Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilya Tsiklin | -- | 4715 | 2022-08-17 15:13:45 | | | |
| 2 | Camila Xu | -1 word(s) | 4714 | 2022-08-24 02:40:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsiklin, I.L.; Shabunin, A.V.; Kolsanov, A.V.; Volova, L.T. In Vivo Bone Tissue Engineering Advances. Encyclopedia. Available online: https://encyclopedia.pub/entry/26378 (accessed on 07 February 2026).
Tsiklin IL, Shabunin AV, Kolsanov AV, Volova LT. In Vivo Bone Tissue Engineering Advances. Encyclopedia. Available at: https://encyclopedia.pub/entry/26378. Accessed February 07, 2026.
Tsiklin, Ilya L., Aleksey V. Shabunin, Alexandr V. Kolsanov, Larisa T. Volova. "In Vivo Bone Tissue Engineering Advances" Encyclopedia, https://encyclopedia.pub/entry/26378 (accessed February 07, 2026).
Tsiklin, I.L., Shabunin, A.V., Kolsanov, A.V., & Volova, L.T. (2022, August 23). In Vivo Bone Tissue Engineering Advances. In Encyclopedia. https://encyclopedia.pub/entry/26378
Tsiklin, Ilya L., et al. "In Vivo Bone Tissue Engineering Advances." Encyclopedia. Web. 23 August, 2022.
Copy Citation
Bone tissue engineering (BTE) has emerged as a novel approach to guided bone tissue regeneration. BTE focuses on in vitro manipulations with seed cells, growth factors and bioactive scaffolds using bioreactors. The successful clinical translation of BTE requires overcoming a number of significant challenges.
bone tissue engineering
bone regeneration
scaffold
stem cells
1. Introduction
Critical-sized bone defects reconstruction remains a tremendous challenge for surgeons and a burden for the healthcare system worldwide. These defects cause considerable functional and cosmetic disorders, and negatively impact the quality of life [1][2]. Despite the huge armamentarium of surgical techniques, current bone repair strategies demonstrate significant limitations.
Among the ample variety of methods, the use of autologous bone grafts is the gold standard for bone defects reconstruction. Bone autografts demonstrate osteoinductive and osteoconductive properties due to their containing growth factors and recruitment of stem cells, however, their usage concerns donor-site morbidity, poor anatomical match, insufficient bone volume, and bone graft resorption [3][4].
Although vascularized bone flaps (e.g., fibula, scapula, or iliac crest) show a predictably high survival rate due to reliable blood supply, their harvesting can result in significant donor-site morbidity including chronic pain, lameness, hernia, ankle instability, etc. Moreover, such complications, as complete or partial flap failure, postoperative fistula, dehiscence, and bone exposure can occur [5][6][7]. Free flap harvest and revascularization substantially increase intraoperative blood loss volume, complexity, and duration of surgeries [8].
Allogeneic and xenogeneic bone graft materials are the most commonly-used alternatives to autologous bone. Along with off-the-shelf availability, human-derived or deproteinized bovine-derived bone substitutes have a long shelf life [9].
The development of computer 3D planning and prototyping methods contributed to the clinical application of patient-specific titanium and synthetic implants for extensive bone defects reconstructions [10][11]. Despite high-precision manufacturing and optimal anatomical match of the implants, the complication rate is significant. Complications predominantly include fractures, instability, extrusion, and rejection of implants. Furthermore, custom-made implants are associated with consequential financial costs [12][13][14].
Distraction osteogenesis has become particularly popular in treating patients with long bones defects; however, it is technically challenging and not widely used in craniofacial bone reconstruction due to the complex three-dimensional configuration of the defects and high surgical complications rate [15][16].
Therefore, it is of critical need for improved bone defects reconstruction methods nowadays. Current trends in bone reconstructive surgery include reducing functional donor-site morbidity, overcoming bone graft volume limitations, and improving the geometrical match of the graft for the recipient site. Bone tissue engineering (BTE) has emerged as a novel approach to bone defects repair and regeneration. This approach is based on in vitro manipulation of seed cells, growth factors and bioactive scaffolds using various bioreactors. The application of BTE is one of the promising trends for researchers globally due to recent advances in the development of various biocompatible scaffolds and cell technologies [17][18][19][20]. Currently, in vitro and in vivo experimental studies on BTE are widely presented, however, the routine clinical application is associated with certain limitations.
2. In Vivo Bone Tissue Engineering Advances
Although the above-presented critical components of BTE have been thoroughly investigated in recent decades, their ideal combination to provide predictable and controlled bone tissue regeneration process is still lacking. Current limitations of in vitro BTE, in particular poor vascularization, confirm the critical need for further development of this field. Flap prefabrication has emerged as a bridge between conventional reconstructive surgery and tissue-engineering. It is a promising strategy which allows to use precise customized flaps matching patient-specific needs [21]. Applied to bone reconstructive surgery flap prefabrication is a potential alternative to autologous bone graft harvest, that can significantly decrease donor-site morbidity.
2.1. Historical and Terminological Aspects of Flap Prefabrication
Historically, the term “prefabrication” first appeared in the record of house-building or manufacturing of ships and aircrafts. It initially means the assembling all the necessary components of a structure, and then transporting the assemblies to the site of construction [22]. The preliminary report first presented by Shen in 1981 included results of the experimental study and clinical application of the vascular pedicle implantation into the skin flap [23]. As a result, a skin flap of the desired location and design became axially vascularized. In 1994 Pribaz et al. introduced the concept of prevascularized flaps modification by implantation of tissue or other device into a flap prior to its local transposition or free distant transfer. Researchers of this concept first suggested the term “prelamination” [24]. The most common example of flap prelamination is the rib cartilage subcutaneous implantation at the forearm followed by later harvest and free transfer of the prelaminated forearm composite skin-cartilage flap for ear reconstruction [25][26][27]. Tan (2004) used the term “vascular induction” to describe the phenomenon of an axial blood supply introduction to create new transplantable tissue [25].
2.2. In Vivo Bioreactor Approach to BTE: Experimental Studies
In 2005 Stevens et al. first introduced the term “in vivo bioreactor” (IVB) as a new concept for in vivo BTE. According to this concept large volumes of bone can be created in a predictable way, without the need for cell transplantation and GF administration. In an experimental study in rabbits, researchers created a space between the surface of the long bone and the inner layer of the periosteum and filled this space with a biocompatible calcium-alginate gel. Radiographical and histological analysis of the bone harvested after a period of 6 weeks confirmed formation of the new bone tissue biomechanically identical to the native one. The researchers emphasized the crucial role of the pluripotent cells of the periosteum in the bone regeneration process [28].
Another historically important experimental study on IVB approach was conducted by Holt et al. in 2005. This research on a rat model presented in vivo ectopic bone formation by combining an axial vascularization and prefabrication of the hydroxyapatite scaffold with a capability of further vascularized tissue transfer. Researchers of this research demonstrated the creation of the rich vascular network inside the scaffold regardless of the administration of the BMP-2, however, supplementation of the BMP-2 could assumedly initiate pluripotent cells recruitment from circulating blood to new bone generation [29].
These two independent groundbreaking studies have become the starting point for further development of in vivo BTE strategies. Even though classical IVB, as it was underlined above, suggested the human body as the main source of progenitor cells and GF, many researchers presented multiple modifications of the IVB by means of scaffolds cell-seeding and supplementation with exogenous GF [30][31][32][33][34][35][36]. IVB has been investigated in variety of small and large animal models [35][36][37][38][39][40][41][42]. Akar et al. presented basic requirements for IVB preclinical models, including the use of clinically translatable surgical techniques, choosing implantation sites with high regenerative potential and low infection risk, allowing quantitative evaluation of results, and availability in a wide range of research centers [37]. The vast majority of these experimental studies present the combination of the IVB critical components, which can be represented as a following formula.
