Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria João Matos | -- | 1493 | 2022-08-10 11:49:41 | | | |
| 2 | Camila Xu | Meta information modification | 1493 | 2022-08-15 03:52:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matos, M.J.; Santana, L.; Uriarte, E.; Borges, F. Thiocoumarins. Encyclopedia. Available online: https://encyclopedia.pub/entry/26025 (accessed on 16 January 2026).
Matos MJ, Santana L, Uriarte E, Borges F. Thiocoumarins. Encyclopedia. Available at: https://encyclopedia.pub/entry/26025. Accessed January 16, 2026.
Matos, Maria J., Lourdes Santana, Eugenio Uriarte, Fernanda Borges. "Thiocoumarins" Encyclopedia, https://encyclopedia.pub/entry/26025 (accessed January 16, 2026).
Matos, M.J., Santana, L., Uriarte, E., & Borges, F. (2022, August 10). Thiocoumarins. In Encyclopedia. https://encyclopedia.pub/entry/26025
Matos, Maria J., et al. "Thiocoumarins." Encyclopedia. Web. 10 August, 2022.
Copy Citation
Thiocoumarins are a particular class of coumarins in which one or two of the oxygen atoms are replaced by a sulfur. They are chemically subdivided in three groups: Thiocoumarins, 2-thioxocoumarins, and dithiocoumarins.
thiocoumarins
2-thioxocoumarins
dithiocoumarins
1. Introduction
The substitution of an oxygen atom by a sulfur is a common strategy in medicinal chemistry and drug discovery, to modulate the chemical properties of a molecule of interest. In fact, the electron deficient and bivalent sulfur atom presents two different areas of positive electrostatic potential due to the low-lying σ* orbitals of the C–S bond, available to interact with different electron donors as nitrogen or oxygen and, possibly, π-systems [1].
In the particular case of the coumarin scaffold, two different oxygen atoms can be replaced by sulfur, providing three different new scaffolds: Thiocoumarins, 2-thioxocoumarins, and dithiocoumarins (Figure 1).
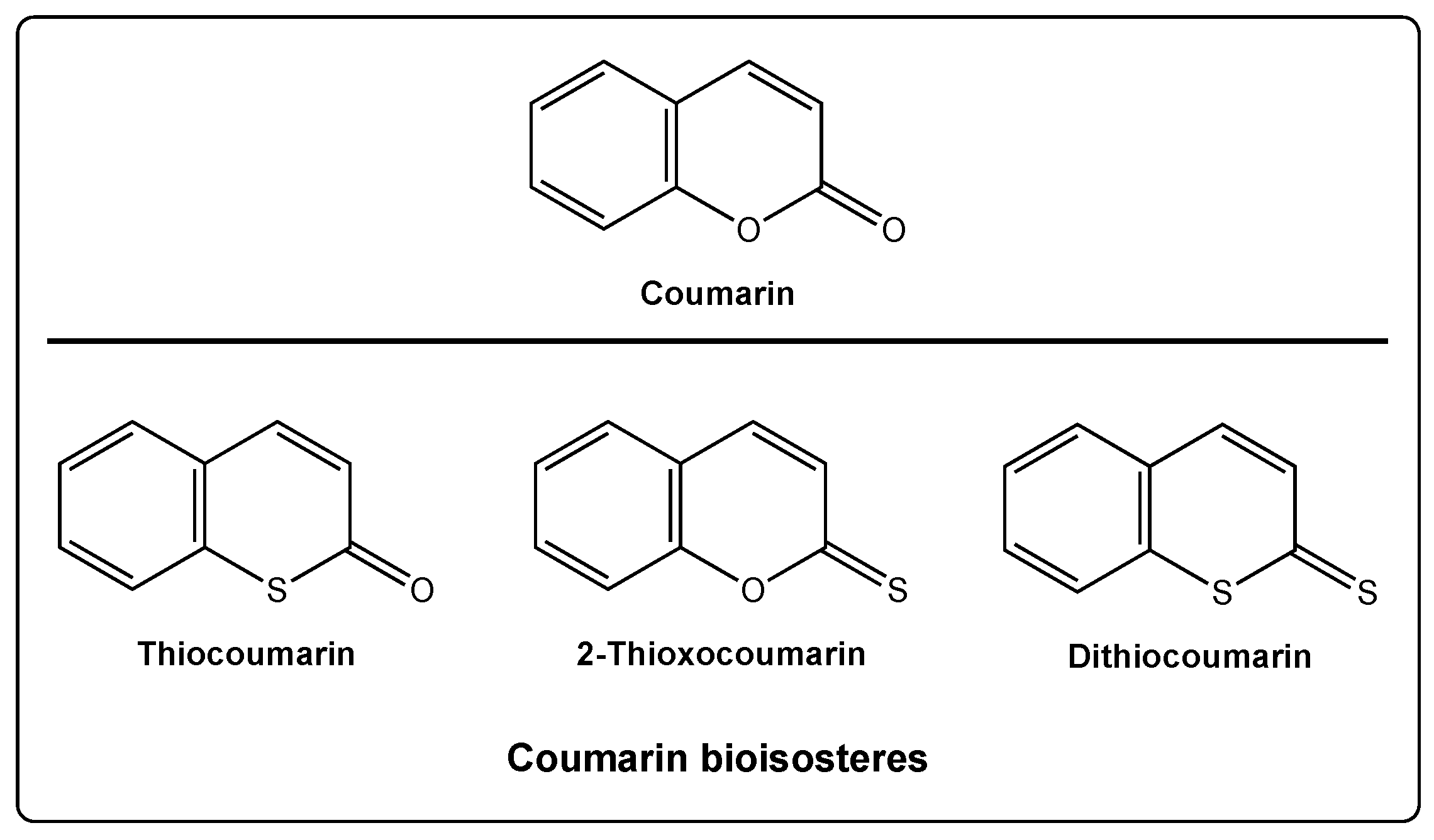
Figure 1. Coumarin and its bioisosteres: Thiocoumarin, 2-thioxocoumarin, and dithiocoumarin.
