Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Moutih Rafei | -- | 2513 | 2022-07-28 18:26:17 | | | |
| 2 | Jessie Wu | + 2 word(s) | 2515 | 2022-07-29 05:54:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
El-Kadiry, A.E.; Beaudoin, S.; Plouffe, S.; Rafei, M. Accum™ Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/25626 (accessed on 08 February 2026).
El-Kadiry AE, Beaudoin S, Plouffe S, Rafei M. Accum™ Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/25626. Accessed February 08, 2026.
El-Kadiry, Abed El-Hakim, Simon Beaudoin, Sebastien Plouffe, Moutih Rafei. "Accum™ Technology" Encyclopedia, https://encyclopedia.pub/entry/25626 (accessed February 08, 2026).
El-Kadiry, A.E., Beaudoin, S., Plouffe, S., & Rafei, M. (2022, July 28). Accum™ Technology. In Encyclopedia. https://encyclopedia.pub/entry/25626
El-Kadiry, Abed El-Hakim, et al. "Accum™ Technology." Encyclopedia. Web. 28 July, 2022.
Copy Citation
Compromised activity is a common impediment for biologics requiring endosome trafficking into target cells. In cancer cells, antibody-drug conjugates (ADCs) are trapped in endosomes or subsequently pumped extracellularly, leading to a reduction in intracellular accumulation. In subsets of dendritic cells (DCs), endosome-engulfed antigens face non-specific proteolysis and collateral damage to epitope immunogenicity before proteasomal processing and subsequent surface presentation. Accum™ is a novel biotechnology exemplifying, in its initial concept, the need to circumvent the biological challenges of ADCs.
Accum
cholic acid
nuclear localization signal
1. Accum™
Assimilating their previous studies [1][2][3][4][5], wherein a synthetic peptide harboring the classical NLS (cNLS) sequence amply dictated the nuclear translocation of a mAb conjugate, Beaudoin and colleagues [6] coupled ChAc to the non-CPP, CGYGPKKKRKVGG [7], generated by an automated peptide synthesizer, then processed and characterized the conjugate via mass spectroscopy and ultra-performance liquid chromatography. PKKKRKV represents the cNLS sequence taken from simian virus 40 (SV-40) large T antigen, while GYG and GG residues serve as N- and C-terminus spacers, respectively [6]. Conjugation of Accum to protein can be performed by using any conjugation technique, such as the cross-linker sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (sulfo-SMCC) reacting with the amine of lysine and the sulfhydryl moiety of cysteine via its NHS-ester and the maleimide reactive group of the cross-linker (Figure 1) [6][8][9]. ChAcNLS, or Accum, is water-soluble; has a positive net charge of +5 and a molecular weight of 1.8 kDa; and is synthetically recovered with ≥94% purity and a molecular mass of 1768.5 g/mol [6][8]. Accum is added to conjugates in the same, or higher, molar excess ratio to yield Accum-modified conjugates [9]. During the modification process, free cross-linker and free Accum are eliminated by multiple centricon filtration and a Sephadex column, while Accum-modified conjugates are subsequently processed in ultrafiltration tubes, concentrated, and biochemically characterized with SDS-PAGE, turbidity assays, differential scanning fluorimetry, and protein concentration assays, followed by functional evaluation with conjugate-receptor affinity assays against naked or non-Accum-modified conjugates [8]. The number of Accum per conjugate is proportional to the number of accessible lysine residues [9] and the intracellular accumulation of conjugates [6][8]. The rate of conjugation is estimated by SDS-PAGE gel [6][8].
