Planned Surgery Versus Conventional Free-Hand Surgery for Reconstruction of the Mandible with Osteocutaneous Free Flaps. Int. J. Oral Maxillofac. Surg. 2020, 49, 1153–1161.
- Craig, E.S.; Yuhasz, M.; Shah, A.; Blumberg, J.; Salomon, J.; Lowlicht, R.; Fusi, S.; Steinbacher, D.M. Simulated Surgery and Cutting Guides Enhance Spatial Positioning in Free Fibular Mandibular Reconstruction. Microsurgery 2015, 35, 29–33.
- Yang, W.F.; Yu, P.; Zhu, W.Y.; Choi, W.S.; Pu, J.J.; Su, Y.X. A Comprehensive Approach for Measuring Spatial Deviations of Computer-Assisted Mandibular Reconstruction. Plast. Reconstr. Surg. 2022, 149, 500e–510e.
- Han, H.H.; Kim, H.Y.; Lee, J.Y. The pros and cons of computer-aided surgery for segmental mandibular reconstruction after oncological surgery. Arch. Craniofac. Surg. 2017, 18, 149–154.
- Mazzoni, S.; Marchetti, C.; Sgarzani, R.; Cipriani, R.; Scotti, R.; Ciocca, L. Evaluation of the accuracy of a surgical guide and custom-made bone plate in oncology patients after mandibular reconstruction. Plast. Reconstr. Surg. 2013, 131, 1376–1385.
- Palla, B.; Callahan, N. Does the Use of Computer-Assisted Surgery Affect the Margin Status in Resections of Ameloblastoma? J. Oral Maxillofac. Surg. 2021, 79, 1467–1473.
- Wilde, F.; Hanken, H.; Probst, F.; Schramm, A.; Heiland, M.; Cornelius, C.P. Multicenter Study on the Use of Patient-Specific CAD/CAM Reconstruction Plates for Mandibular Reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 2035–2051.
- Ma, H.; Shujaat, S.; Van Dessel, J.; Sun, Y.; Bila, M.; Vranckx, J.; Politis, C.; Jacobs, R. Adherence to Computer-Assisted Surgical Planning in 136 Maxillofacial Reconstructions. Front. Oncol. 2021, 11, 713606.
- Yang, W.; Zhang, C.-Y.; Choi, W.; Zhu, W.-Y.; Li, D.; Chen, X.-S.; Du, R.; Su, Y.-X. A Novel ‘Surgeon-Dominated’ Approach to the Design of 3D-Printed Patient-Specific Surgical Plates in Mandibular Reconstruction: A Proof-of-Concept Study. Int. J. Oral Maxillofac. Surg. 2020, 49, 13–21.
- Zhu, W.Y.; Choi, W.S.; Wong, C.M.M.; Pu, J.J.; Yang, W.F.; Su, Y.X. The learning curve of computer-assisted free flap jaw reconstruction surgery using 3D-printed patient-specific plates: A cumulative sum analysis. Front. Oncol. 2021, 11, 737769.
- Marchetti, C.; Bianchi, A.; Mazzoni, S.; Cipriani, R.; Campobassi, A. Oromandibular reconstruction using a fibula osteocutaneous free flap: Four different “preplating” techniques. Plast. Reconstr. Surg. 2006, 118, 643–651.
- Martola, M.; Lindqvist, C.; Hanninen, H.; Al-Sukhun, J. Fracture of titanium plates used for mandibular reconstruction following ablative tumor surgery. J. Biomed. Mater. Res. B 2007, 80, 345–352.
- Crafts, T.D.; Ellsperman, S.E.; Wannemuehler, T.J.; Bellicchi, T.D.; Shipchandler, T.Z.; Mantravadi, A.V. Three-Dimensional Printing and Its Applications in Otorhinolaryngology-Head and Neck Surgery. Otolaryngol. Head Neck Surg. 2017, 156, 999–1010.
- Wilde, F.; Winter, K.; Kletsch, K.; Lorenz, K.; Schramm, A. Mandible reconstruction using patient-specific pre-bent reconstruction plates: Comparison of standard and transfer key methods. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 129–140.
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of Computer-Aided Design and Computer-Aided Manufacturing to Produce Orthognathically Ideal Surgical Outcomes: A Paradigm Shift in Head and Neck Reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 2115–2122.
- Hanasono, M.M.; Skoracki, R.J. Computer-Assisted Design and Rapid Prototype Modelling in Microvascular Mandible Reconstruction. Laryngoscope 2012, 123, 597–604.
- Matros, E.; Albornoz, C.R.; Rensberger, M.; Weimer, K.; Garfein, E.S. Computer-Assisted Design and Computer-Assisted Modeling Technique Optimization and Advantages Over Traditional Methods of Osseous Flap Reconstruction. J. Reconstr. Microsurg. 2014, 30, 289–296.
- Oh, J.H. Recent advances in the reconstruction of cranio-maxillofacial defects using computer-aided design/computer-aided manufacturing. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 2.
