Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Destouni | -- | 2013 | 2022-08-23 20:31:28 | | | |
| 2 | Vivi Li | Meta information modification | 2013 | 2022-08-24 04:39:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Destouni, M.; Lazaris, A.C.; Tzelepi, V. Cribriform Patterned Lesions in the Prostate Gland. Encyclopedia. Available online: https://encyclopedia.pub/entry/26411 (accessed on 07 February 2026).
Destouni M, Lazaris AC, Tzelepi V. Cribriform Patterned Lesions in the Prostate Gland. Encyclopedia. Available at: https://encyclopedia.pub/entry/26411. Accessed February 07, 2026.
Destouni, Maria, Andreas C. Lazaris, Vasiliki Tzelepi. "Cribriform Patterned Lesions in the Prostate Gland" Encyclopedia, https://encyclopedia.pub/entry/26411 (accessed February 07, 2026).
Destouni, M., Lazaris, A.C., & Tzelepi, V. (2022, August 23). Cribriform Patterned Lesions in the Prostate Gland. In Encyclopedia. https://encyclopedia.pub/entry/26411
Destouni, Maria, et al. "Cribriform Patterned Lesions in the Prostate Gland." Encyclopedia. Web. 23 August, 2022.
Copy Citation
Cribriform glandular formations are characterized by a continuous proliferation of cells with intermingled lumina and can constitute a major or minor part of physiologic (normal central zone glands), benign (clear cell cribriform hyperplasia and basal cell hyperplasia), premalignant (high-grade prostatic intraepithelial neoplasia), borderline (atypical intraductal cribriform proliferation) or clearly malignant (intraductal, acinar, ductal and basal cell carcinoma) lesions. Each displays a different clinical course and variability in clinical management and prognosis.
prostate cancer
intraductal carcinoma
cribriform carcinoma
Gleason Score
prognostic grade group
prognosis
1. Introduction
Glandular structures within the prostate may assume a cribrifrom morphology as a major or minor part of physiological, non-neoplastic and neoplastic processes. Cribriform is derived from the Latin word cribrum, which means sieve and is used to describe something that is pierced with small holes. In histology, cribriform is defined as a continuous proliferation of cells with intermingled lumina. That means that there are cells lying one next to another with round or elongated holes interspaced among them at various intervals. Normal prostate glands and benign (clear cell cribriform hyperplasia and basal cell hyperplasia), premalignant (high-grade prostatic intraepithelial neoplasia), borderline (atypical intraductal cribriform proliferation) or clearly malignant (intraductal, acinar, ductal and basal cell carcinoma) lesions can present with a cribriform morphology. These entities display a different clinical course and variability in clinical management and prognosis. Thus, cribriform morphology can cause a diagnostic challenge for the pathologist. In addition, even though cribriform morphology in benign entities is probably the result of cells with glandular differentiation pilling up within a pre-existing space (duct), in malignant entities, it may be a purposeful architectural pattern that offers a survival advantage to cancer cells, as it has been associated with adverse prognosis and therapy resistance.
Overall, the aim of this entry is to summarize the current knowledge regarding the morphological features of cribriform formations within the prostate gland, their differential diagnosis and the utility of immunohistochemical markers (i.e., PTEN loss and ERG expression) in establishing an accurate diagnosis. Areas of controversy regarding the clinical significance and/or management of cribriform entities will also be discussed. In Table 1, the most important morphologic and molecular features of these entities are summarized.
Table 1. Summary of differential diagnosis of entities with cribriform morphology within the prostate gland.
| Entity | Architecture | Cytologic Features | Basal Cell Layer | ERG Expression | PTEN Loss |
|---|---|---|---|---|---|
| Benign cribriform glands | Complex epithelium with cribriform pattern and epithelial bridges located in the central zone | High columnar stratified epithelium, granular cytoplasm, small round nuclei without cytologic atypia or prominent nucleoli | Intact | − | − |
| Basal cell hyperplasia | Nodular lesion, within the transitional zone | Scant cytoplasm, hyperchromatic nuclei without cytologic atypia | The lesion involves basal cells | − | − |
| Clear cell cribriform hyperplasia | Variant of BPH, medium and large sized acini with cribriform morphology | Pale to clear cytoplasm, nuclei lack cytologic atypia or prominent nucleoli | Intact | − | − |
| HGPIN | Normal-sized acini with tufting, micropapillary, or flat growth pattern and without expansion of glands | Cytologic atypia, nuclear enlargement and hyperchromasia with prominent nucleoli, no necrosis | Preserved (can be fragmented) | −/+ | − |
| AIDCP | Loose cribriform lumen-spanning architecture | Moderate nuclear atypia, absence of necrosis, insufficient to meet the criteria for IDC | Preserved (can be fragmented) | +/− | Identified |
| Intraductal carcinoma | Greatly expanded glands with cribriform/solid growth | Nuclear atypia (nuclear enlargement, hyperchromatic nuclei) may be present | Preserved (can be fragmented) | +/− | Identified |
| Invasive cribriform acinar carcinoma | Continuous proliferation of cells with intermingled lumina | Nuclear atypia (prominent nucleoli, hyperchromasia) | Absent | +/− | Identified |
| Ductal carcinoma | Papillary, solid and cribriform growth pattern | Tall columnar cells, nuclear atypia, mitotic figures | Usually absent, may be present | −/+ | Identified |
| Basal cell carcinoma | Irregular cribriform formations containing mucin or basement membrane-like material within the lumina, desmoplastic reaction | Hyperchromatic large nuclei with scant cytoplasm | The entire process involves basal cells | − | Identified |
2. Benign Cribriform Formations
The cribriform pattern is commonly observed in normal tissue and benign processes of the prostate gland. Although these entities can become potential mimickers of atypical and even malignant lesions, under no circumstances must they be confused with cancer, as they have no significant clinical implications. Their features are discussed below.
