Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rajender Boddula | -- | 4324 | 2022-07-22 17:50:02 | | | |
| 2 | Sirius Huang | -2 word(s) | 4322 | 2022-07-25 03:53:38 | | | | |
| 3 | Sirius Huang | Meta information modification | 4322 | 2022-07-25 03:58:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pothu, R.; Tabassum, N.; Pattnaik, A.; Boddula, R.; Balla, P.; Gundeboyina, R.; Challa, P.; Rajesh, R.; Perugopu, V.; Al-Qahtani, N.; et al. Catalysts for Glycerol Reforming. Encyclopedia. Available online: https://encyclopedia.pub/entry/25459 (accessed on 07 February 2026).
Pothu R, Tabassum N, Pattnaik A, Boddula R, Balla P, Gundeboyina R, et al. Catalysts for Glycerol Reforming. Encyclopedia. Available at: https://encyclopedia.pub/entry/25459. Accessed February 07, 2026.
Pothu, Ramyakrishna, Nabila Tabassum, Aishanee Pattnaik, Rajender Boddula, Putrakumar Balla, Raveendra Gundeboyina, Prathap Challa, Rajendiran Rajesh, Vijayanand Perugopu, Noora Al-Qahtani, et al. "Catalysts for Glycerol Reforming" Encyclopedia, https://encyclopedia.pub/entry/25459 (accessed February 07, 2026).
Pothu, R., Tabassum, N., Pattnaik, A., Boddula, R., Balla, P., Gundeboyina, R., Challa, P., Rajesh, R., Perugopu, V., Al-Qahtani, N., & Abdullah, A. (2022, July 23). Catalysts for Glycerol Reforming. In Encyclopedia. https://encyclopedia.pub/entry/25459
Pothu, Ramyakrishna, et al. "Catalysts for Glycerol Reforming." Encyclopedia. Web. 23 July, 2022.
Copy Citation
The valuable products produced from glycerol transformation have become a research route that attracted considerable benefits owing to their huge volumes as well as a myriad of chemical and biological techniques for transforming glycerol into high-value compounds, such as fuel additives, biofuels, precursors, and other useful chemicals, etc. Steam, aqueous, and autothermal reforming processes have been primarily investigated in glycerol reforming. An update on glycerol reforming is given, with an exclusive focus on the various catalyst's performance in designing reaction operation conditions.
glycerol
biodiesel-biowaste
reforming
catalysts
value-added products
1. Introduction
Glycerol transformation carried out through combustion in the past was unsafe, uneconomic, and technically deficient due to the high temperature employed, unwanted gas emission, and complex product [1][2]. Reforming techniques have gained acceptance by industrialists and researchers in view of their efficiencies. Steam, aqueous, and autothermal reforming processes have been primarily investigated in glycerol reforming. The steam reforming reaction is one of the most common reactions used in hydrogen synthesis. Low pressure and very endothermic reactions promote hydrogen selectivity. The aqueous phase reforming (APR) method is regarded as an inexpensive method for producing H2 due to its lower temperature and lack of vaporizing water and fuels [3][4]. Glycerol has a wide product distribution due to its complexity compared to low-chain hydrocarbons or alcohols and to the numerous reactions associated with the reforming process (Figure 1). However, this technique creates H2 without vaporizing the feedstock, resulting in significant energy savings [5]. Supercritical water gasification (SCWG)) is a cost-effective method of turning biomass (such as food waste, paper industrial waste, sewage sludge, agricultural waste, and forestry residue) into hydrogen-rich syngas [6]. Chemical looping steam reforming (CLSR), which uses oxygen carriers (OCs) as a catalyst, has been widely recognized as a highly effective method for generating hydrogen from catalytic steam reforming [7].