2. Thiocoumarins
In the 1980s, thiocoumarins already had clinical use as haemorrhagic agents, anticoagulants (interfering with vitamin K-dependent coagulation factors), and antiallergic agents [2]. In fact, some thiocoumarin derivatives found important applications as anticoagulant rodenticides pesticides [3].
During the decade of the 1980s, the versatile synthesis from the acrylic and propiolic ortho esters and benzenethiols was described [4]. After those first reports, some other evidences on the interest of thiocoumarins as potential bioactive molecules have been published. However, a deeper understanding of biology and biological functions, and how these molecules really work, has to be acquired before considering thiocoumarins as a trend topic in medicinal chemistry. One of the most recent subjects of interest related to this scaffold is the role of thiocoumarins as carbonic anhydrase inhibitors [5][6][7][8][9]. The described thiocoumarins, in particular the 6-hydroxy-2-thioxocoumarin, proved to bound to the human carbonic anhydrase II active site in a completely different mode compared with coumarins, commonly hydrolyzed by the esterase carbonic anhydrase to the corresponding 2-hydroxycinnamic acids. The role of thiocoumarins inhibiting carcinogenesis has also been studied over the last decade [10]. Moreover, few indole derivatives were tested as potential drugs for PUVA photochemotherapy [11]. Furthermore, ethers of thiocoumarin, at position 4, have been reported as nitric oxide synthase inhibitors in the nanomolar range [12].
Synthetically, most of the described thiocoumarins are obtained from the 4-hydroxythiocoumarin. Recently, a review collected the relevant information on the utility of this scaffold in organic chemistry [13]. Detailed synthetic methodologies, structures, and chemical properties of the 4-hydroxythiocoumarin were mentioned in this overview, as well as its most interesting bioactivities. In fact, this compound is easily obtained and modified to achieve key educts for the synthesis of heterocyclic systems.
Few papers per year describe some new advances in the field, related to both synthesis and biological applications. In 2019, there are only four relevant references on this topic, with the first one being a chemical mechanistic approach on the formation of two-center three-electron bonds by the hydroxyl radical induced reaction of thioesculetin [14][15][16][17]. The second study is focused on the anticonvulsant activity of a new series of synthetic 4-amino-3-nitrothiocoumarins [15]. The traditional synthesis starting from the thiophenol and the malonic acid, in the presence of POCl3 and AlCl3 with prolonged heating, is commonly used to afford the 4-hydroxythicoumarin, in low yield (Figure 2) [18]. The yields described for the first time for this traditional reaction were between 50 and 90%. However, further reports for other substituted thiocoumarins were lower than these. In this recent manuscript [16], an alternative two-step method has been proposed by the researchers (Figure 2).