IVB = S + FP + AV
(S—scaffold; FP—flap prefabrication, AV—axial vascularization).
According to the above-mentioned IVB formula it is logical to review and discuss this approach as an inseparable combination of axial vascularization and flap prefabrication methods. However, AV, as a separate component, may be optional if axially vascularized flap is used for scaffold prefabrication. Prior to discussion of the IVB strategies presented in literature in recent years, it is worth highlighting current tendencies in choosing scaffolds for in vivo BTE purposes.
2.2.1. Scaffolds for In Vivo BTE
While reviewing multiple studies related to in vivo BTE, the spectrum of biocompatible materials most commonly used for in vivo BTE typically includes the bioceramic [30][31][33][37][43][44][45][46][47], allogeneic or xenogeneic bone-derived [35][39][40][41][48][49], and composite scaffolds [36][50][51]. This literature review evidently demonstrates the tendency to use mechanically stable materials with long controlled biodegradation rate for in vivo BTE purposes. Furthermore, some researchers present efforts to improve properties of the scaffolds by combining various bioactive materials. Thus, Abu-Shahba used biohybrid bone blocks consisting of bovine-derived bone matrix in combination with PCL biodegradable polymer and collagen fragments for surface activation and scaffold reinforcement [50]. Kuzmenka et al. presented sol-gel hybrid glass scaffold integrated with calcium sources with the aim to create a bioactive implant with long-lasting calcium release while preserving its mechanical properties [52].
2.2.2. In Vivo Vascularization and Prefabrication Strategies in BTE
Viability and growth support of the in vivo tissue engineered construct strictly depend on its constant and reliable blood supply. Despite the huge arsenal of in vitro angiogenesis methods, the lack of adequate vascularization remains the prevalent challenge and limitation in up-to-date BTE [47][53][54][55][56][57][58]. This fact resulted in the development of various in vivo vascularization strategies. Furthermore, local soft tissues at the bone defect site often have inappropriate quality and volume, and can be compromised due to cicatricial or post-radiation changes. According to this fact, ectopic bone graft prefabrication at the intact anatomic site can be an effective method to overcome such challenges.
The axial vascularization strategy has emerged as a powerful tool for rapid vascular network formation within a bioactive scaffold. This concept of vascularization has been expanded through the use of flap-based and vessel-based approaches [39][53] (Figure 1).
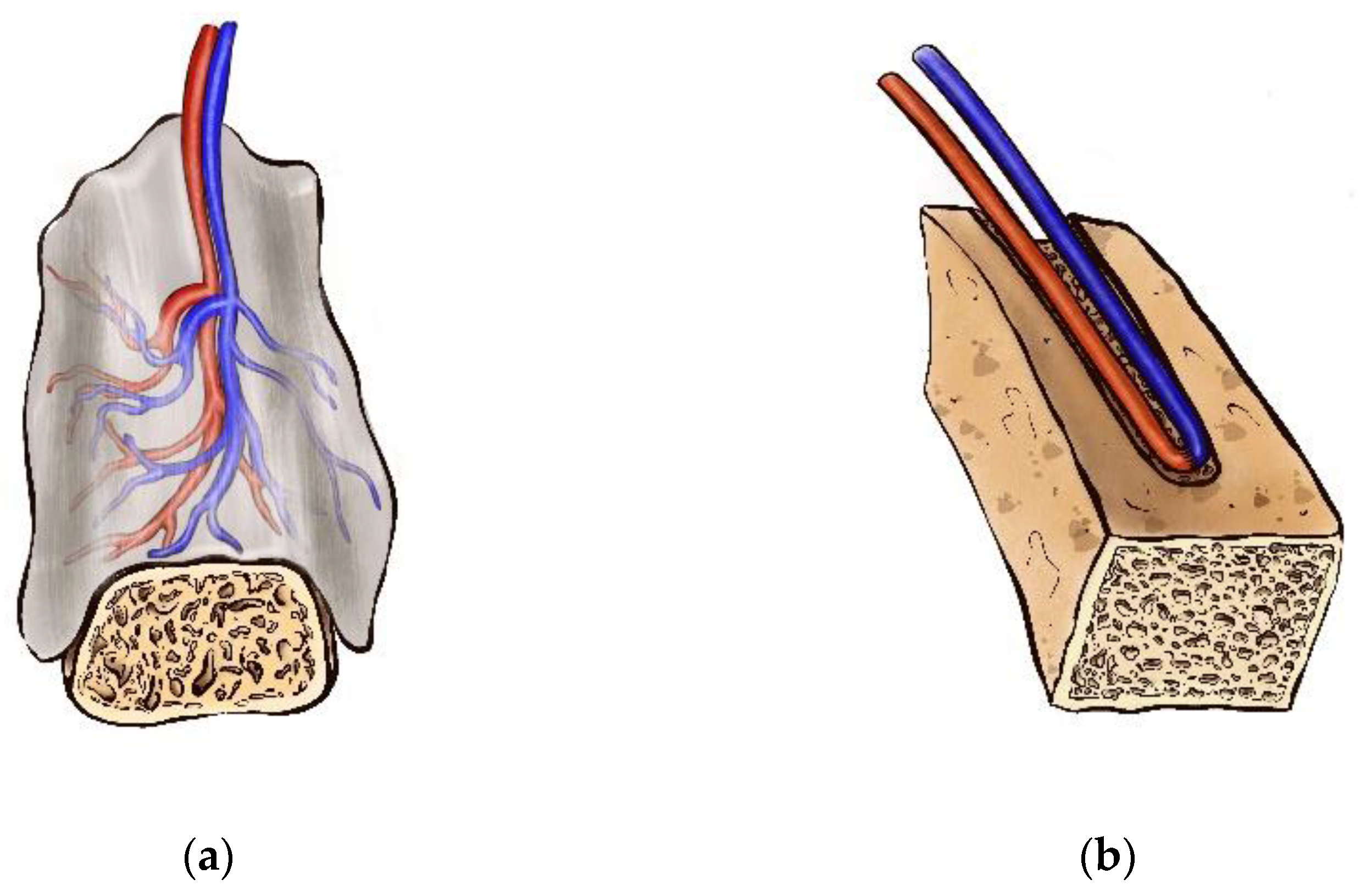 Figure 1. Scaffold axial vascularization strategy—(a) flap-based approach; (b) vessel-based approach.
Figure 1. Scaffold axial vascularization strategy—(a) flap-based approach; (b) vessel-based approach.In the early seventies, the foundation of the axial pattern flaps revolutionized reconstructive surgery [59]. In contrast to random pattern flap approach, which predominantly relies on the angiogenesis within the host recipient site, anatomically stable arteriovenous system allows to induce predictable neo-vascularization of various scaffold biomaterials [53]. Thus, flap-based vascularization, that is also known as the “extrinsic” mode of neovascularization, can be classified in to random flap-based and axial flap-based types. While using random flaps, the neovascular bed originates from the periphery of the construct after its implantation into a highly vascularized environment [59]. According to this principle, subcutaneous, intramuscular, and intraperitoneal implantation have been reported [44][48][60]. At the same time, the presence of the stable arteriovenous axis provides constant and reliable blood supply of the flap, and progenitor cells and GF delivery. Furthermore, axially vascularized flap can be transferred to the defect site via its arteriovenous pedicle [53]. However, extrinsic vascularization demonstrates limited success for the vascular network creation in the central part of large scaffolds [61][62]. Kneser et al. described “intrinsic” vascularization mode as a tool for adequate perfusion of the large tissue-engineered construct and uniform vasculature distribution within its structure [63]. Extrinsic and intrinsic vascularization modes have been widely used in the experimental and clinical studies [48][62][63][64][65]. Various tissue types have been applied for bone grafts or scaffolds prefabrication in recent years, including subcutaneous pocket, periosteal, fascial, muscular, and omental flaps [39][44][50][60][66][67][68][69][70].