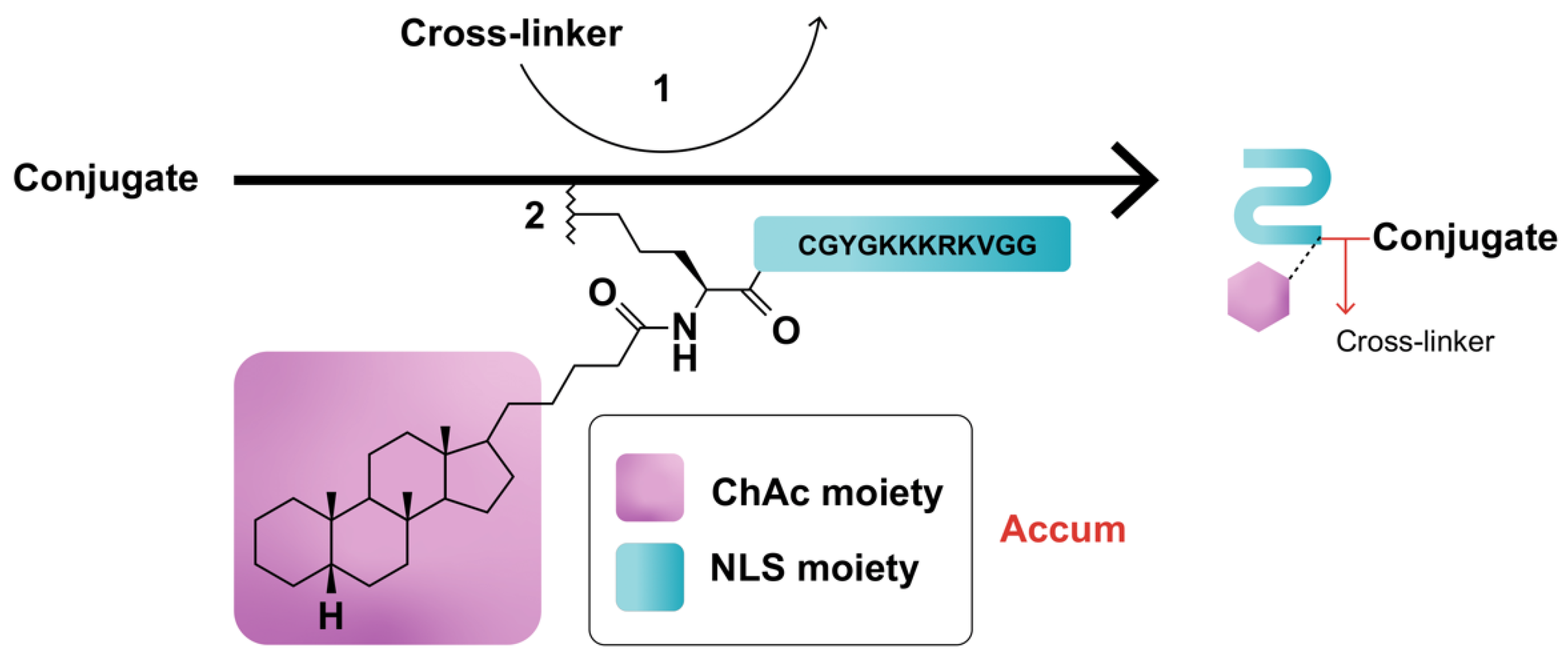
Figure 1. Schematic representation of Accum-modification of protein conjugates. In step (1), the protein reacts with a cross-linker, yielding a maleimide-activated conjugate. In step (2), the sulfhydryl group of the N-terminus cysteine cap of the NLS moiety of Accum reacts with the maleimide-activated conjugate, yielding an Accum-conjugate.
Accum can be likened to a polymath primer: it is well-informed about the biological limitations of CPP- and NLS-equipped conjugates [10] and highly adaptable for functionalizing different conjugates for different therapeutic purposes [6][8][9][10][11][12]. For instance, Beaudoin et al. [6] designed 7G3-Accum—Accum coupled to 7G3—a mAb specifically targeting interleukin-3 receptor-α (IL-3Rα), a cell-surface antigen expressed by leukemic cells. In the context of muscle invasive bladder cancer (MIBC), the same team [8][12] designed A14-Accum, where A14 is a mAb specific for interleukin-5 receptor α-subunit (IL-5Rα). In another therapeutic context, Accum was coupled to the clinically approved ADC trastuzumab-emtansine (T-DM1) [10]. Recently, Bikorimana et al. [9] introduced Accum to the setting of cellular vaccines, coupling ChAcNLS to the xenoantigen ovalbumin (OVA) with the rationale of triggering potent antigen presentation by DCs. The same group further linked Accum to lymphoma tumor lysate proteins to instill potent anti-tumor immunity in DCs. Overall, these studies attest to the pliability of Accum as a biotechnology that primes the function of various molecular conjugates, thereby enhancing therapeutic outcomes.