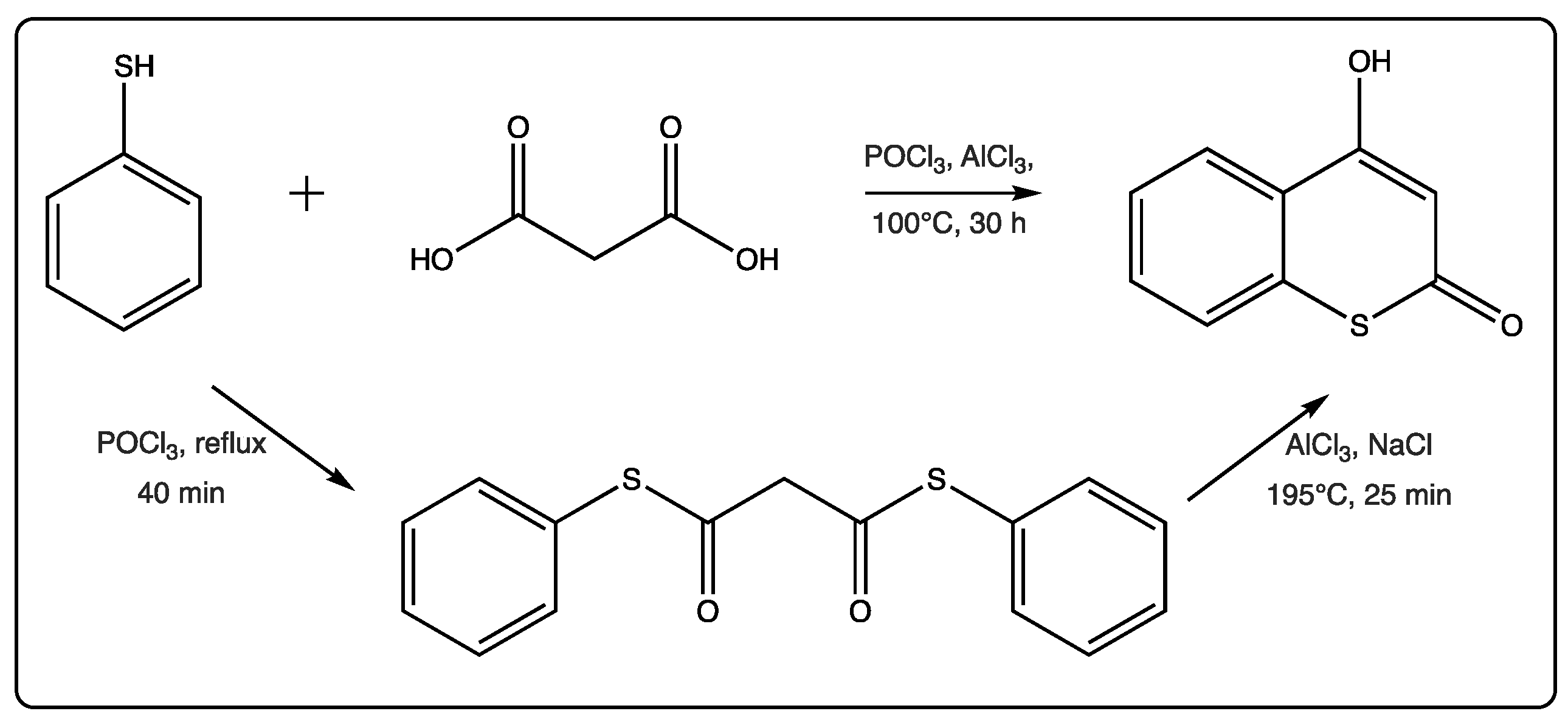
Figure 2. Alternative method of cyclocondensation of thiocoumarins.
The third paper published in 2019 is focused on the design and synthesis of new anticonvulsants [16]. 3-Nitrocoumarins proved to be more active than the studied thiocoumarins. Finally, the synthesis of 4-sulfonylthiocoumarins was also described in 2019 [17]. Starting from the 4-hydroxythiocoumarin, via DABCO-catalyzed direct sulfonylation of 1-sulfonyl-1,2,3-triazoles, new 4-sulfonylthiocoumarins were efficiently obtained. The main advantage of this method is the lack of transition metal catalysts and extra oxidants.
As previously mentioned, most of the traditional synthetic routes to afford the thiocoumarin scaffold involve a Lewis acid, as AlCl3 (Figure 3A) [19]. This reaction may be followed by nickel-catalyzed intramolecular recombination fragment coupling of the thioester to afford the corresponding benzothiophene.
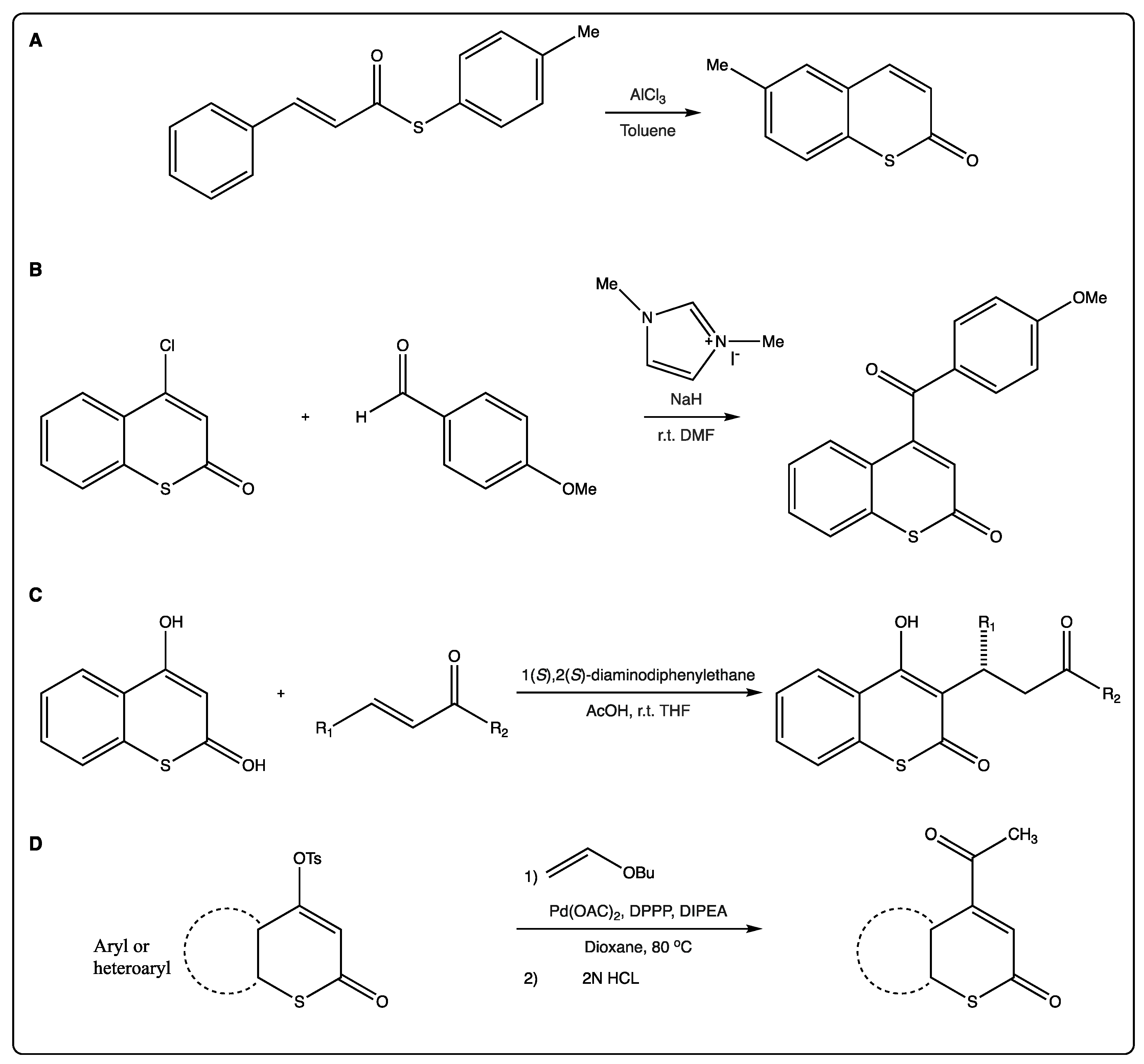
Figure 3. (A) Synthesis of thiocoumarins via traditional Lewis acid formation. (B) A straightforward organocatalytic synthesis of 4-aroylcoumarins. (C) Michael reactions at position 3 of the scaffold. (D) α-Regioselective Heck coupling of tosylates.
Complex thiocoumarins have been synthetized from simple ones, and not only 4-hydroxythiocoumarin is used as starting material for the synthesis of more complex thiocoumarins. 