2.1. Normal Cribriform Glands
The epithelial tissue of the central zone of the prostate gland near its base can form cribriform glandular structures [1]. These structures are composed of complex papillary epithelium with cribriform morphology, Roman Arches and epithelial bridges [1][2] lined by columnar, pseudostratified epithelial cells with granular eosinophilic cytoplasm and a small round nucleus. Of note, the cytologic atypia or prominent nucleoli are consistently absent. In addition, the presence of an intact and frequently prominent basal cell layer is characteristic and can help to identify the benign nature of the lesion (Figure 1a). This totally normal cribriform morphology has been well understood and is easily distinguishable from other cribriform lesions of the prostate gland—albeit, its differential diagnosis may be more challenging in biopsies.
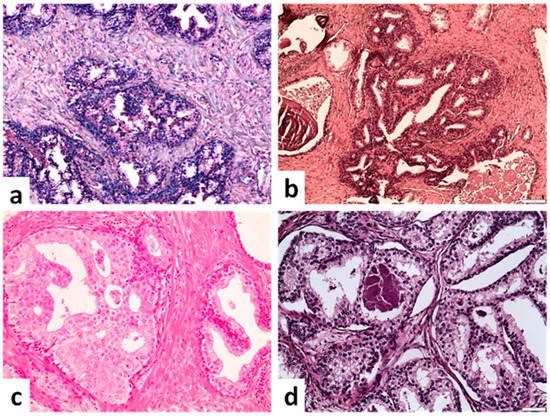
Figure 1. Benign cribriform formations. (a) Glands in the base of the prostate with luminal tufting (upper part) and nuclear pseudostratification. A cribriform architectural pattern is observed, but lack of cytologic atypia and presence of basal cell layer excludes malignancy (×100). (b) Basal cell hyperplasia with a cribriform pattern (×100). (c) Hyperplastic cribriform gland lacking cytologic atypia and displaying abundant pale to clear cytoplasm similar to acinar cells of adjacent prostatic gland (right side) (×200). (d) Clear cell cribriform hyperplasia with bland monotonous nuclei (×200) (scale bar is 50 μm).
2.2. Basal Cell Hyperplasia
Basal cell hyperplasia constitutes a focal, nodular process composed of an expansion of basal cells within the acini of prostate gland [3]. Small clusters of proliferating round basal cells that are two or more layers in thickness are formed within a compressed stroma. These cell clusters may grow in a solid, cystically dilated or cribriform pattern [3][4]. The cribriform architectural pattern is composed of irregular round luminal spaces. Cytologically, basal cells have scant cytoplasm with hyperchromatic nuclei and lack cytologic atypia (Figure 1b). Immunohistochemically, the cells in basal cell hyperplasia are positive for basal cell markers (p63, HMWCK) and negative for AMACR [3]. Basal cell hyperplasia is not associated with an adverse prognosis or a higher risk of prostate cancer.
2.3. Clear Cell Cribriform Hyperplasia
Clear cell cribriform hyperplasia represents a rare variant in the histologic spectrum of benign nodular prostatic hyperplasia (BPH), is typically located in the transitional zone and is composed of a nodular cluster of medium- and large-sized acini with cribriform morphology. The hyperplastic epithelial cells have a pale-to-clear granular cytoplasm, and their nuclei lack cytologic atypia or prominent nucleoli. A clearly visible basal cell layer is always present (Figure 1c,d). Cribriform hyperplasia does not constitute a risk factor for prostate cancer and the treatment is the same as in BPH.