Subcutaneous pocket creation has gained popularity as a method of ectopic scaffold implantation due to simplicity of surgical technique, variety of potential prefabrication sites for random flap-based vascularization, and minimal donor-site morbidity [39]. This approach has been investigated by many researchers in small and large animal models. Lee et.al created subcutaneous pockets in rats for cell-seeded and cell-free tibial condyle scaffolds implantation. The scaffolds were harvested and analyzed 6 weeks after implantation. Histomorphometry and immunohistochemical analysis demonstrated blood vessels and mineralized tissue formation within in vivo engineered bone grafts [61]. Wu et al. used cell-seeded synthetic b-TCP and coral-derived HA scaffolds for ectopic subcutaneous implantation at the back of Nude Mice. Twelve weeks after implantation, researchers revealed the formation of the vascularized new bone tissue via histological analysis and micro-computed tomography [68]. Hypothetically, subcutaneously prefabricated bone flaps can be axially vascularized by means of anatomically consistent or transposed vascular pedicle if simultaneous bone and soft tissue repair is needed.
Periosteal flaps have been widely used for bone grafts prefabrication due to well-known high osteogenic potential and rich vasculature of the periosteum [37][39][40][44][50]. The inner cambium layer of the periosteum is well-vascularized and represents the rich source of clinically useful osteoprogenitor cells, including osteoblasts and multipotent stem cells. Despite the fact that osteoblasts may be absent in a cambial layer in adults, they can appear whenever required, for instance, for fracture healing [71]. The outer fibrous layer contains highly organized and directional collagen fibers (Sharpey’s fibers), and a smaller number of cells, mostly fibroblasts and pericytes [72][73]. As mentioned earlier, the first introduction of the IVB concept was based on the use of the periosteum cambial layer as the source of progenitor cells for bone regeneration [28]. Periosteal flaps have been predominantly investigated as an extrinsic approach to scaffolds vascularization and bone grafts prefabrication [39][44][74][75]. However, Sparks et al. suggested to use axially vascularized inverted corticoperiosteal flap. This approach provides an intrinsic axial blood supply, while the osteogenic surface of the periosteum can be located inside the scaffold [53]. Huang et al. presented a rabbit model of the bone graft prefabrication using a skull periosteal flap based on the supraorbital vessels, and confirmed new bone formation after 16 weeks of the graft prefabrication [39]. Ersoy used the combination of the periosto-fasciocutaneous flap transposed to the abdomen for bioactive glass and hydroxyapatite scaffolds prefabrication, and confirmed the osteogenic capacity of the vascularized periosteum [44]. Han et.al presented the preclinical model of the IVB in rabbits by combining β-TCP scaffold, tibial periosteal flap and the saphenous vascular pedicle, and confirmed the presence of the rich vascular network and new bone formation 4 weeks after prefabrication. New bone formation was mainly seen in peripheral aspects of the scaffold, while microvascular infusion and immunohistochemical staining showed direct revascularization of β-TCP scaffold [31]. Nau et al. used a rat model to evaluate the efficacy of periosteal flaps in bone defects healing. In various study groups, researchers used periosteal flaps alone or in combination with β-TCP scaffold, bone marrow-derived mononuclear cells, and vascular pedicle. Prefabrication terms of four and eight weeks were analyzed. This strategy resulted in significantly improved bone healing [75]. Abu-Shahba et al. investigated the regenerative potential of the periosteal grafts and vascularized periosteal flaps in combination with muscle flaps in sheep model mandible defects reconstruction. The researchers revealed enhanced new bone formation and enhanced vascularization after 13 and 23 weeks after alloplastic scaffolds implantation in both study groups by means of micro-CT and histological analysis [50].
Prelaminated fascial flaps have been successfully applied for complex tracheal, laryngeal and ear defects by many researchers, by means of cartilage or alloplastic materials implantation underneath a radial forearm or temporoparietal fascia [26][76][77]. Prefabrication of bone grafts using fascial flaps has been also presented in series of preclinical studies. Fan et al. demonstrated efficacy of segmental bone defects repair in rhesus monkey using prevascularized cell-seeded scaffolds. These scaffolds were vascularized by saphenous arteriovenous bundle and covered with the fascial flap. Such vascularization and prefabrication approach resulted in new bone formation and capillary vessel in-growth [78]. Brey et al. conducted comparative analysis of periosteal and fascial flaps use for bone grafts prefabrication and vascularization. The analysis showed no significant difference in vascularization of the scaffolds and volume or shape of tissue formed. However, the use of the fascial flaps resulted in formation of predominantly fibrovascular tissue, while scaffolds that contacted with periosteal flaps demonstrated endochondral, direct, and appositional bone growth [79]. For clinical purposes random or axially vascularized fascial flaps for extrinsic vascularization of the scaffold can be harvested in a variety of anatomical locations, with minimal donor-site morbidity, however, osteogenic potential of fascia requires further investigations. Hypothetically, combination of fascial flaps with periosteal flaps and/or intrinsic vessel-based vascularization strategies can be considered as a logical future direction of the IVB concept development.
Muscle flaps have been effectively applied for in vivo BTE purposes [64][69][80][81]. It is well established that muscle tissue is a rich source of progenitor cells, including cells with osteogenic properties. Covering the fracture with muscle flaps provides a suitable environment for osteogenesis and reduces bone healing time [82].
Spalthoff et al. used prefabricated β-TCP scaffolds with or without a vascular bundle, combined with autologous bone marrow by implantation in the latissimus dorsi muscle in sheep. Histomorphometric analysis exhibited ectopic bone growth in all study groups with no significant difference between three- or six-months terms of prefabrication [81]. Kokemüller et al. reported experimental study on IVB approach based on prefabrication of the β-TCP scaffolds with a latissimus dorsi muscle flap and thoracodorsal vascular pedicle for axial construct perfusion in sheep. The researchers confirmed considerably induced ectopic bone growth in all implanted scaffolds, and significantly increased bone growth, ceramic resorption and angiogenesis in scaffolds with axial perfusion [65]. Liu et al. presented IVB for vascularized bone graft engineering by implanting the composite bovine-derived scaffolds supplemented with recombinant human bone morphogenetic protein-7 (rhBMP-7) and/or recombinant human vascular endothelial growth factor 165 (rhVEGF165) in latissimus dorsi muscle in pigs. Histomorphometric analysis after twelve weeks of prefabrication revealed new lamellar and trabecular bone formation with higher bone density in scaffolds supplemented with rhVEGF165 [70]. Zhou et al. used demineralized freeze-dried bone allografts and coralline hydroxyapatite scaffolds with or without BMP stimulation, loaded into customized titanium meshes and prefabricated with latissimus dorsi muscle flaps in monkeys. Prefabricated bone grafts were used for mandible reconstruction thirteen weeks after implantation, and were observed in situ for another thirteen weeks. The researchers analyzed the optimal time for prefabricated bone flap transfer via technetium-99m-methyl diphosphonate (Tc-MDP) single-photon emission computed tomography/computed tomography (SPECT/CT). According to the study results, researchers suggested to transfer the flap at an interval of 8 to 13 weeks. At these terms the bone density gradually increased, while the uptake of 99 m Tc-MDP started to decrease from its peak at 8 weeks [83].