4-Chlorothiocoumarin is also used for acylating thiocoumarins to afford γ-ketoenones (Figure 3B) [20]. The researchers described a one-step organocatalytic synthesis of 4-acylcoumarins from 4-chlorocoumarin, reporting the first examples of nucleophilic substitutions at the β-carbons of enones to afford γ-ketoenones [21].
Few thiocoumarins have also been explored in the design of novel carrier systems. The development of egg shell-like nanovesicles using the thiocoumarin-3-carboxylate has been described [22]. These drug delivery vehicles have been used as a potential carrier for the bacteriostatic antibiotic sulfamethoxazole. The spectroscopic studies as well as the growth inhibition of E. coli exhibit that this formulation leads to pH responsive sustained release of the drug.
Thiocoumarins have been recently prepared using microwave irradiation, in a versatile Lewis acid-catalyzed reaction [23]. Functional groups as pyrimidine and 1,3,4-oxadiazole have been introduced in the scaffold. The technique has been described as efficient, selective, fast, and clean. The series of compounds has been reported for the anti-microbial and antioxidant activities.
Thiocoumarins can also be converted to dithiocoumarins using Lawesson’s reagent, in a reaction with ~30–40% yield (Figure 4) [6][8][24]. This reaction may contribute to deeply exploring these analogues, which are less studied due to the scarcity and rarity of starting materials.
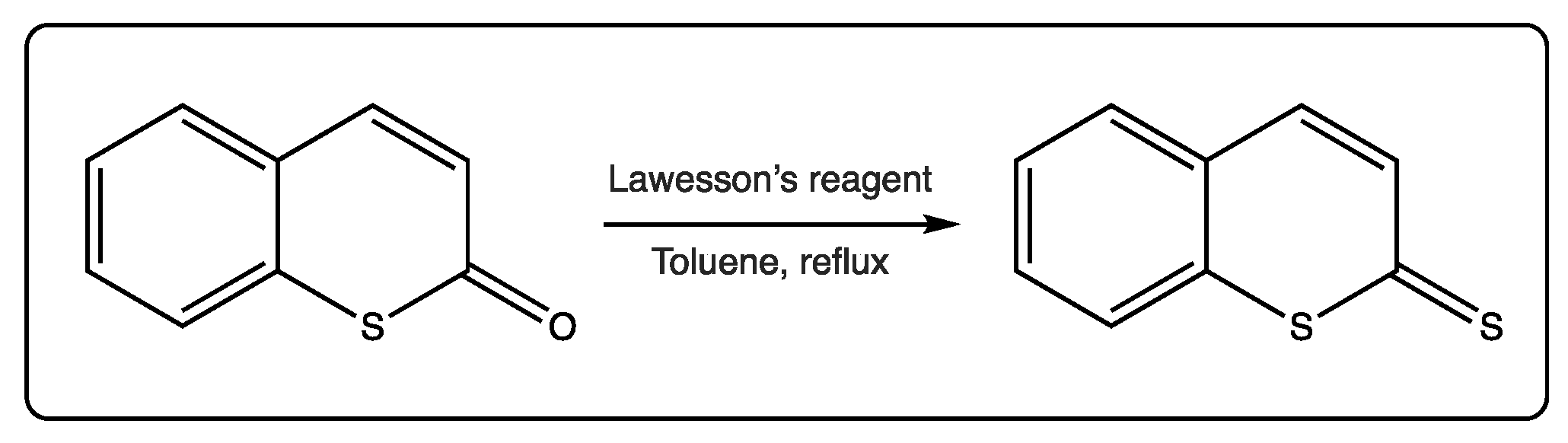
Figure 4. Conversion of thiocoumarins to dithiocoumarins.