2. Characterization, Validation, and Efficacy Data
Most ADCs undergo receptor-mediated intracellular transport to lysosomes, where they exploit pH-sensitive proteases for the catabolism, release, and intracellular diffusion of their cytotoxic payloads [13]. However, this transport system faces a backlash from cancer cell mechanisms, including receptor downregulation/recycling and increased expression of multidrug resistance pumps, that either entrap drug payloads in endosomes or counteract their intracellular accumulation [14][15]. On another note, several new ADC modifications, including ADCs coupled to synthetic CPPs or pH-sensitive polymers, have been effective in evading endosome entrapment or routing to alternative cellular compartments; however, they have failed to either produce intracellular retention [16][17][18] or achieve target cell specificity and sufficient tumor uptake [19][20][21][22][23][24]; otherwise, they have induced high toxicity by indiscriminately penetrating off-target cells [18][20]. The Accum fusion compound ChAcNLS was thus devised with the aim of enabling ADCs to (i) dodge entrapment in the endosomal-lysosomal pathway, (ii) partake in nuclear routing, and (iii) accumulate intracellularly while retaining target cell selectivity and evading off-target payload effects [6]. Optimum endosome escape and intracellular accumulation was hypothesized to be the result of synergism between (a) the ChAc moiety, which selectively disrupts endosomal membranes by triggering ceramide formation while leaving plasma membranes intact [6] in the same way it allows nonenveloped viruses to escape from endosomes into the cytoplasm [25][26][27][28][29]; and (b) the NLS moiety, which directs molecular conjugates toward the nucleus [6]. To validate these hypotheses, different in vitro and in vivo studies were performed using different Accum-modified conjugates. Additional studies were also conducted to evaluate whether the distinctive cellular delivery mechanism of Accum provides superior therapeutic advantages compared to control counterparts.
2.1. 7G3-Accum
Beaudoin et al. [6][12] initially designed 7G3-Accum, an Accum-modified mAb specific for IL-3Rα. In characterization assays, the number of Accum copies per antibody was shown to correspond with the sulfo-SMCC:antibody ratio, reaching a 50:1 ratio with an average of 8.5 and 2.6 copies per heavy and light chain of 7G3, respectively [6][9][12]. In proof-of-concept experiments, IL-3Rα-expressing TF-1a leukemic cells were used to study the intracellular trafficking of 7G3-ChAcNLS against different controls, including unmodified 7G3, 7G3-ChAc, 7G3-NLS, 7G3-ChAcLeu, and 7G3-ChAcDap (ChAcLeu and ChAcDap enable endosome escape—but with less efficacity—without nuclear trafficking, and possess, respectively, structural and net charge similarities to ChAcNLS). Indeed, confocal microscopy revealed a distinctive cellular distribution pattern for 7G3-ChAcNLS, which was centered and homogenously radiating throughout the target cell, suggesting intracellular antibody retention [6][12]. Contrarily, 7G3- and 7G3-NLS-treated cells both showed similar distribution patterns with clusters proximal to the intracellular surface of the plasma membrane, typical of endosome entrapment [6]. On the other hand, 7G3-ChAc was detected in the cytoplasm with a homogeneous distribution throughout, but not in the nucleus. In quantitative analyses, flow cytometry showed significantly higher intracellular accumulation of 7G3-ChAcNLS compared to all controls [6]. Intracellular accumulation levels compared to unmodified 7G3 were significantly higher for 7G3-ChAcNLS (3-fold) and 7G3-ChAc (1.8-fold), whereas no significant change was detected for 7G3-NLS. The higher intracellular accumulation of 7G3-ChAcNLS compared to 7G3-ChAc, 7G3-ChAcLeu, and 7G3-ChAcDap revealed that the combination of the ChAc moiety with the NLS moiety is responsible for the endosome escape and intracellular accumulation of ChAcNLS [6]. In fact, if ChAc had only endosome escape activity and NLS only nuclear trafficking activity, the level of intracellular accumulation should have been the same as 7G3-ChAc, 7G3-ChAcLeu, or 7G3-ChAcDap [6]. To further evaluate target cell specificity, intracellular and nuclear accumulation of 7G3-ChAcNLS versus unmodified 7G3 were imaged with confocal microscopy in IL-3Rα-positive and -negative cells. The data revealed that ChAcNLS modification resulted in ample specific accumulation of 7G3 in target cells and nuclei, with a margin of 10–13% nonspecific localization. Similarly, the authors used flow cytometry, western blot, and radioactivity assays to demonstrate that Accum could enhance the intracellular and nuclear accumulation of its conjugate by a margin of 21.1-fold [6][12]. These data showed that the complementary functions of the Accum moieties—ChAc and NLS—are equally indispensable for maintaining effective intracellular conjugate accumulation and target cell selectivity.