In addition to representing a rich source of progenitor cells, vasculogenic and osteogenic environment, vascularized muscle flaps can be found in a range of anatomic sites. In a clinical setting, latissimus dorsi, pectoralis, rectus abdominis, and rectus femoris muscles are widely used as free flaps due to having anatomically consistent vascular pedicles of an appropriate caliber and length.
Omental flaps have demonstrated osteogenic and vasculogenic potential for BTE purposes in multiple experimental studies [48][84][85]. Kamei et al. presented an experimental study in a rabbit model based on wrapping omentum with a periosteal graft followed by harvesting and analyzing omentum samples in 1, 2, 4, 6, 8, 12, or 24 weeks after surgery. Within 1 week after surgery, researchers revealed the presence of osteoblasts clusters, while 8 weeks after prefabrication, medullization, including the presence of granulocytes, was observed [85]. Similarly, Sadegh et al. used free periosteal graft loaded with adipose tissue-derived stem cells for wrapping the pedicled omental flap in dog model. Such a tissue engineering approach lead to ectopic new bone formation [86]. Wiltfang et al. proposed the IVB strategy for ectopic bone formation based on the combination of titanium cages filled with bone mineral blocks, supplemented with recombinant human BMP-2, and bone marrow aspirate. These scaffolds were implanted into the gastrocolic omentum for a period of 3 months. Later, a free composite flap was harvested and transferred to the mandibular defect. Bone remodeling and mineralization both at the prefabrication and at the defect sites was confirmed by in vivo SPECT/CT [48]. Jacinto-Tinajero et al. presented the dog model of the IVB for BTE. In this research the scaffold consisted of collagen type 1 sponge, demineralized bone powder, calcium chloride, thrombin and PRP. The scaffold was wrapped with omentum and prefabricated for four months. As a result, a heterotopic trabecular bone formation was revealed [66]. Applied to clinical settings, omental flaps have been typically used for pharyngeal and esophageal defects reconstruction [86]. The feasibility of omental flaps clinical application for in vivo BTE purposes remains controversial and requires further experimental investigations.
While summarizing the efficacy of the flap-based techniques for in vivo BTE, it is mandatory to underline that the use of different tissue flaps for the scaffolds prefabrication demonstrates both osteogenic and vasculogenic capacity. Therefore, flap-based approach can be considered as an essential component of IVB structure. Axially vascularized flaps initiate bone tissue and blood vessels ingrowth within ectopically implanted scaffold and allow its consequent transposition or free transfer.
Vessel-based axial vascularization has revolutionized BTE due to the possibility to create rich and stable vascular network within a scaffold in a rapid and predictable manner [41][47][53][54][62][87][88][89]. As mentioned above, the presence of the vascular pedicle allows for the transposition of the flap locally, or to transfer it to the distant recipient site. Three main strategies of vessel-based axial vascularization include arteriovenous bundle (AVB), flow-through vascular bundle (FTVB), and arteriovenous loop (AV-loop).
Arteriovenous bundle (AVB) approach to tissue-engineered construct axial vascularization is based on the use of anatomically consistent vascular pedicle. It has emerged as a reliable method to provide a scaffold with a stable axial blood supply and constant perfusion. Moreover, AVB strategy allows to transfer the bone flap after a certain period of prefabrication using microsurgical techniques [32][88][90]. Polykandriotis et al. presented the experimental study on the use of AVB for BTE in rats. In this research the researchers made an effort to create vascularized tissue-engineered construct suitable for microsurgical transfer. For this purpose, AVB was used as the source of an axial blood supply by inserting the pedicle into a specially designed channel in to the bovine cancellous bone-derived scaffold. The scaffold vascularization was evaluated at 2, 4, and 8 weeks after implantation by means of histological, histomorphometric analysis, scanning electron microscopy, and micro-magnetic resonance angiography for in vivo evaluation of the vascularized scaffolds. The vascularized constructs demonstrated well organized sprouting vascular network of high density and degree of maturation, with organization into vessels of different orders [89]. Li et al. used AVB for axial vascularization of the composite PLGA/ β-TCP scaffolds supplemented with rhBMP-2 in minipigs. The researchers concluded that AVB significantly improved scaffold vascularization and new bone formation. Furthermore, defined vascular pedicle allows to transfer the flap to the bone defect by means of microvascular anastomoses [91].
Flow-through vascular bundle (FTVB) is an effective and relatively simple option for intrinsic vascularization of the scaffold. The main difference from the AVB approach is that the vascular pedicle is not ligated [41][91][92]. Yamaguchi et al. reported results of the vascularized allogeneic bone graft prefabrication in a rat model using flow-through saphenous bundle as a vascular carrier. Histological evaluations confirmed angiogenesis and bone formation in the group of axially vascularized scaffolds [41]. Yao et al. conducted an experimental study on IVB strategy in rabbits. Free radial bone grafts were harvested and vascularized by the external maxillary artery pedicle passed through the bone marrow cavity. India ink perfusion revealed high microvessel density comparing to the control group without axial blood supply. A peak of angiogenesis was indicated at four weeks postoperatively by means of integrated optical density of tetracycline fluorescence labelling. The researchers concluded that increased angiogenesis enhanced osteogenesis in the tissue engineered construct [92].
Arteriovenous loop (AVL) has become the most commonly applied and perspective axial vascularization strategy described in multiple experimental studies on soft and bone tissue regeneration in recent decades [32][34][47][54][62][92][93][94]. The AVL approach to axial vascularization is based on the creation of the arteriovenous shunt with a purpose of rapid sprouting of the vascular network. This technique has been investigated in small and large animal models as the IVB approach to bone tissue regeneration. Kneser et al. reported the use of the AVL including artery, vein and vein graft, for bovine cancellous bone blocks axial vascularization in rats. Significant vascularization of the porous bone matrix occurred by the 8th week of observation and was confirmed by the intraaortic India ink perfusion [63]. Beier et al. presented results of the bovine cancellous bone block axial vascularization via AVL in a sheep model. AVL was microsurgically created in an isolation chamber. The tissue engineered constructs displayed increased axial vascularization, which was quantified by histomorphometric analysis and micro-computed tomography. In vivo sequential MRI demonstrated a significant progressive increase in the scaffold perfusion volume. Immunohistochemical analysis confirmed new blood vessels formation [95]. In 2011 Eweida et al. introduced the results of the cadaveric and surgical pilot studies in goats to test the potential model for axial vascularization of the mandible tissue engineered constructs. The aim of this research was to define the optimal vascular axis to create the AVL in the mandibular region. Facial artery and vein were considered as vessels of choice to vascularize scaffolds for mandible defects reconstruction. Later, in 2014, researchers applied this model in vivo by means of successful facial artery—facial vein AVL creating in goats, and confirmed significantly enhanced vascularization in the central part of the scaffold comparing to non-vascularized scaffolds in the control group [90][96]. Ma et al. reported the rabbit model of the IVB for bone defects reconstruction. Researchers used a combination of β-TCP scaffold, saphenous artery—saphenous vein AVL, and osteogenic cell sheet based on bone marrow-derived stem cells. The AVL was inserted into the specially prepared lateral grooves of the scaffold. The scaffold was then wrapped with the cell sheet. After eight weeks of prefabrication, histological and histomorphometric examinations revealed a formation of the trabecular bone tissue at the central part of the axially vascularized scaffold with the presence of osteoblasts, osteocytes, and bone marrow cavity-like structures surrounded by a dense matrix. The control group included scaffolds without axial blood supply, and displayed lamellar bone formation, predominantly at the edge of the construct, and sporadic bone formation in the central part of the scaffold [47].