As some simple coumarins, thiocoumarins have been recently described as fluorescent probes and sensors. A thiocoumarin, named 7-(N,N-diethylamino)-4-trifluoromethyl-2-thiocoumarin, has been used as sensor for heavy metal pollution and bacterial contamination. Its specific thiocarbonyl scaffold as donor-π-accepter electronic properties, provides intriguing optical properties and several applicable functions [25]. A thiocoumarin-containing ratiometric fluorescent probe has been described for the simultaneous detection of hypochlorite and singlet oxygen [26]. Once again, the thiocarbonyl moiety has been highlighted as an excellent alternative to traditional probes. Moreover, these applications have been described for live-cell imaging, with the thiocoumarin fluorescent probe turned-on once inside the cells [27]. The potential of these molecules for imaging has been recently claimed in a patent [28].
3. 2-Thioxocoumarins
As previously described for the thiocoumarins, the 2-thioxocoumarins have been studied as carbonic anhydrase inhibitors [7][29]. The researchers presented the X-ray crystal structure of the 6-hydroxy-2-thioxocoumarin on the human carbonic anhydrase II active site, and these results revealed an unprecedented and unexpected inhibition mechanism for these new inhibitors when compared with isostructural coumarins. It was proved that the exo-sulfur atom can link to a zinc-coordinated water molecule, while other parts of the scaffold can establish important interactions with different amino acids of the binding pocket.
7-Diethylamino-4-hydroxymethyl-thiocoumarin (thio-DEACM) caged molecules are recently attracting attention. The thio-DEACM as a caging group has great properties, such as rapid blue-cyan light responsiveness and avoiding UV irradiation of cells in combination with the absence of toxicity of the released photocage [30].
4. Dithiocoumarins
Dithiocoumarins were more explored more than the above mentioned 2-thioxocoumarins. Few reports on new synthetic routes to achieve this scaffold have been recently reported [31]. Thiocoumarins, presenting a variety of functional groups, were prepared from the corresponding thiophenols and diketene via cyclocondensation [24].
These compounds are also used as starting materials to obtain more complex molecules. For most of the reactions involving dithiocoumarins as starting materials, the 4-hydroxy derivative is the selected molecule. This molecule is being used since the 1980s, when an efficient protocol was described to prepare it [32]. Following this protocol, 2’-chloroacetophenones react with carbon disulfide in the presence of sodium hydride to form 4-hydroxydithiocoumarin anions (Figure 5). Kinetic protonation provides the desired 4-hydroxydithiocoumarins. Alkylation of these precursors may provide S-alkyl derivatives.
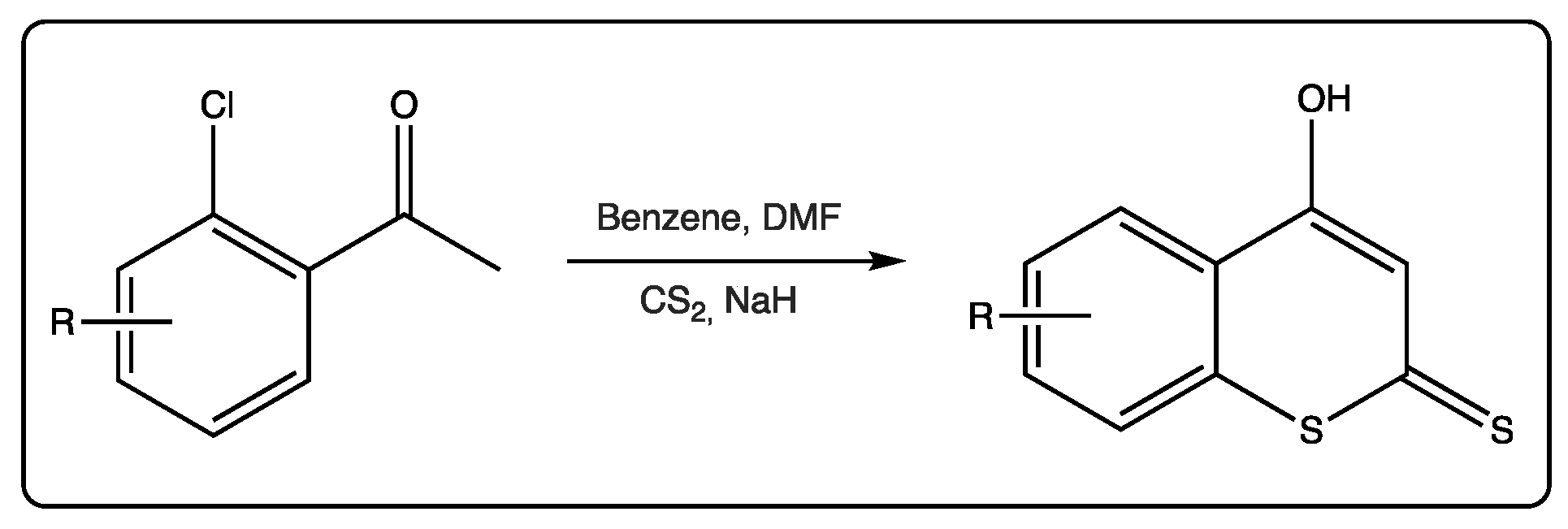
Figure 5. Synthesis of 4-hydroxydithiocoumarins starting from 2’-chloroacetophenones.