2.2. A14-Accum
Paquette et al. [11] have previously shown that the rapid internalization of IL-5Rα, whose levels are preferentially elevated in MIBC tumors, renders the use of ADCs with specificity for IL-5Rα strategic for the intracellular accumulation of payloads, including the positron emitter copper-64 (64Cu) used for imaging. Therefrom, the same group [8] designed A14-Accum, an Accum-modified mAb against IL-5Rα, to characterize its use as a tumor cell accumulator. To further assess its use as an efficient drug delivery system, A14-Accum was coupled to the radioactive diagnostic agent 64Cu [12]. 64Cu-A14-Accum indeed revealed a purity of preparation within the 95–99% range and had an affinity for IL-5Rα in the nanomolar spectrum [8]. Unpublished data also revealed that Accum augmented the kinetics of receptor internalization. When incubated with MIBC cell lines and assessed with flow cytometry (for quantifying ceramide levels) and confocal microscopy (for visualizing endosome disruption and quantifying nuclear localization), A14-ChAcNLS demonstrated marked endosome escape with nuclear routing compared to A14 [8]. In radioactivity assays investigating cargo deposition, A14-ChAcNLS significantly increased the intracellular accumulation of 64Cu cargo by 3.3–9.4- and 3.2–4.6-fold compared to A14 and A14-NLS, respectively, in high- and low-density IL-5Rα-expressing MIBC cell lines [8]. In the same cell lines, A14-ChAcNLS also increased the nuclear accumulation of 64Cu cargo by 1.7–5.3- and 2.5–2.6-fold compared to A14 and A14-NLS, respectively. When target selectivity was analyzed against an IgG-ChAcNLS control showed, A14-ChAcNLS showed only trace non-specificity for intracellular 64Cu cargo accumulation [8]. Beaudoin et al. [12] also showed that Accum resulted in significant intracellular and nuclear specificity and accumulation (≥3-fold) of 64Cu in IL-5Rα-positive invasive bladder cancer cells. These in vitro data demonstrated that both Accum moieties are necessary for increasing the intracellular accumulation of cargo-bearing conjugates and maintaining target specificity.
Paquette and colleagues [8] further characterized the pharmacokinetic (PK) profile of 64Cu-A14-ChAcNLS injected into mouse models of human high- or low-density IL-5Rα MIBC. Blood sampling revealed an estimated half-life (t1/2) of 54.4 h, and a study of the organs indicated major hepatic metabolism and clearance with early transient glomerular accumulation. In comparative analyses, the t1/2 of 64Cu-A14-ChAcNLS was 45% lower compared to 64Cu-A14 (t1/2 = 99.5 h), indicating increased blood clearance. Nonetheless, ChAcNLS maintained the levels of 64Cu-A14 tumor uptake. Moreover, organ dissection 96 h post-injection showed that the biodistribution of 64Cu-A14-ChAcNLS was reduced in most healthy organs compared to 64Cu-A14 with significant reductions in the liver and heart [8]. Biodistribution of 64Cu-A14-ChAcNLS was also visualized with positron emission tomography (PET) and revealed equivalent tumor uptake with a higher tumor/adjacent muscle uptake ratio compared to 64Cu-A14 [8][12]. These preclinical PK and PET analyses demonstrate that Accum technology provides ACs with (i) comparable high rates of tumor uptake, (ii) elevated intracellular accumulation, and (iii) intermediate clearance rates from healthy tissues, for an overall improvement in selective tumor targeting.
