Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tauqeer Hussain Mallhi | -- | 2745 | 2022-08-23 14:10:08 | | | |
| 2 | Conner Chen | Meta information modification | 2745 | 2022-08-24 08:42:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H.; et al. Viral Vectors for Gene Delivery. Encyclopedia. Available online: https://encyclopedia.pub/entry/26397 (accessed on 05 March 2026).
Butt MH, Zaman M, Ahmad A, Khan R, Mallhi TH, Hasan MM, et al. Viral Vectors for Gene Delivery. Encyclopedia. Available at: https://encyclopedia.pub/entry/26397. Accessed March 05, 2026.
Butt, Muhammad Hammad, Muhammad Zaman, Abrar Ahmad, Rahima Khan, Tauqeer Hussain Mallhi, Mohammad Mehedi Hasan, Yusra Habib Khan, Sara Hafeez, Ehab El Sayed Massoud, Md. Habibur Rahman, et al. "Viral Vectors for Gene Delivery" Encyclopedia, https://encyclopedia.pub/entry/26397 (accessed March 05, 2026).
Butt, M.H., Zaman, M., Ahmad, A., Khan, R., Mallhi, T.H., Hasan, M.M., Khan, Y.H., Hafeez, S., Massoud, E.E.S., Rahman, M.H., & Cavalu, S. (2022, August 23). Viral Vectors for Gene Delivery. In Encyclopedia. https://encyclopedia.pub/entry/26397
Butt, Muhammad Hammad, et al. "Viral Vectors for Gene Delivery." Encyclopedia. Web. 23 August, 2022.
Copy Citation
Over the course of millions of years, viruses have evolved and adapted to changes in the biological environment which has allowed them to survive and replicate in host cells. Using this feature of viruses, gene therapy research has developed new approaches utilizing viruses and their different genomes as carriers and vectors for the delivery of genes, nucleic acids, and other genetic material to cell target sites.
gene delivery
viral vectors
gene therapy
pharmacogenetics
pharmacogenomics
pharmacy
1. Viral Vectors for Gene Delivery
Over the course of millions of years, viruses have evolved and adapted to changes in the biological environment which has allowed them to survive and replicate in host cells. Using this feature of viruses, gene therapy research has developed new approaches utilizing viruses and their different genomes as carriers and vectors for the delivery of genes, nucleic acids, and other genetic material to cell target sites. The major advantage that the use of viral vectors has brought to gene therapy is their ability to protect transgenes from biological degradation and the capability to efficiently cross cellular barriers. The first clinical trial conducted on gene therapy in 1999 utilized viral vectors for the treatment of severe combined immunodeficiency disorder and proved to be successful. According to The Journal of Gene Medicine, up until November 2017, more than 68% of the clinical trials conducted on gene therapy utilized viruses as vectors for gene delivery [1]. The promising use of viral vectors in gene therapy has recently been backed by the approval of several gene products based on viral vectors by the US FDA. Many more products using viruses as delivery vehicles are in the phases of clinical trials or process of approval.
Although the broad application of viral vectors in gene therapy has led to the belief that they are safe for human use despite their infection potential, their harmful nature is still of a major safety concern, which is important to address while developing gene therapies. For this purpose, scientists have come up with some engineering strategies that are aimed at enhancing the safe use of viral vectors without limiting their efficiency. These include avoidance of viral replication, promotion of viral inactivation, and attenuation of natural toxicity of viruses [2].
Another feature of viruses that makes them suitable for gene delivery is their occurrence in a wide range of types and species that have varying properties of size, morphology, type of genetic material, and natural tropism, allowing more variety to choose from as per the requirements of the specific gene therapy. There are several criteria on the basis of which viruses can be classified. These include the presence of envelope, symmetry of viral capsid, nature of viral genetic material, i.e., DNA or RNA, replication site of the virus, i.e., nucleus or cytoplasm, and virion size [3].
The choice of a specific viral vector for gene therapy is made by keeping in view the above criteria of viral classification and the advantages and disadvantages of viral vectors in order to ensure efficient gene delivery. Some advantages and disadvantages of viral vectors in gene delivery are mentioned in Table 1 [4].