As previously mentioned, reactions using different dithiocoumarins as starting materials have been described. An oxidative cross-coupling reaction of 4-hydroxydithiocoumarin and amines/thiols using a combination of iodine and tert-butyl hydroperoxide (TBHP) to provide access to lead molecules for biomedical applications was recently reported (Figure 5) [33].
A reaction starting from different dithiocoumarins, in the presence of nitroalkenes allowed for the formation of tricyclic molecules [34]. This reaction is catalyzed by potassium carbonate, which allows the closure of the new five members ring. This is an unprecedented and efficient method via a thio[3+2] cyclization reaction of 4-hydroxydithiocoumarins and trans-β-nitrostyrenes. This protocol has been described as faster than the previously reported, following mild conditions, presenting good yields, allowing for the formation of C–C and C–S bonds in a regioselective manner (Figure 6).
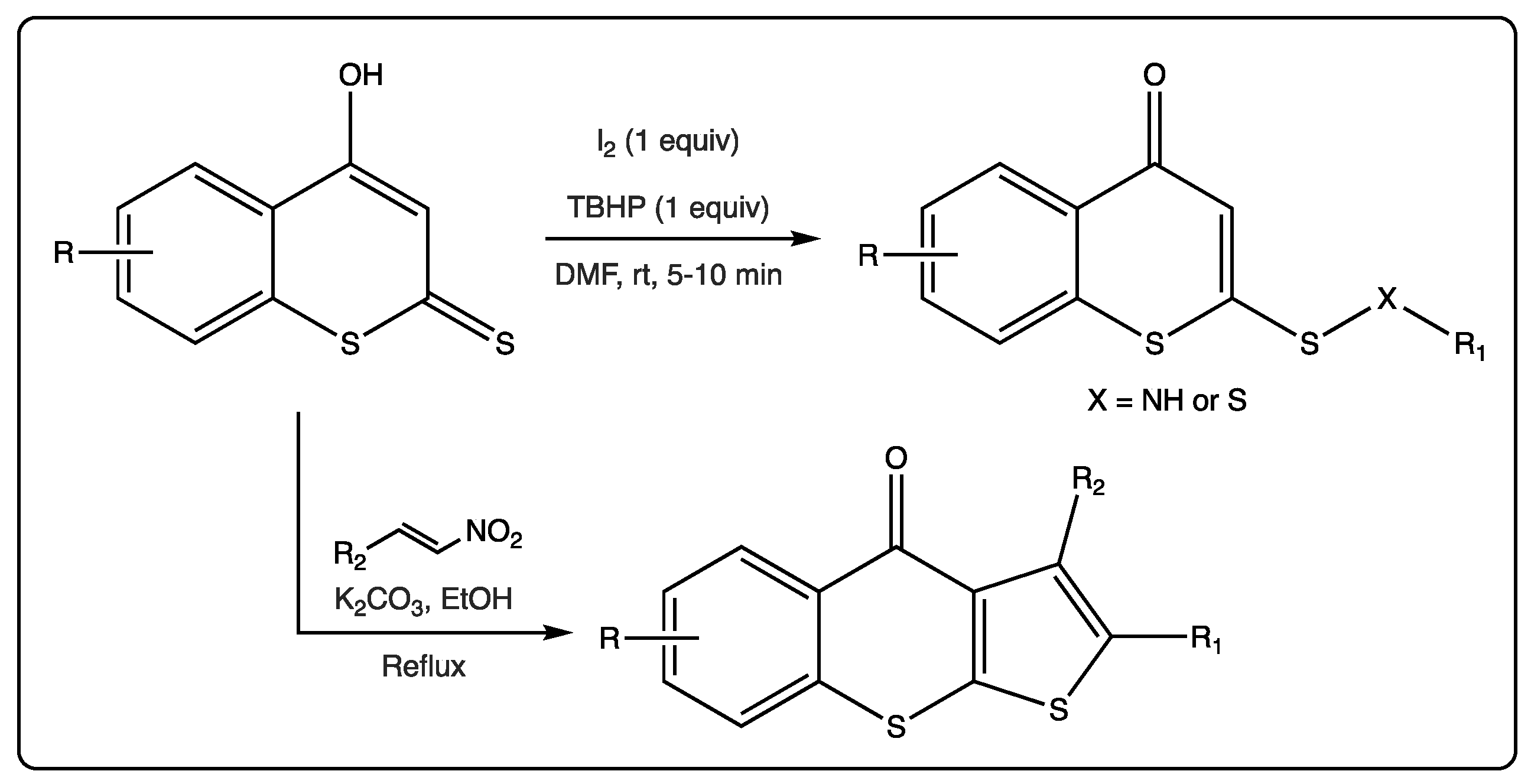
Figure 6. Thio[3+2] cyclization reaction of 4-hydroxydithiocoumarins and trans-β-nitrostyrenes.