As far as a differential diagnosis is concerned, the normal and benign cribriform formations described above may be confused with cribriform adenocarcinoma, intraductal carcinoma (IDC) and high-grade prostatic intraepithelial neoplasia (HGPIN), especially when a prostate needle biopsy specimen is evaluated. In this case, the benign cytologic features (absence of prominent nucleoli and lack of cytologic atypia) and the presence of a prominent and continuous basal cell layer support the diagnosis of a benign entity. In addition, the similarity in cellular morphology between the cribriform structure in question and adjacent normal acini is helpful to confirm the non-malignant nature of these cribriform glands.
3. Premalignant Cribriform Lesions
High-Grade Prostatic Intraepithelial Neoplasia (HGPIN)
HGPIN is regarded as a precursor lesion to invasive adenocarcinoma. This lesion shares phenotypic and genetic alterations with invasive carcinoma but lacks invasion into the fibromuscular stroma [5]. In HGPIN, luminal epithelial growth takes place within ducts/glands of normal size without expansion of their lumina. This lack of expansion of the involved glandular spaces is contrasted to intraductal carcinoma which typically involves expanded acini and ducts. Cytologic atypia is present and includes nuclear enlargement, hyperchromatic nuclei, prominent nucleoli, slightly amphophilic cytoplasm, and nuclear stratification. Necrosis is absent and the basal cell layer is preserved, although it can be fragmented (Figure 2a). Four main growth patterns were initially described in HGPIN: tufting, micropapillary, cribriform, and flat [6]. However, the cribriform pattern is no longer considered acceptable in HGPIN. Lesions with a loose cribriform pattern and no necrosis or significant pleomorphism are considered atypical cribriform lesions (see below), whereas lesions with dense cribriform pattern and/or necrosis or nuclear enlargement/pleomorphism are diagnosed as IDC.
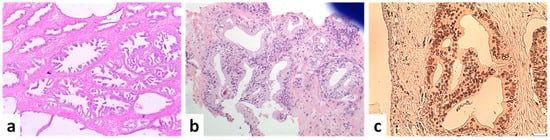
Figure 2. Preneoplastic and borderline neoplastic cribriform lesions. (a) Multiple foci of high-grade PIN (×40). (b) Atypical intraductal cribriform proliferation in a biopsy. Proliferation is present in <50% of the gland, located at the edge of the core and not accompanied by atypia or necrosis (×100). (c) Atypical intraductal cribriform proliferation. An expanded gland with a loose cribriform architecture is observed. Immunohistochemical expression of the marker ERG is suggestive of the malignant nature of neoplastic cells but is not enough to change the diagnosis to IDC (×100) (scale bar is 50 μm).
The distinction of HGPIN from IDC is particularly important, especially in biopsies, as the two entities have different clinical implications and a different subsequent approach is followed for each of them [7][8]. The distinction is usually straightforward because, as mentioned above, dense cribriform architecture, nuclear pleomorphism and necrosis are not features that are consistent with the diagnosis of HGPIN. Gland expansion, the involvement of >6 glands, presence of an irregular contour and identification of a biphasic cell population favor IDC [8][9]. In cases where it is difficult to distinguish HGPIN from IDC morphologically (i.e., in flat lesions with moderate nucleomegaly), immunohistochemistry can be of use; ERG expression and PTEN loss is observed in IDC, whereas ERG is less frequently expressed and PTEN expression is preserved in HGPIN [7][10][11][12].
4. Cribriform Lesions with Borderline Clinical Significance
Atypical Intraductal Cribriform Proliferation (AIDCP)
Atypical intraductal cribriform proliferation (AIDCP) is a relatively new term that describes a cribriform lesion with a phenotype more atypical than HGPIN but without meeting all the diagnostic features of IDC. The characteristics of AIDCP on needle biopsies include (a) loose cribriform lumen-spanning architecture beyond that of HGPIN, but lacking significant nuclear pleomorphism or necrosis to meet the criteria for IDC; (b) atypical nuclei with significant pleomorphism but insufficient for a diagnosis of IDC; and/or (c) dense cribriform or solid proliferation of atypical cells partially present in large ducts on the edge of core biopsy specimens (Figure 2b,c) [10]. Basal cells are retained in AIDCP.
The differential diagnosis of AIDCP from HGPIN is usually straightforward, as cribriform architecture is no longer permissible in HGPIN. AIDCP is also easily distinguished from invasive cribriform carcinoma by the presence of basal cells in the case of the former. Immunohistochemistry can help to confirm the presence of basal cells in difficult cases.