Table 1. Advantages and disadvantages of viral vectors.
| Advantages | Disadvantages |
|---|---|
| Provide greater gene transfer efficiency in both in vivo and in vitro environments | Can trigger severe immune responses and inflammatory reactions |
| Persist for longer periods of time in most cases | Their cloning capacity is very limited |
| Can target a large number of cells | Produced by complex production methods |
| A large variety of viruses are available to choose from | Low capability of tropism to some specific target cells |
| Innate ability of tropism toward infection | Can cause mutagenesis by inserting their exogenous DNA into the host genome |
| Capable of evading endosomes by various mechanisms learned by evolution of viruses | Research is needed to further understand the mechanisms of molecular infection by viruses |
The mechanism of viral gene delivery (Figure 1) starts with the incorporation of the transgene into the viral DNA, and then this modified DNA is injected into the viral vector. This vector, upon reaching the target site, attaches to the receptors found on the cell membrane of the target cells. After cellular internalization, the vector is packed into endosomes, followed by an acid breakdown of these endosomes that release the capsid containing the modified DNA. This capsid then travels to the nucleus and binds to nuclear pores to enter the nucleus, where the modified gene is integrated into the DNA of the target cell. After that, transcription and translation occur, which form the protein of interest and bring about gene expression [5]. This mechanism is followed by Lentiviruses and most Retroviruses [6]. However, some viral vectors do not bring about gene delivery by integration into host genome such as Adenoviruses; they simply deliver the genetic material into the cytoplasm or nucleus, and transgene expression occurs from there [7].
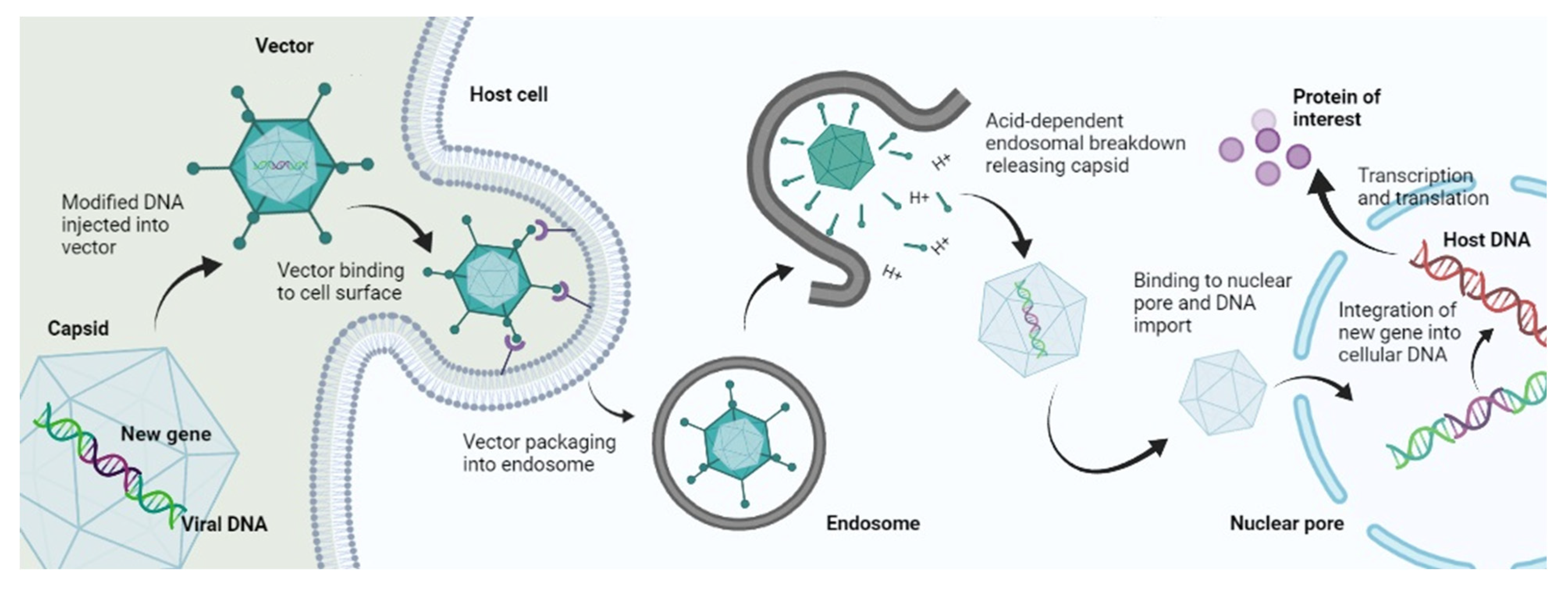
Figure 1. Mechanism of viral gene delivery.
The different types of viral vectors used in gene therapy are further explained below.