As for thiocoumarins, 4-hydroxy-dithiocoumarins are the most studied starting materials for obtaining new and more complex molecules. The reactions and yields are similar to the previously described analogues.
References
- Beno, B.R.; Yeung, K.-S.; Bartberger, M.D.; Pennington, L.D.; Meanwell, N.A. A survey of the role of noncovalent sulfur interactions in drug design. J. Med. Chem. 2015, 58, 4383–4438.
- Meth-Cohn, O.; Tarnowski, B. Thiocoumarins. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 1980; pp. 115–133.
- Fourel, I.; Berlioz-Barbier, A.; Benoit, E. Mass spectrometry characterization of anticoagulant rodenticides and hydroxyl metabolites. Rapid Commun. Mass Spectrom. 2020, 34, e8871.
- Panetta, J.A.; Rapoport, H. Synthesis of thiocoumarins from acrylic and propiolic ortho esters and benzenethiols. J. Org. Chem. 1982, 47, 2626–2628.
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 2010, 53, 335–344.
- Supuran, C.T. Carbonic anhydrase inhibition/activation: Trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr. Pharm. Des. 2010, 16, 3233–3245.
- Ferraroni, M.; Carta, F.; Scozzafava, A.; Supuran, C.T. Thioxocoumarins show an alternative carbonic anhydrase inhibition mechanism compared to coumarins. J. Med. Chem. 2016, 59, 462–473.
- Zalubovskis, R. In a search for selective inhibitors of carbonic anhydrases: Coumarin and its bioisosteres—Synthesis and derivatization. Chem. Heterocycl. Compd. 2015, 51, 607–612.
- Monti, S.M.; Supuran, C.T.; De Simone, G. Anticancer carbonic anhydrase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2013, 23, 737–749.
- Haywood, R.D.; Franks, M.A. Synthetic design of coumarin and thiocoumarin derivatives to inhibit carcinogénesis. In Proceedings of the 245th ACS National Meeting & Exposition, New Orleans, LA, USA, 18 March 2013.
- Barraja, P.; Sciabica, L.; Diana, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G.; Disaro, S.; Basso, G.; et al. Synthesis and photochemotherapeutic activity of thiopyranoindol-2-ones. Bioorgan. Med. Chem. Lett. 2005, 15, 2291–2294.
- Jackson, S.A.; Sahni, S.; Lee, L.; Luo, Y.; Nieduzak, T.R.; Liang, G.; Chiang, Y.; Collar, N.; Fink, D.; He, W.; et al. Design, synthesis and characterization of a novel class of coumarin-based inhibitors of inducible nitric oxide synthase. Bioorgan. Med. Chem. 2005, 13, 2723–2739.
- Abdou, M.M. Utility of 4-hydroxythiocoumarin in organic synthesis. Arab. J. Chem. 2017, 10, S3955–S3961.
- Shinde, R.G.; Khan, A.A.; Barik, A. Formation of two centre three electron bond by hydroxyl radical induced reaction of thiocoumarin: Evidence from experimental and theoretical studies. Free Radic. Res. 2019, 53, 629–640.
- Mokrov, G.V.; Voronina, T.A.; Litvinova, S.A.; Kovalev, I.G.; Nerobkova, L.N.; Durnev, A.D.; Gudasheva, T.A.; Seredenin, S.B. Synthesis and anticonvulsant activity of 4-amino-3-nitro-1-thiocoumarins and 4-amino-3-nitroquinolin-2-ones. Pharm. Chem. J. 2019, 53, 194–200.
- Mokrov, G.V.; Litvinova, S.A.; Voronina, T.A.; Nerobkova, L.N.; Kutepova, I.S.; Kovalev, I.G.; Gudasheva, T.A.; Durnev, A.D. Design, synthesis, and anticonvulsant evaluation of 4-GABA-3-nitrocoumarines, 1-thiocoumarines, quinolone-2-ones, and their derivatives. Med. Chem. Res. 2019, 28, 1901–1911.
- He, X.; Wu, Y.; Zuo, Y.; Xie, M.; Li, R.; Shang, Y. Transition metal- and oxidant-free sulfonylation of 1-sulfonyl-1H-1,2,3-triazoles to enols for the synthesis of sulfonate derivatives. Synth. Commun. 2019, 49, 959–972.