2.3. T-DM1-Accum
T-DM1 clinically hinders breast cancer growth, yet several patients still experience disease progression [30] due to insufficient intracellular accumulation [31][32]. Lacasse et al. [10] thus investigated whether Accum-modification enhanced intracellular accumulation of T-DM1 by using the human epidermal growth factor receptor 2 (HER2)-positive breast cancer cell line SKBR3. Confocal microscopy with temporal imaging revealed that T-DM1-Accum first undergoes endosome entrapment, then diffuses throughout the cytoplasm before slowly localizing in the nucleus, where it is proteolyzed and T-DM1 is released [10]. In subsequent analyses, nuclear transport of T-DM1-Accum was shown to be mediated by the nuclear transport receptor importin-7 (IPO7) based on electrostatic interactions fueled by the cationic charge build-up on NLS moieties [10]. Contrarily, unmodified T-DM1 localized near the plasma membrane [10] due to the rapid recycling of HER2 back to the cell surface of SKBR3 [31][33][34]. Additionally, T-DM1-Accum had stronger cytotoxic potency compared to T-DM1-NLS and unmodified T-DM1 by several-fold. Importantly, the authors showed that the specificity of T-DM1 for HER2 was not altered by Accum modification, and Accum did not perturb HER2 binding and its internalization processes [10]. These data validated that Accum modifications prime the cytotoxicity of molecular payloads by overriding endosome entrapment and directing nuclear localization, all while retaining target cell specificity. Next, the same group [10] investigated the therapeutic potency of T-DM1-Accum in HER2-positive SKBR3 cells. T-DM1-Accum demonstrated marked cytotoxicity, achieving 50% tumor growth inhibition at concentrations >9- and >3.2-fold lower compared to T-DM1 and T-DM1-NLS, respectively [10]. In gene knockdown studies, this cytotoxicity relied on IPO7-mediated nuclear transport, likely driven by electrostatic interactions between the NLS moiety of Accum (positive charge) and IPO7 nuclear receptor (negative charge) [10]. Notably, T-DM1-NLS demonstrated >1.8-fold more cytotoxicity compared to T-DM1, and Accum was not cytotoxic itself, but rather enhanced the cytotoxicity of T-DM1, since Trastuzumab (Tmab)-Accum was as equally non-toxic as Tmab. Overall, these data corroborate that both Accum moieties operate with a functional synergism that results in enhanced cellular accumulation and therapeutic efficacy [10].
2.4. OVA- and Lymphoma Lysate Proteins-Accum
Antigen presenting cells such as DCs engulf soluble antigens and sort them into endosomes for limited degradation before exportation to the cytosol [35]. Therein, the proteasome further processes antigen fragments into short amino acid sequences that are presented on the cell surface by Major Histocompatibility Complex (MHC) class I molecules for T-cell activation, a process termed cross-presentation [36][37]. However, non-specific endosomal degradation of antigens in certain subsets of DCs, such as monocyte-derived CD8− DCs, can compromise antigen immunogenicity and reduce anti-tumoral immunity [38]. Drawing on Accum’s innovative mechanism of action validated with 7G3 [6], A14 [8], and T-DM1 [10], Bikorimana and colleagues [9] conjugated Accum to soluble antigens to investigate whether its ADC-functionalizing biotechnology could prime the antigen cross-presentation properties of CD8− DCs. Accum modification increased the resistance of OVA to thermal denaturation [9]. Unlike OVA, OVA-Accum uptake by DCs induced endosome rupture, as revealed by confocal microscopy. Using primary bone-marrow derived DCs, it was further shown that OVA-Accum was processed in the cytosol, as per flow cytometry analysis [9]. Complementary antigen presentation assays using primary DCs co-cultured with T cells also showed that OVA-Accum induced marked immune cell activation, resulting in heightened inflammatory cytokine secretion [9]. Similarly, Accum-modified proteins derived from lymphoma lysate significantly enhanced the immunogenicity of allogeneic DCs in tumor-bearing mice [9]. Combined, these data validate the peculiarity of endosome-to-cytosol translocation, where Accum enables antigens to escape endosomal damage, undergo efficient proteasomal processing into immunogenic peptides, and arrive at the cell surface for potent antigen cross-presentation and subsequent T-cell activation.