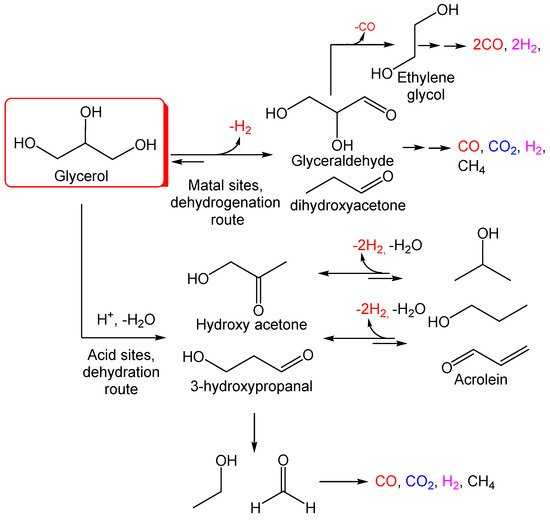
Figure 1. Potential reaction pathways in glycerol aqueous phase reforming (APR).
Bio-derived liquids steam reforming produces hydrogen and becomes interested due to the environment and raises hydrogen demand, specifically in proton-exchange membrane fuel cells (PEMFC) [8]. The low temperature and high-pressure process conditions, concentrated feed, and acidic catalysts are used for the supercritical water reforming process. High temperature and low pressure, diluted feed, and alkali catalyst are used for gasification [9].
The glycerol reforming and endothermic reactions took place. Hence, the heat needed for the decomposition of glycerol (Equation (1)) is significantly larger than the water–gas shift (WGS) reaction (Equation (2)), where heat is produced as energy. As operated under atmospheric pressure, glycerol steam reforming (Equation (3)) becomes desirable with less fear of protection. Considerably, 1 mol of glycerol converts to produce 7 mol of hydrogen (Equation (2)). Vast amounts of steam and elevated temperature shift a reaction forward to equilibrium, and hence much more H2 is generated [10].
C3H8O3 ↔ 4H2 + 3CO Decomposition Reaction (1)
CO + H2O ↔ H2 + CO2 Water Gas Shift Reaction (2)
C3H8O3 + 3H2O ↔ 7H2 + 3CO2 Steam Reforming Reaction (3)
The mentioned noble metal catalysts, Pt [11][12], Ir [13], Ru [14], and Rh [15][16], have shown good resistance to coke and catalytic activity. Vaidya and Rodrigues studied Ni, Pt, and Ru catalysts that promote hydrogen production but cause deactivation by dehydration [17].
2. Platinum Catalysts for Glycerol Reforming
Doukkali et al. investigated Pt and/or Ni supported over γ-alumina catalyst for the generation of hydrogen by a method of APR through three preparation methods of catalyst: impregnation method, and sol-gel in acidic and basic medium. Total glycerol transformation occurred with maximum hydrogen production by sol-gel in basic method over PtNiAl (T = 250 °C, P = 30 bar, and WHSV = 2.6 hr−1). The acidity of the superficial hydroxyl groups on the -AlOOH surface could be to blame for the lower glycerol conversion into gaseous products, favoring dehydration processes and resulting in more OHCs in the liquid phase. In fact, by blocking surface hydroxyl sites on -Al2O3, structural alterations in hydrothermal settings were explored to create efficient alumina catalysts for APR processes. The drop in H2 production could be explained by Ni particle aggregation and reoxidation of Ni particles. These behaviors combined with the adsorption of carbonaceous molecules/organic compounds identified on several of the catalysts could be the primary causes of their deactivation [11].
Pompeo et al. investigated SiO2 catalyst supported with Pt and Ni through the ion-exchange method for steam reforming to produce hydrogen. The experiment for the steam reforming reaction of glycerol has demonstrated that using 1 Pt and 2 Pt catalysts. The more active and stable catalyst was Pt compared to Ni catalyst. At 350 °C, a complete conversion to gas was achieved with a space-time of 1.66 and 0.88 min, respectively. Due to the fact that the Ni catalyzed the WGS reaction to remove the CO adsorbed on the surface and turn it into CO2, the selectivity of gas products was significantly different. Only 3% CO was obtained using the 5 Ni catalyst at 450 °C for 1.66 min. During 40 hr activity test on stream at 350 °C, the 2 Pt catalyst allowed entire conversion to gas without deactivation. It was also suggested that bimetallic catalyst research is required to obtain a system with high selectivity for H2 and good stability [12].