2.3. In Vivo Bioreactor Approach to BTE: Clinical Application
Despite that numerous experimental studies on IVB in BTE using small and large animal models have confirmed efficiency of this strategy for ectopic bone prevascularization and regeneration, reports on the clinical application of this principle are still rare. Researchers have reviewed clinical reports related to in vivo BTE and IVB principle presented in past decades. In 2016 Huang et al. summarized eight clinical reports on IVB approach to bone defects reconstruction between 1999 and 2014 [97]. The majority of the defects presented in this research included mandible defects repair using IVB principle and various prefabrication strategies [63][64][65][70][75][98][99]. In two of the eight mentioned cases, researchers presented in situ tibial and radial bones defects repair, and for the first time used AVL in the clinical settings with excellent long-term results [67]. Additionally, the review of publications that could belong to the IVB approach to bone reconstruction for the same period of time revealed several more clinical cases.
Thus, in 2000, Safak et al. reported experimental studies on bone flap prefabrication, followed by two clinical cases of successful use of prefabricated iliac osteomyocutaneous flaps. The researchers elevated pedicled split-inner cortex iliac bone flap and implanted it into the subcutaneous pocket in the medial groin region. After four weeks of prefabrication neovascularized composite flap was harvested and transferred to the defect based on the deep circumflex iliac vessels [100]. In 2001, Vacanti et al. presented a clinical report on reconstruction of an avulsed phalanx using in vivo BTE approach. As a result of the car accident, dorsal skin, nail bed, extensor tendon, and distal phalanx of the thumb had been lost. At the first stage the injured thumb was debrided and placed in to subcutaneous pocket at the abdomen for nineteen days. The skin flap healed successfully and was completely divided on nineteenth day. At the second stage porous HA scaffold was seeded with autologous radial periosteum-derived cells, incubated in vitro for nine weeks. Cell-seeded scaffold was implanted to the defect site twelve weeks after injury. MRI examination, conventional radiographs, and light-microscopical examination of the biopsy specimens, confirmed adequate vascularization of the scaffold and new lamellar bone formation within the tissue-engineered construct. However, histomorphometric analysis revealed that 5 percent of the construct incorporated lamellar bone and ossified endochondral tissue, while most of the volume was represented with soft tissue and blood vessels [101]. In 2004, Gronthos described a clinical case of the human mandible reconstruction using IVB approach. The researcher used prefabricated composite bone–muscle-flap. The scaffold used, consisted of a titanium mesh loaded with HA blocks seeded with bone marrow-derived stem cells, and supplemented by rhBMP-7. The tissue engineered construct was implanted into the latissimus dorsi muscle. After seven weeks of prefabrication the composite flap was transferred via microvascular anastomoses with the branches of external carotid artery and cephalic vein with good initial clinical results. During the follow up of the patient, a fracture of the titanium mesh, as well as partial necrosis of the bone flap were documented. The follow up was limited due to patient’s death from the cardiac arrest fifteen months after tissue engineered flap transfer [63][102][103]. In 2004 Yamada et al. successfully applied injectable tissue-engineered bone, based on bone marrow-derived stem cells and PRP, for maxilla and mandible augmentation in three partially edentulous patients [104]. In 2007 Marcacci et al. presented a new tissue engineering approach to surgical treatment of patients with extensive bone diaphysis defects. The researchers applied porous HA ceramic scaffolds seeded with bone marrow-derived stem cells. Seven years follow-up demonstrated complete integration of the scaffold confirmed by conventional radiographs and computed tomography scans [105].
Despite the variety of the IVB strategies used by the researchers of the above-mentioned studies, the philosophy of the basic approach in all the studies relies on the effort to effectively combine the critical components of the bone tissue regeneration process.
References
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61.
- Toogood, P.; Miclau, T. Critical-Sized Bone Defects: Sequence and Planning. J. Orthop. Trauma 2017, 31 (Suppl. S5), S23–S26.
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. S2), S203–S217.
- Barone, A.; Ricci, M.; Mangano, F.; Covani, U. Morbidity associated with iliac crest harvesting in the treatment of maxillary and mandibular atrophies: A 10-year analysis. J. Oral Maxillofac. Surg. 2011, 69, 2298–2304.
- Ritschl, L.M.; Mücke, T.; Hart, D.; Unterhuber, T.; Kehl, V.; Wolff, K.D.; Fichter, A.M. Retrospective analysis of complications in 190 mandibular resections and simultaneous reconstructions with free fibula flap, iliac crest flap or reconstruction plate: A comparative single centre study. Clin. Oral Investig. 2021, 25, 2905–2914.
- Wu, C.C.; Lin, P.Y.; Chew, K.Y.; Kuo, Y.R. Free tissue transfers in head and neck reconstruction: Complications, outcomes and strategies for management of flap failure: Analysis of 2019 flaps in single institute. Microsurgery 2014, 34, 339–344.
- Ling, X.F.; Peng, X. What is the price to pay for a free fibula flap? A systematic review of donor-site morbidity following free fibula flap surgery. Plast. Reconstr. Surg. 2012, 129, 657–674.
- Chen, X.F.; Chen, Y.M.; Gokavarapu, S.; Shen, Q.C.; Ji, T. Free flap reconstruction for patients aged 85 years and over with head and neck cancer: Clinical considerations for comprehensive care. Br. J. Oral Maxillofac. Surg. 2017, 55, 793–797.
- Al-Moraissi, E.A.; Alkhutari, A.S.; Abotaleb, B.; Altairi, N.H.; Del Fabbro, M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 107–120.
- Darwich, K.; Ismail, M.B.; Al-Mozaiek, M.Y.A.; Alhelwani, A. Reconstruction of mandible using a computer-designed 3D-printed patient-specific titanium implant: A case report. Oral Maxillofac. Surg. 2021, 25, 103–111.
- Nguyen, P.D.; Khechoyan, D.Y.; Phillips, J.H.; Forrest, C.R. Custom CAD/CAM implants for complex craniofacial reconstruction in children: Our experience based on 136 cases. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1609–1617.
- Almansoori, A.A.; Choung, H.W.; Kim, B.; Park, J.Y.; Kim, S.M.; Lee, J.H. Fracture of Standard Titanium Mandibular Reconstruction Plates and Preliminary Study of Three-Dimensional Printed Reconstruction Plates. J. Oral Maxillofac. Surg. 2020, 78, 153–166.
- Hirohata, H.; Yanagawa, T.; Takaoka, S.; Yamagata, K.; Sasaki, K.; Shibuya, Y.; Uchida, F.; Fukuzawa, S.; Tabuchi, K.; Hasegawa, S.; et al. A small number of residual teeth after the mandibular resection of oral cancer is associated with titanium reconstruction plate exposure. Clin. Exp. Dent. Res. 2019, 5, 469–475.
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115.
- Thakeb, M.F.; Fayyad, T.A.; ElGebeily, M.A.; Diab, R.A.; El Zahlawy, H.; Sharafeldin, M.S.; Al Kersh, M.A. Bifocal Compression-Distraction for Combined Bone and Soft-Tissue Defects in Post-traumatic Tibial Nonunion. J. Orthop. Trauma 2019, 33, e372–e377.
- Goldstein, J.A.; Paliga, J.T.; Taylor, J.A.; Bartlett, S.P. Complications in 54 frontofacial distraction procedures in patients with syndromic craniosynostosis. J. Craniofacial Surg. 2015, 26, 124–128.
- Kumar, P.; Saini, M.; Dehiya, B.S.; Sindhu, A.; Kumar, V.; Kumar, R.; Lamberti, L.; Pruncu, C.I.; Thakur, R. Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications. Nanomaterials 2020, 10, 2019.
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Berakdar, G.A.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75.
- Shahabipour, F.; Ashammakhi, N.; Oskuee, R.K.; Bonakdar, S.; Hoffman, T.; Shokrgozar, M.A.; Khademhosseini, A. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl. Res. 2019, 216, 57–76.