Deploying these proof-of-principle data, Bikorimana and colleagues investigated whether the enhanced immunogenicity of OVA-Accum in DCs translates to a potent cellular vaccine [9]. In the context of prophylactic vaccination, two doses of OVA-Accum-pulsed primary DCs were administered subcutaneously 2 weeks apart in mice challenged thereafter with three ascending doses of an OVA-expressing T-cell lymphoma. Compared to OVA-pulsed DCs, OVA-Accum-pulsed DCs completely counteracted tumor growth, maintaining full and durable survival of all mice at 96 days post-immunization. Concomitantly, serum analysis showed higher antibody titers, and immunophenotyping revealed markedly higher levels of CD4 effector and CD8 central and effector memory T cells. Pro-inflammatory mediators were also more heightened [9]. As for therapeutic vaccination, lymphoma-bearing mice were administered two subcutaneous injections of OVA-Accum-pulsed syngeneic or allogeneic DCs one week apart. Compared to OVA-pulsed DCs, OVA-Accum-pulsed DCs delayed tumor growth and prolonged survival. Co-administration of the immune-checkpoint inhibitor anti-PD-1 over 2 weeks further enhanced these outcomes, producing at 54 days post-immunization 50% overall survival (OS), 30–40% partial response, and 10–20% complete response (CR) [9]. Allogeneic DCs pulsed with Accum-modified non-OVA-expressing lymphoma lysate proteins were also examined as a broader-spectrum vaccine for therapeutic efficacy. In lymphoma-bearing mice, there was only a minor delay in tumor growth. However, combined with anti-PD-1, this treatment resulted in marked tumor growth delays resulting in 70% OS and 30% CR compared to standard lysate-pulsed DCs co-administered with anti-PD-1 [9]. With Accum-lysate-pulsed DCs/anti-PD-1, immunophenotyping also showed elevated levels of immune effector cells and diminished levels of regulatory CD4 T cells [9].
References
- Fasih, A.; Fonge, H.; Cai, Z.; Leyton, J.V.; Tikhomirov, I.; Done, S.J.; Reilly, R.M. 111In-Bn-DTPA-nimotuzumab with/without modification with nuclear translocation sequence (NLS) peptides: An Auger electron-emitting radioimmunotherapeutic agent for EGFR-positive and trastuzumab (Herceptin)-resistant breast cancer. Breast Cancer Res. Treat. 2012, 135, 189–200.
- Leyton, J.V.; Gao, C.; Williams, B.; Keating, A.; Minden, M.; Reilly, R.M. A radiolabeled antibody targeting CD123(+) leukemia stem cells-initial radioimmunotherapy studies in NOD/SCID mice engrafted with primary human AML. Leuk Res. Rep. 2015, 4, 55–59.
- Leyton, J.V.; Hu, M.; Gao, C.; Turner, P.V.; Dick, J.E.; Minden, M.; Reilly, R.M. Auger electron radioimmunotherapeutic agent specific for the CD123+/CD131− phenotype of the leukemia stem cell population. J. Nucl. Med. 2011, 52, 1465–1473.
- Zereshkian, A.; Leyton, J.V.; Cai, Z.; Bergstrom, D.; Weinfeld, M.; Reilly, R.M. The human polynucleotide kinase/phosphatase (hPNKP) inhibitor A12B4C3 radiosensitizes human myeloid leukemia cells to Auger electron-emitting anti-CD123 111In-NLS-7G3 radioimmunoconjugates. Nucl Med. Biol 2014, 41, 377–383.