2. Adenoviral Vectors
Adenoviruses are among viruses that were studied first and foremost for the purpose of gene therapy. They were proposed to be used as gene delivery vectors about 20 years ago [8]. They contain a DNA genome that is double-stranded and has a size of 35 kb. They are nonenveloped viruses. Attenuation of adenoviruses is achieved by deletion of fragments of their genome that specifically code for early proteins. There are three generations of adenoviral vectors that are based on the level of attenuation achieved by the deletion of genes. In the first-generation adenoviral vectors, the E1A and E1B genes are deleted. In the second-generation adenoviral vectors, a large number of the early genes are deleted. In third-generation adenoviral vectors, the complete genome or genetic information of the virus is deleted, which is why they are also called gutless vectors [9].
The adenoviral genome is quite large in size, and the prospect of complete genome deletion greatly renders these viruses a high coding capacity. First-generation vectors can allow ~3.2 kb of genome insertion, while third-generation vectors allow up to 30 kb. An advantage of adenoviral vectors is that there is a very negligible possibility of integration of their genome into the genome of the host cell, which makes them rather safe and nontoxic for use. However, long or sustainable gene expression is difficult to achieve with adenoviruses because their life cycle is not adapted to it [10].
Originally, scientists believed that adenoviruses could serve as vectors for a large variety of therapies ranging from gene delivery to hereditary disorders and regenerative therapy. However, it turned out that several toxic properties of adenoviruses render them unsuitable for this purpose. These include their ability to trigger severe immune responses due to their highly immunogenic capsids. They are also more vulnerable to attachment with blood-circulating proteins, ligands, and other blood cells, which can cause viral inactivation and hinder the system delivery of transgenes [11]. Moreover, if these viruses are administered via a systemic route in large doses, then they can result in a severe inflammatory response which can be life-threatening. This toxic potential of these viruses has limited their use as vectors in gene therapy where local administration of transgene is required, for example, against malignant tumors [12].
Despite these disadvantages of adenoviral vectors, researchers have devised new ways of modifying adenoviral vector systems to improve their gene transfection ability. These include extensive global genome modification with the deletion of almost all genes except those required for replication and packaging. This reduces the toxicity of adenoviral vectors, rendering them with a very high cloning capacity, i.e., ~36 kb, and the ability to deliver multiple transgenes or genomic loci at a time. The formed adenoviral vectors are called helper-dependent or high-capacity adenoviral vectors (HAdVs). Several studies reported a very prolonged transgene expression and high reduced immunogenicity of adenoviral vectors by employing this technique [13][14][15]. However there are several challenges associated with the use of HAdVs for gene transfer which include increased immunogenicity, transient expression of transgene, triggering of potent immune and inflammatory reactions, and pre-existing immunity in cancer patients [16][17]. Researchers have, however, developed some ways of overcoming these challenges which include altering the tropism of HAdVs, preparation of vector chimeras, and combination immunotherapy treatments [18][19][20][21].
3. Retroviruses and Lentiviruses
These are RNA viruses, the replication of which is based on reverse transcription of RNA to DNA followed by its integration into the host genome. Earlier in the 1990s, retroviruses were studied for their potential to be used in gene therapy for the treatment of diseases caused by defects in a single gene and not a segment of a genome. An example of this is the use of these viruses in gene therapy for the treatment of severe combined immunodeficiency caused by a problem in the gene that codes for the enzyme adenosine deaminase [22]. For this purpose, the γ-retrovirus murine leukemia (MLV) virus was used as a vector. The specificity of retroviruses to integrate at a specific portion of the host genome resulted in their choice as vectors being a failure. This is because MLV had a different genome insertion focal point than the target, which led to oncogene expression leading to genotoxicity and the development of leucosis in five out of 20 patients who participated in the clinical trial. To overcome this problem, another group of retroviruses called lentiviruses was explored for their potential to be used as vectors in gene therapy. The genomic insertion point of these viruses was different from MLV vectors; thus, they showed a decreased occurrence of genotoxicity [23][24].