- Kohli, N.; Sharma, V.; Orera, A.; Sawadkar, P.; Owji, N.; Frost, O.G.; Bailey, R.J.; Snow, M.; Knowles, J.C.; Blunn, G.W.; et al. Pro-angiogenic and osteogenic composite scaffolds of fibrin, alginate and calcium phosphate for bone tissue engineering. J. Tissue Eng. 2021, 12, 20417314211005610.
- Krakowczyk, L.; Maciejewski, A.; Szymczyk, C.; Wierzgoń, J.; Szumniak, R.; Jędrzejewski, P.; Grajek, M.; Dobrut, M.; Ulczok, R.; Półtorak, S. The use of prefabrication technique in microvascular reconstructive surgery. Contemp. Oncol. 2012, 16, 546–550.
- Wei, X.; Li, Q. Prefabrication as a term in flap surgery: Do we need a broader definition? J. Reconstr. Microsurg. 2013, 29, 559–560.
- Shen, T.Y. Vascular implantation into skin flap: Experimental study and clinical application: A preliminary report. Plast. Reconstr. Surg. 1981, 68, 404–410.
- Pribaz, J.J.; Fine, N. Prelamination: Defining the prefabricated flap—A case report and review. Microsurgery 1994, 15, 618–623.
- Tan, B.K.; Chen, H.C.; He, T.M.; Song, I.C. Flap prefabrication—The bridge between conventional flaps and tissue-engineered flaps. Ann. Acad. Med. Singap. 2004, 33, 662–666.
- Trung, V.T.; Van Long, P.; Van, H.T. Restoration of Ear Defects by Prefabricated Radial Forearm Flap. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2616.
- Flaherty, F.; Militelli, F.; Vizcay, M. Partial Ear Reconstruction with a Prelaminated Induced Expanded Radial Artery Flap. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3344.
- Stevens, M.M.; Marini, R.P.; Schaefer, D.; Aronson, J.; Langer, R.; Shastri, V.P. In vivo engineering of organs: The bone bioreactor. Proc. Natl. Acad. Sci. USA 2005, 102, 11450–11455.
- Holt, G.E.; Halpern, J.L.; Dovan, T.T.; Hamming, D.; Schwartz, H.S. Evolution of an in vivo bioreactor. J. Orthop. Res. 2005, 23, 916–923.
- Buehrer, G.; Balzer, A.; Arnold, I.; Beier, J.P.; Körner, C.; Bleiziffer, O.; Brandl, A.; Weis, C.; Horch, R.E.; Kneser, U.; et al. Combination of BMP2 and MSCs significantly increases bone formation in the rat arterio-venous loop model. Tissue Eng. A 2015, 21, 96–105.
- Han, D.; Guan, X.; Wang, J.; Wei, J.; Li, Q. Rabbit tibial periosteum and saphenous arteriovenous vascular bundle as an in vivo bioreactor to construct vascularized tissue-engineered bone: A feasibility study. Artif. Organs. 2014, 38, 167–174.
- Yang, P.; Huang, X.; Shen, J.; Wang, C.; Dang, X.; Mankin, H.; Duan, Z.; Wang, K. Development of a new pre-vascularized tissue-engineered construct using predifferentiated rADSCs, arteriovenous vascular bundle and porous nano-hydroxyapatide-polyamide 66 scaffold. BMC Musculoskelet. Disord. 2013, 14, 318.
- Sever, C.; Uygur, F.; Kose, G.T.; Urhan, M.; Haholu, A.; Kulahci, Y.; Sinan, O.; Cihan, S.; Omer, O. Prefabrication of vascularized bone graft using an interconnected porous calcium hydroxyapatite ceramic in presence of vascular endothelial growth factor and bone marrow mesenchymal stem cells: Experimental study in rats. Indian J. Plast. Surg. 2012, 45, 444–452.
- Boos, A.M.; Loew, J.S.; Weigand, A.; Deschler, G.; Klumpp, D.; Arkudas, A.; Bleiziffer, O.; Gulle, H.; Kneser, U.; Horch, R.E.; et al. Engineering axially vascularized bone in the sheep arteriovenous-loop model. J. Tissue Eng. Regen. Med. 2013, 7, 654–664.
- Runyan, C.M.; Vu, A.T.; Rumburg, A.; Bove, K.; Racadio, J.; Billmire, D.A.; Taylor, J.A. Repair of a Critical Porcine Tibial Defect by Means of Allograft Revitalization. Plast. Reconstr. Surg. 2015, 136, 461e–473e.
- Tatara, A.M.; Shah, S.R.; Demian, N.; Ho, T.; Shum, J.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016, 45, 72–84.
- Akar, B.; Tatara, A.M.; Sutradhar, A.; Hsiao, H.Y.; Miller, M.; Cheng, M.H.; Mikos, A.G.; Brey, E.M. Large Animal Models of an In Vivo Bioreactor for Engineering Vascularized Bone. Tissue Eng. B Rev. 2018, 24, 317–325.
- Top, H.; Aygit, C.; Sarikaya, A.; Cakir, B.; Cakir, B.; Unlu, E. Bone flap prefabrication: An experimental study in rabbits. Ann. Plast. Surg. 2005, 54, 428–434.
- Huang, R.-L.; Tremp, M.; Ho, C.-K.; Sun, Y.; Liu, K.; Li, Q. Prefabrication of a functional bone graft with a pedicled periosteal flap as an in vivo bioreactor. Sci. Rep. 2017, 7, 18038.
- Watson, E.; Tatara, A.M.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. An Ovine Model of In Vivo Bioreactor-Based Bone Generation. Tissue Eng. C Methods 2020, 26, 384–396.
- Yamaguchi, K.; Kaji, Y.; Nakamura, O.; Tobiume, S.; Yamamoto, T. Prefabrication of Vascularized Allogenic Bone Graft in a Rat by Implanting a Flow-Through Vascular Pedicle and Basic Fibroblast Growth Factor Containing Hydroxyapatite/Collagen Composite. J. Reconstr. Microsurg. 2017, 33, 367–376.
- Zhang, H.; Han, D. Research progress of in vivo bioreactor as vascularization strategies in bone tissue engineering. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2014, 28, 1173–1176.
- Aliyev, A.; Ekin, Ö.; Bitik, O.; Korkusuz, P.; Yersal, N.C.; Çelik, H.H.; Tunçbilek, G. A Novel Method of Neo-osseous Flap Prefabrication: Induction of Free Calvarial Periosteum with Bioactive Glass. J. Reconstr. Microsurg. 2018, 34, 307–314, Erratum in J. Reconstr. Microsurg. 2018, 34, e1.
- Ersoy, B.; Bayramiçli, M.; Ercan, F.; Şirinoğlu, H.; Turan, P.; Numanoğlu, A. Comparison of bone prefabrication with vascularized periosteal flaps, hydroxyapatite, and bioactive glass in rats. J. Reconstr. Microsurg. 2015, 31, 291–299.
- Dong, Z.; Li, B.; Zhao, J.; Ma, Q.; Bai, S.; Yang, W.; Li, G.; Ma, G.; Liu, Y. Prefabrication of vascularized bone grafts using a combination of bone marrow mesenchymal stem cells and vascular bundles with β-tricalcium phosphate ceramics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114 (Suppl. S5), S153–S159.
- Omar, O.; Engstrand, T.; Linder, L.K.B.; Åberg, J.; Shah, F.A.; Palmquist, A.; Birgersson, U.; Elgali, I.; Pujari-Palmer, M.; Engqvist, H.; et al. In situ bone regeneration of large cranial defects using synthetic ceramic implants with a tailored composition and design. Proc. Natl. Acad. Sci. USA 2020, 117, 26660–26671.