- Leyton, J.V.; Williams, B.; Gao, C.; Keating, A.; Minden, M.; Reilly, R.M. MicroSPECT/CT imaging of primary human AML engrafted into the bone marrow and spleen of NOD/SCID mice using 111In-DTPA-NLS-CSL360 radioimmunoconjugates recognizing the CD123+/CD131− epitope expressed by leukemia stem cells. Leuk Res. 2014, 38, 1367–1373.
- Beaudoin, S.; Rondeau, A.; Martel, O.; Bonin, M.A.; van Lier, J.E.; Leyton, J.V. ChAcNLS, a Novel Modification to Antibody-Conjugates Permitting Target Cell-Specific Endosomal Escape, Localization to the Nucleus, and Enhanced Total Intracellular Accumulation. Mol. Pharm. 2016, 13, 1915–1926.
- Sarko, D.; Beijer, B.; Garcia Boy, R.; Nothelfer, E.M.; Leotta, K.; Eisenhut, M.; Altmann, A.; Haberkorn, U.; Mier, W. The pharmacokinetics of cell-penetrating peptides. Mol. Pharm. 2010, 7, 2224–2231.
- Paquette, M.; Beaudoin, S.; Tremblay, M.A.; Jean, S.; Lopez, A.F.; Lecomte, R.; Guerin, B.; Bentourkia, M.; Sabbagh, R.; Leyton, J.V. NLS-Cholic Acid Conjugation to IL-5Ralpha-Specific Antibody Improves Cellular Accumulation and In Vivo Tumor-Targeting Properties in a Bladder Cancer Model. Bioconjugate Chem. 2018, 29, 1352–1363.
- Bikorimana, J.-P.; Salame, N.; Beaudoin, S.; Balood, M.; Crosson, T.; Abusarah, J.; Talbot, S.; Löbenberg, R.; Plouffe, S.; Rafei, M. Promoting antigen escape from dendritic cell endosomes potentiates anti-tumoral immunity. Cell Rep. Med. 2022, 3, 100534.
- Lacasse, V.; Beaudoin, S.; Jean, S.; Leyton, J.V. A Novel Proteomic Method Reveals NLS Tagging of T-DM1 Contravenes Classical Nuclear Transport in a Model of HER2-Positive Breast Cancer. Mol. Ther Methods Clin. Dev. 2020, 19, 99–119.
- Paquette, M.; Vilera-Perez, L.G.; Beaudoin, S.; Ekindi-Ndongo, N.; Boudreaut, P.L.; Bonin, M.A.; Battista, M.C.; Bentourkia, M.; Lopez, A.F.; Lecomte, R.; et al. Targeting IL-5Ralpha with antibody-conjugates reveals a strategy for imaging and therapy for invasive bladder cancer. Oncoimmunology 2017, 6, e1331195.
- Beaudoin, S.; Paquette, M.; Fafard-Couture, L.; Tremblay, M.A.; Lecomte, R.; Guerin, B.; Leyton, J.V. Initial Evaluation of Antibody-Conjugates Modified with Viral-Derived Peptides for Increasing Cellular Accumulation and Improving Tumor Targeting. J. Vis. Exp. 2018, 133, e55440.
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344.
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209.
- Chen, R.; Hou, J.; Newman, E.; Kim, Y.; Donohue, C.; Liu, X.; Thomas, S.H.; Forman, S.J.; Kane, S.E. CD30 Downregulation, MMAE Resistance, and MDR1 Upregulation Are All Associated with Resistance to Brentuximab Vedotin. Mol. Cancer Ther. 2015, 14, 1376–1384.
- Berguig, G.Y.; Convertine, A.J.; Shi, J.; Palanca-Wessels, M.C.; Duvall, C.L.; Pun, S.H.; Press, O.W.; Stayton, P.S. Intracellular delivery and trafficking dynamics of a lymphoma-targeting antibody-polymer conjugate. Mol. Pharm. 2012, 9, 3506–3514.
- Lackey, C.A.; Press, O.W.; Hoffman, A.S.; Stayton, P.S. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjugate Chem. 2002, 13, 996–1001.