Retroviruses have various benefits over different vectors. The main benefit that retroviral vectors offer is their capacity to change their ssRNA genome into a dsDNA particle that steadily incorporates into the genome of its host cells. This element empowers the retroviral vectors to alter the nuclear genome of host cells and bring about gene expression [25][26]. At present, retroviral vector-mediated gene therapy has been revived with the improvement of a new retroviral vector class called Lentiviruses (LV). The LV have the special capacity among RV to taint noncycling cells. Vectors obtained from LV have given a significant jump in gene editing and gene transfer, and they present new roads to accomplish huge degrees of gene delivery in vivo. Lentiviruses include the human immunodeficiency virus HIV. Although lentiviruses show a much lower extent of mutagenesis than MLV retrovirus vectors, their safety level is still of great concern for use as vectors in gene delivery [27][28]
Control levels for the utilization of retroviruses are resolved in light of the cell types they infect. BSL-1 is fitting for RV that do not contaminate human cells. BSL-2 is important if they are utilized to contaminate human cells [29]. The essential danger with the utilization of RV emerges from their capacity to coordinate into the host cell chromosome, which raises the chance of insertional mutagenesis and oncogene initiation [30]. Formation of RV capable of replicating in target cells or tissues is the essential disadvantage connected with the utilization of retroviral vectors. Appraisal of this chance is basic in deciding the security related with the utilization of retroviral vector frameworks. Furthermore, the scope of the target cell accession of the vector is also a security issue [31].
Use of a viral envelope that can infect cells from numerous species increases both the risk of forming RV capable of replicating and the likely risk of any subsequent infection, which could spread from one animal varieties to another. Future examinations that use retroviral vectors in quality treatment tests should investigate more secure methodologies that center around the dangers related with in vivo recombination, age of mosaic RV, and storage of viral genetic data for longer periods of time [32].
The utilization of LVVs in research is as yet connected with potential risks, and the drawn-out security of these clinical mediations is as yet being assessed. While lentiviral frameworks are obtained from HIV, their association across various plasmids and the erasure of numerous HIV proteins brings down the probability of creating a replication-competent virus. These vector frameworks are dealt with at BSL-2 [33]. The impediments of involving LVVs in preclinical trials today are for the most part because of deficient strategies for the creation of high-titer infection stocks and the security concerns connected with their HIV origin, notwithstanding the designing of packaging cell lines and erasures of viral replication genes. One way to deal with these security issues has been to foster LV vectors unequipped for replication in human cells. Despite the fact that LVVs are less connected with insertional mutagenesis than other RV, these vectors actually give proof of off-target effects [34].
4. Adeno-Associated Viruses
Belonging to the viral family of Parvoviridae, adeno-associated viruses (AAV) are DNA viruses having a single strand, which are small and nonenveloped. These viruses are nonautonomous, which makes them incapable of replicating when adenovirus is not present. Naturally, these viruses do not integrate into host cell genomes and remain inactive after infecting humans. AAV genomes integrate into the host’s genome in 0.1% of the cases via insertion into a specific portion of chromosome 19. Vectors based on these viruses have not yet been shown to cause genotoxicity because of the lack of insertion of viral genome into the host cell genome. However, this property has resulted in a side-effect against use as gene delivery vectors, i.e., the level of transgene expression is reduced in dividing cells where the AAV genome is decreased. However, this property has allowed them to be used for gene therapy where target cells are slow-dividing, such as cardiomyocytes [35].
These viruses also have a less immunogenic and toxic capsid as compared to other types of viral vectors such as adenoviruses or poxviruses. AAV shows a negligible immune response upon systemic administration and is stable in blood to a great extent. The low level of AAV vector side-effects and decreased toxicity potential have led to them becoming the safest viral vectors for gene therapy that can provide a good transgene expression [36].
5. Poxviruses
Belonging to the Poxviridae family of viruses, they are the most complicated and largest of all viruses that infect humans and cause diseases. Their genome is double-stranded and has a size of approximately 180–220 kb. The smallpox virus is the most popular virus belonging to this family and represents its group very well. The vaccinia virus belonging to the poxvirus family is the virus which was used for the development of smallpox vaccine. There are two features unique to this virus: its capability to carry out its life cycle in the cell cytoplasm due to which it does not insert its genome into the host cell genome, and its occurrence in two different infectious variants i.e., an intracellular mature virus (IMV) and an extracellular enveloped virus (EEV) [37].
The efficient life cycle of the vaccinia virus allows its use in gene therapy for cancer. Moreover, the virus also has a reasonable cloning capacity. Its capacity is up to 25 kb if no part of the viral genome is removed, and this capacity can increase up to 75 kb if some parts of the viral genome are deleted. However, the complexity of the viral structure and genome makes it difficult to be used in gene therapy. Nevertheless, there are several ongoing clinical trials of recombinant vaccinia viral vectors for oncolytic gene therapy [38].