- Ma, D.; Ren, L.; Cao, Z.; Li, J.; Cao, J.; Tian, W.; Yao, H. Prefabrication of axially vascularized bone by combining β-tricalciumphosphate, arteriovenous loop, and cell sheet technique. Tissue Eng. Regen. Med. 2016, 13, 579–584.
- Wiltfang, J.; Rohnen, M.; Egberts, J.H.; Lützen, U.; Wieker, H.; Açil, Y.; Naujokat, H. Man as a Living Bioreactor: Prefabrication of a Custom Vascularized Bone Graft in the Gastrocolic Omentum. Tissue Eng. C Methods 2016, 22, 740–746.
- Nakamura, O.; Kaji, Y.; Imaizumi, Y.; Yamagami, Y.; Yamamoto, T. Prefabrication of vascularized bone allograft in a recipient rat using a flow-through vascular pedicle, bone morphogenetic protein, and bisphosphonate. J. Reconstr. Microsurg. 2013, 29, 241–248.
- Abu-Shahba, A.G.; Wilkman, T.; Kornilov, R.; Adam, M.; Salla, K.M.; Lindén, J.; Lappalainen, A.K.; Björkstrand, R.; Seppänen-Kaijansinkko, R.; Mannerström, B. Periosteal Flaps Enhance Prefabricated Engineered Bone Reparative Potential. J. Dent. Res. 2021, 101, 220345211037247.
- Hokugo, A.; Sawada, Y.; Sugimoto, K.; Fukuda, A.; Mushimoto, K.; Morita, S.; Tabata, Y. Preparation of prefabricated vascularized bone graft with neoangiogenesis by combination of autologous tissue and biodegradable materials. Int. J. Oral Maxillofac. Surg. 2006, 35, 1034–1040.
- Kuzmenka, D.; Sewohl, C.; König, A.; Flath, T.; Hahnel, S.; Schulze, F.P.; Hacker, M.C.; Schulz-Siegmund, M. Sustained Calcium(II)-Release to Impart Bioactivity in Hybrid Glass Scaffolds for Bone Tissue Engineering. Pharmaceutics 2020, 12, 1192.
- Sparks, D.S.; Savi, F.M.; Saifzadeh, S.; Schuetz, M.A.; Wagels, M.; Hutmacher, D.W. Convergence of Scaffold-Guided Bone Reconstruction and Surgical Vascularization Strategies-A Quest for Regenerative Matching Axial Vascularization. Front. Bioeng. Biotechnol. 2020, 7, 448.
- Weigand, A.; Beier, J.P.; Arkudas, A.; Al-Abboodi, M.; Polykandriotis, E.; Horch, R.E.; Boos, A.M. The Arteriovenous (AV) Loop in a Small Animal Model to Study Angiogenesis and Vascularized Tissue Engineering. J. Vis. Exp. 2016, 117, e54676.
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in Craniofacial Bone Tissue Engineering. J. Dent. Res. 2018, 97, 969–976.
- Wang, J.H.; Chen, J.; Kuo, S.M.; Mitchell, G.M.; Lim, S.Y.; Liu, G.S. Methods for Assessing Scaffold Vascularization In Vivo. Methods Mol. Biol. 2019, 1993, 217–226.
- Stephens, C.J.; Spector, J.A.; Butcher, J.T. Biofabrication of thick vascularized neo-pedicle flaps for reconstructive surgery. Transl. Res. 2019, 211, 84–122.
- Später, T.; Ampofo, E.; Menger, M.D.; Laschke, M.W. Combining Vascularization Strategies in Tissue Engineering: The Faster Road to Success? Front. Bioeng. Biotechnol. 2020, 8, 592095.
- McGregor, I.A.; Morgan, G. Axial and random pattern flaps. Br. J. Plast. Surg. 1973, 26, 202–213.
- Cassell, O.C.; Hofer, S.O.; Morrison, W.A.; Knight, K.R. Vascularisation of tissue-engineered grafts: The regulation of angiogenesis in reconstructive surgery and in disease states. Br. J. Plast. Surg. 2002, 55, 603–610.
- Lee, C.H.; Marion, N.W.; Hollister, S.; Mao, J.J. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng. A 2009, 15, 3923–3930.
- Horch, R.E.; Kneser, U.; Polykandriotis, E.; Schmidt, V.J.; Sun, J.; Arkudas, A. Tissue engineering and regenerative medicine -where do we stand? J. Cell. Mol. Med. 2012, 16, 1157–1165.
- Kneser, U.; Polykandriotis, E.; Ohnolz, J.; Heidner, K.; Grabinger, L.; Euler, S.; Amann, K.U.; Hess, A.; Brune, K.; Greil, P.; et al. Engineering of vascularized transplantable bone tissues: Induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006, 12, 1721–1731.
- Warnke, P.; Springer, I.; Wiltfang, J.; Acil, Y.; Eufinger, H.; Wehmöller, M.; Russo, P.; Bolte, H.; Sherry, E.; Behrens, E.; et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004, 364, 766–770.
- Kokemueller, H.; Spalthoff, S.; Nolff, M.; Tavassol, F.; Essig, H.; Stuehmer, C.; Bormann, K.H.; Rücker, M.; Gellrich, N.C. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: Experimental pilot study in sheep and first clinical application. Int. J. Oral Maxillofac. Surg. 2010, 39, 379–387.
- Jacinto-Tinajero, J.C.; Ascencio, D.; Marquina, B.; Barrios-Payán, J.; Gutierrez, M.C.; Lim, M.G.; Pando, R.H. Induction of bone formation in abdominal implants constituted by collagen sponges embedded with plant-based human transforming growth factor family proteins in ectopic dog model. J. Exp. Orthop. 2014, 1, 11.
- Horch, R.E.; Beier, J.P.; Kneser, U.; Arkudas, A. Successful human long-term application of in situ bone tissue engineering. J. Cell. Mol. Med. 2014, 18, 1478–1485.
- Wu, H.; Kang, N.; Wang, Q.; Dong, P.; Lv, X.; Cao, Y.; Xiao, R. The dose-effect relationship between the seeding quantity of human marrow mesenchymal stem cells and in vivo tissue-engineered bone yield. Cell Transplant. 2015, 24, 1957–1968.
- Okuda, T.; Uysal, A.C.; Tobita, M.; Hyakusoku, H.; Mizuno, H. Prefabrication of tissue engineered bone grafts: An experimental study. Ann. Plast. Surg. 2010, 64, 98–104.
- Liu, Y.; Möller, B.; Wiltfang, J.; Warnke, P.H.; Terheyden, H. Tissue engineering of a vascularized bone graft of critical size with an osteogenic and angiogenic factor-based in vivo bioreactor. Tissue Eng. A 2014, 20, 3189–3197.
- Heliotis, M.; Lavery, K.M.; Ripamonti, U.; Tsiridis, E.; di Silvio, L. Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest. Int J. Oral Maxillofac. Surg. 2006, 35, 265–269.
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50.
- Chang, H.; Knothe Tate, M.L. Concise review: The periosteum: Tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl. Med. 2012, 1, 480–491.
- Squier, C.A.; Ghoneim, S.; Kremenak, C.R. Ultrastructure of the periosteum from membrane bone. J. Anat. 1990, 171, 233–239.
- Nau, C.; Henrich, D.; Seebach, C.; Schröder, K.; Fitzsimmons, S.-J.; Hankel, S.; Barker, J.H.; Marzi, I.; Frank, J. Treatment of large bone defects with a vascularized periosteal flap in combination with biodegradable scaffold seeded with bone marrow-derived mononuclear cells: An experimental study in rats. Tissue Eng. A 2016, 22, 133–141.