- Tolstikov, V.V.; Cole, R.; Fang, H.; Pincus, S.H. Influence of endosome-destabilizing peptides on efficacy of anti-HIV immunotoxins. Bioconjugate Chem. 1997, 8, 38–43.
- Kameyama, S.; Horie, M.; Kikuchi, T.; Omura, T.; Takeuchi, T.; Nakase, I.; Sugiura, Y.; Futaki, S. Effects of cell-permeating peptide binding on the distribution of 125I-labeled Fab fragment in rats. Bioconjugate Chem. 2006, 17, 597–602.
- Kameyama, S.; Okada, R.; Kikuchi, T.; Omura, T.; Nakase, I.; Takeuchi, T.; Sugiura, Y.; Futaki, S. Distribution of immunoglobulin Fab fragment conjugated with HIV-1 REV peptide following intravenous administration in rats. Mol. Pharm. 2006, 3, 174–180.
- Zhao, Y.; Lou, D.; Burkett, J.; Kohler, H. Chemical engineering of cell penetrating antibodies. J. Immunol. Methods 2001, 254, 137–145.
- Anderson, D.C.; Nichols, E.; Manger, R.; Woodle, D.; Barry, M.; Fritzberg, A.R. Tumor cell retention of antibody Fab fragments is enhanced by an attached HIV TAT protein-derived peptide. Biochem. Biophys. Res. Commun. 1993, 194, 876–884.
- Cornelissen, B.; Hu, M.; McLarty, K.; Costantini, D.; Reilly, R.M. Cellular penetration and nuclear importation properties of 111In-labeled and 123I-labeled HIV-1 tat peptide immunoconjugates in BT-474 human breast cancer cells. Nucl. Med. Biol. 2007, 34, 37–46.
- Hu, M.; Chen, P.; Wang, J.; Chan, C.; Scollard, D.A.; Reilly, R.M. Site-specific conjugation of HIV-1 tat peptides to IgG: A potential route to construct radioimmunoconjugates for targeting intracellular and nuclear epitopes in cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 301–310.
- Shivanna, V.; Kim, Y.; Chang, K.O. The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology 2014, 456–457, 268–278.
- Basanez, G.; Ruiz-Arguello, M.B.; Alonso, A.; Goni, F.M.; Karlsson, G.; Edwards, K. Morphological changes induced by phospholipase C and by sphingomyelinase on large unilamellar vesicles: A cryo-transmission electron microscopy study of liposome fusion. Biophys. J. 1997, 72, 2630–2637.
- Gulbins, E.; Dreschers, S.; Wilker, B.; Grassme, H. Ceramide, membrane rafts and infections. J. Mol. Med. 2004, 82, 357–363.
- Montes, L.R.; Ruiz-Arguello, M.B.; Goni, F.M.; Alonso, A. Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol. Chem. 2002, 277, 11788–11794.
- Siskind, L.J.; Colombini, M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 2000, 275, 38640–38644.
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791.
- Austin, C.D.; De Maziere, A.M.; Pisacane, P.I.; van Dijk, S.M.; Eigenbrot, C.; Sliwkowski, M.X.; Klumperman, J.; Scheller, R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 2004, 15, 5268–5282.
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R.; et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-DM1). Mol. Cancer Ther. 2018, 17, 243–253.
- Hommelgaard, A.M.; Lerdrup, M.; van Deurs, B. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol. Biol. Cell 2004, 15, 1557–1567.
- Longva, K.E.; Pedersen, N.M.; Haslekas, C.; Stang, E.; Madshus, I.H. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int. J. Cancer 2005, 116, 359–367.
- Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976, 143, 1283–1288.
- Carbone, F.R.; Heath, W.R. Cross-priming: Its beginnings. J. Immunol 2010, 185, 1353–1354.
- Trombetta, E.S.; Ebersold, M.; Garrett, W.; Pypaert, M.; Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 2003, 299, 1400–1403.
- Savina, A.; Peres, A.; Cebrian, I.; Carmo, N.; Moita, C.; Hacohen, N.; Moita, L.F.; Amigorena, S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009, 30, 544–555.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
29 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