6. Other Virus
Most human, animal, and bird viruses can now be subjected to genetic engineering and modifications of the viral genome, collectively called reverse genetics. This property makes them feasible for genome changes and engineering for transgene delivery in gene therapy. Some herpes viruses are being used in treatment of gene-related CNS disorders due their property of being neurotropic [39]. Moreover, some baculoviruses that belong to the Baculoviridae family are also being explored for their potential to be used as vectors in gene delivery. These viruses possess a reasonable cloning capacity of about 38 kb, and they allow insertion of about 100 kb of genomic material in their capsid. These viruses also do not replicate in mammalian cells, which reduces their risk of causing toxicity. Several studies are exploring the use of these viruses for gene delivery in treatment of certain lymphomas [40].
Several vector-shielding strategies have been devised by researchers to protect the viral vectors from interacting with blood components and causing unwanted immunogenic reactions. One of them includes chemical capsid modification with compounds, e.g., thiol-directed genetic capsid modification. Others include attachment of adapter molecules for targeting purposes, introduction of cysteine moieties or peptides in hexon or fiber of viral capsid, introduction of point mutations, or fiber pseudotyping with whole fiber or knob of different serotype. These techniques have proven to be successful in reducing liver tropism and immunogenicity potential of viral vectors, most importantly adenoviral vectors [41].
References
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015.
- Lundstrom, K. Viral vectors in gene therapy. Diseases 2018, 6, 42.
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral vectors for gene therapy: Translational and clinical outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89.
- Lukashev, A.; Zamyatnin, A. Viral vectors for gene therapy: Current state and clinical perspectives. Biochemistry 2016, 81, 700–708.
- Ju, D.; Santos, A.; Freeman, A.; Daniele, E. Neuroscience; eCampus: Toronto, ON, Canada, 2018.
- Desfarges, S.; Ciuffi, A. Viral integration and consequences on host gene expression. In Viruses: Essential Agents of Life; Springer: Berlin/Heidelberg, Germany, 2012; pp. 147–175.
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53.
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on adenoviruses: Epidemiology, pathogenicity, and gene therapy. Biomedicines 2019, 7, 61.
- Giacca, M. Introduction to gene therapy. In Gene Therapy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–7.
- Tsai, S.; Schillinger, K.; Ye, X. Adenovirus-mediated transfer of regulable gene expression. Curr. Opin. Mol. Ther. 2000, 2, 515–523.
- Lyons, M.; Onion, D.; Green, N.K.; Aslan, K.; Rajaratnam, R.; Bazan-Peregrino, M.; Phipps, S.; Hale, S.; Mautner, V.; Seymour, L.W. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006, 14, 118–128.
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.-p.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158.
- Bandara, R.A.; Chen, Z.R.; Hu, J. Potential of helper-dependent Adenoviral vectors in CRISPR-cas9-mediated lung gene therapy. Cell Biosci. 2021, 11, 145.
- Cao, H.; Ouyang, H.; Laselva, O.; Bartlett, C.; Zhou, Z.P.; Duan, C.; Gunawardena, T.; Avolio, J.; Bear, C.E.; Gonska, T. A helper-dependent adenoviral vector rescues CFTR to wild-type functional levels in cystic fibrosis epithelial cells harbouring class I mutations. Eur. Respir. J. 2020, 56, 2000205.
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-capacity adenoviral vectors: Expanding the scope of gene therapy. Int. J. Mol. Sci. 2020, 21, 3643.
- Singh, S.; Kumar, R.; Agrawal, B. Adenoviral vector-based vaccines and gene therapies: Current status and future prospects. Adenoviruses 2019, 4, 53–91.
- Chandler, R.J.; Venditti, C.P. Gene therapy for metabolic diseases. Transl. Sci. Rare Dis. 2016, 1, 73–89.
- Pereboev, A.V.; Nagle, J.M.; Shakhmatov, M.A.; Triozzi, P.L.; Matthews, Q.L.; Kawakami, Y.; Curiel, D.T.; Blackwell, J.L. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol. Ther. 2004, 9, 712–720.
- Stoff-Khalili, M.A.; Rivera, A.A.; Stoff, A.; Michael Mathis, J.; Rocconi, R.P.; Matthews, Q.L.; Numnum, M.T.; Herrmann, I.; Dall, P.; Eckhoff, D.E. Combining high selectivity of replication via CXCR4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer. Int. J. Cancer 2007, 120, 935–941.