- Yang, W.F.; Choi, W.S.; Leung, Y.Y.; Curtin, J.P.; Du, R.; Zhang, C.Y.; Chen, X.S.; Su, Y.X. Three-dimensional printing of patient-specific surgical plates in head and neck reconstruction: A prospective pilot study. Oral Oncol. 2018, 78, 31–36.
- Yang, W.F.; Choi, W.S.; Wong, M.C.; Powcharoen, W.; Zhu, W.Y.; Tsoi, J.K.; Chow, M.; Kwok, K.W.; Su, Y.X. Three-Dimensionally Printed Patient-Specific Surgical Plates Increase Accuracy of Oncologic Head and Neck Reconstruction Versus Conventional Surgical Plates: A Comparative Study. Ann. Surg. Oncol. 2021, 28, 363–375.
- Rana, M.; Chin, S.-J.; Muecke, T.; Kesting, M.; Groebe, A.; Riecke, B.; Heiland, M.; Gellrich, N.-C. Increasing the Accuracy of Mandibular Reconstruction with Free Fibula Flaps Using Functionalized Selective Laser-Melted Patient-Specific Implants: A Retrospective Multicenter Analysis. J. Cranio-Maxillofac. Surg. 2017, 45, 1212–1219.
- Du, R.; Su, Y.-X.; Yan, Y.; Choi, W.S.; Yang, W.F.; Zhang, C.; Chen, X.; Curtin, J.P.; Ouyang, J.; Zhang, B. A Systematic Approach for Making 3D-Printed Patient-Specific Implants for Craniomaxillofacial Reconstruction. Engineering 2020, 6, 1291–1301.
- Murtezani, I.; Sharma, N.; Thieringer, F.M. Medical 3D Printing with a focus on Point-of-Care in Cranio- and Maxillofacial Surgery. A systematic review of literature. Ann. 3D Print. Med. 2022, 6, 100059.
- Available online: https://www.fda.gov/medical-devices/3d-printing-medical-devices/3d-printing-medical-devices-point-care-discussion-paper (accessed on 20 June 2022).
- Zavattero, E.; Bolzoni, A.; Dell’Aversana, G.; Santagata, M.; Massarelli, O.; Ferri, A.; Della Monaca, M.; Copelli, C.; Gessaroli, M.; Valsecchi, S.; et al. Accuracy of Fibula Reconstruction Using Patient-Specific Cad/Cam Plates: A Multicenter Study on 47 Patients. Laryngoscope 2021, 131, E2169–E2175.
- Schepers, R.H.; Kraeima, J.; Vissink, A.; Lahoda, L.U.; Roodenburg, J.L.; Reintsema, H.; Raghoebar, G.M.; Witjes, M.J. Accuracy of Secondary Maxillofacial Reconstruction with Prefabricated Fibula Grafts Using 3D Planning and Guided Reconstruction. J. Cranio-Maxillofac. Surg. 2016, 44, 392–399.
- Levine, J.P.; Bae, J.S.; Soares, M.; Brecht, L.E.; Saadeh, P.B.; Ceradini, D.J.; Hirsch, D.L. Jaw in a Day: Total Maxillofacial Reconstruction Using Digital Technology. Plast. Reconstr. Surg. 2013, 131, 1386–1391.
- Zweifel, D.; Bredell, M.; Essig, H.; Gander, T.; Lanzer, M.; Rostetter, C.; Rücker, M.; Studer, S. Total Virtual Workflow in CAD-CAM Bony Reconstruction with a Single Step Free Fibular Graft and Immediate Dental Implants. Br. J. Oral Maxillofac. Surg. 2018, 56, 859–863.
- Zweifel, D.; Bredell, M.G.; Lanzer, M.; Rostetter, C.; Rücker, M.; Studer, S. Precision of Simultaneous Guided Dental Implantation in Microvascular Fibular Flap Reconstructions with and without Additional Guiding Splints. J. Oral Maxillofac. Surg. 2019, 77, 971–976.
- Schepers, R.H.; Raghoebar, G.M.; Vissink, A.; Lahoda, L.U.; Van der Meer, W.J.; Roodenburg, J.L.; Reintsema, H.; Witjes, M.J. Fully 3-Dimensional Digitally Planned Reconstruction of a Mandible with a Free Vascularized Fibula and Immediate Placement of an Implant-Supported Prosthetic Construction. Head Neck 2013, 35, E109–E114.
- Zhu, W.Y.; Su, Y.X.; Pow, E.H.N.; Yang, W.F.; Qin, L.; Choi, W.S. “Three-In-One” Patient-Specific Surgical Guides for Simultaneous Dental Implants in Fibula Flap Jaw Reconstruction: A Prospective Case Series. Clin. Implant Dent. Relat. Res. 2020, 23, 43–53.
- Pu, J.J.; Choi, W.S.; Yeung, W.K.; Yang, W.F.; Zhu, W.Y.; Su, Y.X. A Comparative Study on a Novel Fibula Malleolus Cap to Increase the Accuracy of Oncologic Jaw Reconstruction. Front. Oncol. 2022, 11, 743389.
More
Information
Subjects:
Surgery; Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
920
Revisions:
3 times
(View History)
Update Date:
29 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