- Jamkhandi, P.; Rajagopal, S. The chemistry of anticoagulants. Synthesis of aryl-substituted 4-hydroxythiocoumarin. Monatsh. Chem. 1963, 94, 1271–1273.
- Lee, S.-C.; Liao, H.-H.; Chatupheeraphat, A.; Rueping, M. Nickel-catalyzed C-S bond formation via decarbonylative thioetherification of esters, amides and intramolecular recombination fragment coupling of thioesters. Chem. Eur. J. 2018, 24, 3608–3612.
- Yang, S.-M.; Reddy, G.M.; Liu, M.-H.; Wang, T.-P.; Yu, J.-K.; Lin, W. Diastereoselective synthesis of Rauhut-Currier-type adducts via an unexpected α-addition of α,β-unsaturated γ-butyrolactams to coumarin derivatives. J. Org. Chem. 2017, 82, 781–789.
- Suzuki, Y.; Ando, A.; Nakagawa, M. Synthesis of 4-acylcoumarins by NHC-catalyzed nucleophilic substitution. Tetrahedron Lett. 2018, 59, 4276–4278.
- Debnath, M.; Sasmal, S.; Haldar, D. Fabrication of egg shell-like nanovesicles from a thiocoumarin-based ε-amino ester: A potential carrier. J. Mater. Chem. B 2017, 5, 5450–5457.
- Tiwari, M.R.; Patel, N.B. Synthesis and pharmacological activities of oxadiazole and pyrimidine bearing thiocoumarin derivatives. Curr. Microw. Chem. 2021, 8, 33–43.
- Voss, J.; Edler, R.; Adiwidjaja, G. Preparation of new tert-butyl substituted coumarins, thiocoumarins and dithiocoumarins. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1893–1905.
- Xu, Z.Y.; He, X.D.; Han, L.; Wang, X.H.; Huang, S.L.; Chen, J.R.; Xu, L.Q.; Luo, H.Q.; Li, N.B. Engineering of a multifunctional small molecule enables dual-channel fluorescence visualizing of environmental unamiable heavy metal ions as well as photoinactivation-based and ultra-efficient eliminating of human pathogens. Chem. Eng. J. 2022, 444, 136601.
- Cho, M.; Nguyen, V.-N.; Yoon, J. Simultaneous detection of hypochlorite and singlet oxygen by a yhiocoumarin-based ratiometric fluorescent probe. ACS Meas. Sci. Au 2022, 2, 219–223.
- Nguyen, V.-N.; Heo, S.; Kim, S.; Swamy, K.M.K.; Ha, J.; Park, S.; Yoon, J. A thiocoumarin-based turn-on fluorescent probe for hypochlorite detection and its application to live-cell imaging. Sens. Actuators B Chem. 2020, 317, 128213.
- Li, D.; Zhao, S.; Zhao, B.; Xia, Y.; Liu, A.; Wang, K.; Hou, R. Fluorescent Probe Containing Coumarin for Selective Recognition of Mercury Ion and Preparation Thereof. CN113666898A, 29 March 2017. (In Chinese).
- Nocentini, A.; Angeli, A.; Carta, F.; Winum, J.-Y.; Zalubovskis, R.; Carradori, S.; Capasso, C.; Donald, W.A.; Supuran, C.T. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J. Enzym. Inhib. Med. Chem. 2021, 36, 561–580.
- Ma, J.; Ripp, A.; Wassy, D.; Dürr, T.; Qiu, D.; Häner, M.; Haas, T.; Popp, C.; Bezold, D.; Richert, S.; et al. Thiocoumarin caged nucleotides: Synthetic access and their photophysical properties. Molecules 2020, 25, 5325.
- Nelson, A. Product class 9: Benzothiopyranones and benzothiopyranthiones. Sci. Synth. 2003, 14, 787–816.
- Anderson-McKay, J.E.; Liepa, A.J. The synthesis of 4-hydroxydithiocoumarins: A case of unusual tautomer stability. Aust. J. Chem. 1987, 40, 1179–1190.
- Mahato, K.; Arora, N.; Bagdi, P.R.; Gattu, R.; Ghosh, S.S.; Khan, A.T. An oxidative cross-coupling reaction of 4-hydroxydithiocoumarin and amines/thiols using a combination of I2 and TBHP: Access to lead molecules for biomedical applications. Chem. Commun. 2018, 54, 1513–1516.
- Mahato, K.; Bagdi, P.R.; Khan, A.T. K2CO3 catalyzed regioselective synthesis of thienothiochromen-4-one oximes: Access to the corresponding amine and nitroso derivatives. Org. Biomol. Chem. 2017, 15, 5625–5634.
- Dobelmann-Mara, L.; Riedmueller, S.; Schraub, M. Compounds for optically active devices. WO2017032442A1, 2 March 2017.
- Dobelmann-Mara, L.; Riedmueller, S.; Schraub, M. Hydrophilic Compounds for Optically Active Devices. WO 2017032444A1, 2 March 2017.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
15 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