- Matthews, Q.L.; Sibley, D.A.; Wu, H.; Li, J.; Stoff-Khalili, M.A.; Waehler, R.; Mathis, J.M.; Curiel, D.T. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol. Imaging 2006, 5, 7290.2006.00029.
- Tang, Y.; Le, L.P.; Matthews, Q.L.; Han, T.; Wu, H.; Curiel, D.T. Derivation of a triple mosaic adenovirus based on modification of the minor capsid protein IX. Virology 2008, 377, 391–400.
- Cattoglio, C.; Pellin, D.; Rizzi, E.; Maruggi, G.; Corti, G.; Miselli, F.; Sartori, D.; Guffanti, A.; Di Serio, C.; Ambrosi, A. High-definition mapping of retroviral integration sites identifies active regulatory elements in human multipotent hematopoietic progenitors. Blood J. Am. Soc. Hematol. 2010, 116, 5507–5517.
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003, 300, 1749–1751.
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142.
- Ghosh, S.; Brown, A.M.; Jenkins, C.; Campbell, K. Viral vector systems for gene therapy: A comprehensive literature review of progress and biosafety challenges. Appl. Biosaf. 2020, 25, 7–18.
- Vogt, P.K. Retroviral oncogenes: A historical primer. Nat. Rev. Cancer 2012, 12, 639–648.
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Ponzoni, M.; Bartholomae, C.; Sergi, L.S.; Benedicenti, F.; Ambrosi, A.; Di Serio, C. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006, 24, 687–696.
- Ramezani, A.; Hawley, R.G. Overview of the HIV-1 lentiviral vector system. Curr. Protoc. Mol. Biol. 2002, 60, 16.21. 11–16.21. 15.
- Collins, D.E.; Reuter, J.D.; Rush, H.G.; Villano, J.S. Viral vector biosafety in laboratory animal research. Comp. Med. 2017, 67, 215–221.
- Romano, G. Development of safer gene delivery systems to minimize the risk of insertional mutagenesis-related malignancies: A critical issue for the field of gene therapy. Int. Sch. Res. Not. 2012, 2012, 616310.
- Romano, G.; Marino, I.R.; Pentimalli, F.; Adamo, V.; Giordano, A. Insertional mutagenesis and development of malignancies induced by integrating gene delivery systems: Implications for the design of safer gene-based interventions in patients. Drug News Perspect. 2009, 22, 185–196.
- David, R.M.; Doherty, A.T. Viral vectors: The road to reducing genotoxicity. Toxicol. Sci. 2017, 155, 315–325.
- Rodríguez, A.S.S.; Montes, A.M.S.; Armendáriz-Borunda, J. Viral vectors in gene therapy. Advantages of the adenoassociated vectors. Rev. Gastroenterol. Mex. 2005, 70, 192–202.
- Schlimgen, R.; Howard, J.; Wooley, D.; Thompson, M.; Baden, L.R.; Yang, O.O.; Christiani, D.C.; Mostoslavsky, G.; Diamond, D.V.; Duane, E.G. Risks associated with lentiviral vector exposures and prevention strategies. J. Occup. Environ. Med. 2016, 58, 1159.
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451.
- Gao, G.-P.; Alvira, M.R.; Wang, L.; Calcedo, R.; Johnston, J.; Wilson, J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 11854–11859.
- Conrad, S.J.; Liu, J. Poxviruses as gene therapy vectors: Generating Poxviral vectors expressing therapeutic transgenes. In Viral Vectors for Gene Therapy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 189–209.
- Mell, L.K.; Brumund, K.T.; Daniels, G.A.; Advani, S.J.; Zakeri, K.; Wright, M.E.; Onyeama, S.-J.; Weisman, R.A.; Sanghvi, P.R.; Martin, P.J. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin. Cancer Res. 2017, 23, 5696–5702.
- Kantor, B.; Bailey, R.M.; Wimberly, K.; Kalburgi, S.N.; Gray, S.J. Methods for gene transfer to the central nervous system. Adv. Genet. 2014, 87, 125–197.
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a tool for gene delivery and gene therapy. Viruses 2018, 10, 510.
- Weklak, D.; Pembaur, D.; Koukou, G.; Jönsson, F.; Hagedorn, C.; Kreppel, F. Genetic and Chemical Capsid Modifications of Adenovirus Vectors to Modulate Vector–Host Interactions. Viruses 2021, 13, 1300.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
24 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