The most problematic differential diagnosis is its distinction from IDC. Immunohistochemistry for basal cell markers is not helpful in this setting as both entities show a present, albeit in some cases fragmented, basal cell layer. Similarly, PTEN loss and ERG expression are not helpful as AIDCP and IDC have shown a similar pattern of PTEN and ERG expression [10]. The distinction is, thus, based on morphology and the discriminative features are more a matter of quantity, rather than their presence or absence. When architecture is not dense and nuclear atypia is not high enough to make an IDC diagnosis, then the cribriform lesion is called AIDCP. In researchers' experience, AIDCP commonly coexists with IDC in prostatectomy specimens, meaning that adjacent to ducts with typical features of IDC are ducts with an intraductal proliferation that is very similar to, but falls short of, IDC diagnosis. Whether the criteria for IDC need to be loosened in those cases to include all cribriform intraductal proliferations remains to be determined. This, however, has mainly academic interest, as areas with definite IDC are usually present as are areas with invasive carcinoma and the nature (and nomenclature) of the not-typical-for-IDC cribriform formations will not have any significant implications for the patient’s further management.
From a practical standpoint, the diagnosis of AIDCP has important clinical implications in biopsy specimens as, when isolated, this has correlated with an increased probability of the presence of an invasive carcinoma in repeat biopsy [10][13]. For this reason, the presence of isolated AIDP in biopsies should be treated with close surveillance and re-biopsy and should not be considered as HGPIN [7][11][14]—an entity that does not usually justify a biopsy repeat.
References
- Srodon, M.; Epstein, J.I. Central zone histology of the prostate: A mimicker of high-grade prostatic intraepithelial neoplasia. Hum. Pathol. 2002, 33, 518–523.
- McNeal, J.E. Prostate. In Histology for Histopathologists; Mills, S.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007.
- Hosler, G.A.; Epstein, J.I. Basal cell hyperplasia: An unusual diagnostic dilemma on prostate needle biopsies. Hum. Pathol. 2005, 36, 480–485.
- Rioux-Leclercq, N.C.; Epstein, J.I. Unusual Morphologic Patterns of Basal Cell Hyperplasia of the Prostate. Am. J. Surg. Pathol. 2002, 26, 237–243.
- Bostwick, D.; Montironi, R.; Sesterhenn, I.A. Diagnosis of Prostatic Intraepithelial Neoplasia: Prostate Working Group 1 Consensus Report. Scand. J. Urol. Nephrol. 2000, 34, 3–10.
- Montironi, R.; Mazzucchelli, R.; Lopez-Beltran, A.; Scarpelli, M.; Cheng, L. Prostatic intraepithelial neoplasia: Its morphological and molecular diagnosis and clinical significance. BJU Int. 2011, 108, 1394–1401.
- Hickman, R.A.; Yu, H.; Li, J.; Kong, M.; Shah, R.B.; Zhou, M.; Melamed, J.; Deng, F.M. Atypical intraductal cribriform proliferations of the prostate exhibit similar molecular and clinicopathologic characteristics as intraductal carcinoma of the prostate. Am. J. Surg. Pathol. 2017, 41, 550–556.
- Magers, M.; Kunju, L.P.; Wu, A. Intraductal carcinoma of the prostate morphologic features, differential diagnoses, significance, and reporting practices. Arch. Pathol. Lab. Med. 2015, 139, 1234–1241.
- Cohen, R.J.; Wheeler, T.M.; Bonkhoff, H.; Rubin, M.A. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch. Pathol. Lab. Med. 2007, 131, 1103–1109.
- Morais, C.L.; Han, J.S.; Gordetsky, J.; Nagar, M.S.; Anderson, A.E.; Lee, S.; Hicks, J.L.; Zhou, M.; Magi-Galluzzi, C.; Shah, R.B.; et al. Utility of PTEN and ERG Immunostaining for Distinguishing High-grade PIN From Intraductal Carcinoma of the Prostate on Needle Biopsy. Am. J. Surg. Pathol. 2015, 39, 169–178.
- Lotan, T.L.; Gumuskaya, B.; Rahimi, H.; Hicks, J.L.; Iwata, T.; Robinson, B.D.; Epstein, J.I.; De Marzo, A.M. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod. Pathol. 2013, 26, 587–603.
- Shah, R.B.; Yoon, J.; Liu, G.; Tian, W. Atypical intraductal proliferation and intraductal carcinoma of the prostate on core needle biopsy: A comparative clinicopathological and molecular study with a proposal to expand the morphological spectrum of intraductal carcinoma. Histopathology 2017, 71, 693–702.
- Jiang, H.; Zhou, Z.; Jin, S.; Xu, K.; Zhang, H.; Xu, J.; Sun, Q.; Wang, J.; Xu, J. PRMT9 promotes hepatocellular carcinoma invasion and metastasis via activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Sci. 2018, 109, 1414–1427.
- Divatia, M.K.; Ro, J.Y. Ymj-57-1054. Yonsei Med. J. 2016, 57, 1054–1062.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
24 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