- Cheng, M.-H.; Brey, E.M.; Ulusal, B.G.; Wei, F.-C. Mandible augmentation for osseointegrated implants using tissue engineering strategies. Plast. Reconstr. Surg. 2006, 118, 1e–4e.
- Vranckx, J.J.; Den Hondt, M.; Delaere, P. Prefabrication and prelamination strategies for the reconstruction of complex defects of trachea and larynx. J. Reconstr. Microsurg. 2014, 30, 145–152.
- Jaquet, Y.; Higgins, K.M.; Enepekides, D.J. The temporoparietal fascia flap: A versatile tool in head and neck reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 235–241.
- Fan, H.; Zeng, X.; Wang, X.; Zhu, R.; Pei, G. Efficacy of prevascularization for segmental bone defect repair using β-tricalcium phosphate scaffold in rhesus monkey. Biomaterials 2014, 35, 7407–7415.
- Brey, E.M.; Cheng, M.H.; Allori, A.; Satterfield, W.; Chang, D.W.; Patrick, C.W., Jr.; Miller, M.J. Comparison of guided bone formation from periosteum and muscle fascia. Plast. Reconstr. Surg. 2007, 119, 1216–1222.
- Spalthoff, S.; Jehn, P.; Zimmerer, R.; Möllmann, U.; Gellrich, N.C.; Kokemueller, H. Heterotopic bone formation in the musculus latissimus dorsi of sheep using β-tricalcium phosphate scaffolds: Evaluation of an extended prefabrication time on bone formation and matrix degeneration. Int. J. Oral Maxillofac. Surg. 2015, 44, 791–797.
- Hashimoto, N.; Kiyono, T.; Wada, M.R.; Umeda, R.; Goto, Y.; Nonaka, I.; Shimizu, S.; Yasumoto, S.; Inagawa-Ogashiwa, M. Osteogenic properties of human myogenic progenitor cells. Mech. Dev. 2008, 125, 257–269.
- Zhou, M.; Peng, X.; Mao, C.; Tian, J.H.; Zhang, S.W.; Xu, F.; Tu, J.J.; Liu, S.; Hu, M.; Yu, G.Y. The value of SPECT/CT in monitoring prefabricated tissue-engineered bone and orthotopic rhBMP-2 implants for mandibular reconstruction. PLoS ONE 2015, 10, e0137167.
- Chan, J.K.; Harry, L.; Williams, G.; Nanchahal, J. Soft tissue reconstruction of open fractures of the lower limb: Muscle versus fasciocutaneous flaps. Plast. Reconstr. Surg. 2012, 130, 284e–295e.
- Kamei, Y.; Toriyama, K.; Takada, T.; Yagi, S. Tissue-engineering bone from omentum. Nagoya J. Med. Sci. 2010, 72, 111–117.
- Sadegh, A.B.; Basiri, E.; Oryan, A.; Mirshokraei, P. Wrapped omentum with periosteum concurrent with adipose derived adult stem cells for bone tissue engineering in dog model. Cell Tissue Bank. 2014, 15, 127–137.
- Tokashiki, K.; Okamoto, I.; Okada, T.; Sato, H.; Yamashita, T.; Matsuki, T.; Kondo, T.; Fushimi, C.; Masubuchi, T.; Miura, K.; et al. Postoperative Complications and Swallowing Function after Jejunal and Skin Flap Reconstruction for Hypopharyngeal Carcinoma-A Multicenter Retrospective Study. J. Clin. Med. 2022, 11, 1464.
- Weigand, A.; Beier, J.P.; Hess, A.; Gerber, T.; Arkudas, A.; Horch, R.E.; Boos, A.M. Acceleration of vascularized bone tissue-engineered constructs in a large animal model combining intrinsic and extrinsic vascularization. Tissue Eng. A 2015, 21, 1680–1694.
- Polykandriotis, E.; Arkudas, A.; Beier, J.P.; Hess, A.; Greil, P.; Papadopoulos, T.; Kopp, J.; Bach, A.D.; Horch, R.E.; Kneser, U. Intrinsic axial vascularization of an osteoconductive bone matrix by means of an arteriovenous vascular bundle. Plast. Reconstr. Surg. 2007, 120, 855–868.
- Eweida, A.M.; Nabawi, A.S.; Abouarab, M.; Kayed, M.; Elhammady, H.; Etaby, A.; Khalil, M.R.; Shawky, M.S.; Kneser, U.; Horch, R.E.; et al. Enhancing mandibular bone regeneration and perfusion via axial vascularization of scaffolds. Clin. Oral Investig. 2014, 18, 1671–1678.
- Li, B.; Ruan, C.; Ma, Y.; Huang, Z.; Huang, Z.; Zhou, G.; Zhang, J.; Wang, H.; Wu, Z.; Qiu, G. Fabrication of Vascularized Bone Flaps with Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Arteriovenous Bundle. Tissue Eng. A 2018, 24, 1413–1422.
- Yao, Y.; Hua, C.; Tang, X.; Wang, Y.; Zhang, F.; Xiang, Z. Angiogenesis and osteogenesis of non-vascularised autogenous bone graft with arterial pedicle implantation. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 467–473.
- Tanaka, Y.; Sung, K.C.; Tsutsumi, A.; Ohba, S.; Ueda, K.; Morrison, W.A. Tissue engineering skin flaps: Which vascular carrier, arteriovenous shunt loop or arteriovenous bundle, has more potential for angiogenesis and tissue generation? Plast. Reconstr. Surg. 2003, 112, 1636–1644.
- Mian, R.; Morrison, W.A.; Hurley, J.V.; Penington, A.J.; Romeo, R.; Tanaka, Y.; Knight, K.R. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000, 6, 595–603.
- Beier, J.P.; Horch, R.E.; Hess, A.; Arkudas, A.; Heinrich, J.; Loew, J.; Gulle, H.; Polykandriotis, E.; Bleiziffer, O.; Kneser, U. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J. Tissue Eng. Regen. Med. 2010, 4, 216–223.
- Eweida, A.M.; Nabawi, A.S.; Marei, M.K.; Khalil, M.R.; Elhammady, H.A. Mandibular reconstruction using an axially vascularized tissue-engineered construct. Ann. Surg. Innov. Res. 2011, 5, 2, Erratum in Ann. Surg. Innov. Res. 2012, 6, 4.
- Huang, R.-L.; Kobayashi, E.; Liu, K.; Li, Q. Bone Graft Prefabrication Following the In Vivo Bioreactor Principle. EBioMedicine 2016, 12, 43–54.
- Orringer, J.S.; Shaw, W.W.; Borud, L.J.; Freymiller, E.G.; Wang, S.A.; Markowitz, B.L. Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap. Plast. Reconstr. Surg. 1999, 104, 793–797.
- Mesimäki, K.; Lindroos, B.; Törnwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J. Oral Maxillofac. Surg. 2009, 38, 201–209.
- Safak, T.; Akyürek, M.; Ozcan, G.; Keçik, A.; Aydin, M. Osteocutaneous flap prefabrication based on the principle of vascular induction: An experimental and clinical study. Plast. Reconstr. Surg. 2000, 105, 1304–1313.
- Vacanti, C.A.; Bonassar, L.J.; Vacanti, M.P.; Shufflebarger, J. Replacement of an avulsed phalanx with tissue-engineered bone. N. Engl. J. Med. 2001, 344, 1511–1514, Erratum in N. Engl. J. Med. 2001, 345, 704.
- Gronthos, S. Reconstruction of human mandible by tissue engineering. Lancet 2004, 364, 735–736.
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250.
- Yamada, Y.; Ueda, M.; Hibi, H.; Nagasaka, T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004, 13, 343–355.
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
626
Revisions:
2 times
(View History)
Update Date:
24 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