3. Ruthenium Catalysts for Glycerol Reforming
Ru catalyst supported over core-shell metal-ceramic micro composites, created at low temperature and 1 atm pressure through microwave-induced hydrothermal oxidation technique (MW-HTO) of aluminum (Al) metal particles. The highest glycerol turnover ratio was achieved over Al, and Ru-modified Al2O3 at Al compared with Ru-modified MgAl2O4 and Ru-modified Al2O3 catalysts, suggesting that Al metal particles seem to be more effective in preparing MgAl2O4 at Al and Al2O3 at Al composite structure. The highest selectivity of hydrogen was 70% at Ru/MgAl2O4 at Al catalyst at 550 °C reaction temperature, 1 atm pressure with 870 mm gcat−1 h−1 feed flow rate. The Ru/MgAl2O4@Al and Ru/Al2O3@Al catalysts have 2–3 times the glycerol conversion rates of their Ru/MgAl2O4 and Ru/Al2O3counterparts, owing to enhanced heat transfer via the metal-ceramic composite catalytic architectures [14]. Glycerol steam reforming process over Ru doped Al2O3 catalyst. In total, 89.1% glycerol transformed to produce 0.49 (mol/mol) yield of hydrogen at T = 500 °C, P = 0.1 MPa and 1.98 W/FA0. This research has aided in developing reactor systems for producing hydrogen from glycerol steam reforming [15].
4. Rhodium Catalysts for Glycerol Reforming
Martinez et al. studied hydrogen production from glycerol reforming using fluorite-type oxides CeZr-CoRh prepared by the sol-gel method. 100% glycerol was transformed to produce 86% hydrogen yield at 650 °C. The activity data showed that the catalysts’ ability to activate H2O under reaction circumstances was connected to selective H2 generation. This process guarantees that the by-products are steam reformed to H2. As this capacity deteriorates, H2 generation decreases, steam reforming capability reduces, and glycerol decomposition takes over. In this scenario, the creation of condensable compounds, such as hydroxyacetone, acetaldehyde, and acrolein, was aided by the production of CO, CH4, and C2H4 [18].
Lee and Doohwan synthesized Core−shell MgAl2O4 metal−ceramic composites at Al catalyst by hydrothermal method, and Rh (1, 3, and 5 wt.% loadings) modified MeAl2O4 catalysts were synthesized by incipient wetness impregnation method. Then, 100% glycerol transformed to produce Rh/MgAl2O4 at Al catalyst [T = 450 °C, and WHSV = 34,000 mL g−1 h−1]. The high surface area and complex surface morphological properties of MeAl2O4@Al as a catalyst enhance increased Rh cluster dispersion while hindering their thermal sintering during glycerol steam reforming processes at high temperatures. Rh/MeAl2O4@Al had a 20–30% greater glycerol conversion turnover rate than Rh/MeAl2O4 (both Rh cluster sizes are similar), indicating that the facilitated heat and mass transfer through the unique MeAl2O4@Al microstructures cooperated constructively for the reactions. These are being explored as future research topics [19]. Baca et al. studied Rh, Ni, and Co metal-doped over Al2O3–ZrO2–TiO2 (AZT) for syngas production through carbon dioxide reforming of glycerol. Then, 1 wt.% Rh doped AZT, 5 wt.% Ni-doped AZT, and 5 wt.% Co-doped AZT catalysts were synthesized through the incipient impregnation method. The glycerol transformation was 80% achieved with 24% maximum hydrogen yield on Rh doped AZT catalyst at 750 °C. Glycerol Conversion reduced in the given sequence: 1% Rh doped AZT > 5% Ni doped AZT > 5% Co-doped AZT. Rh doped AZT catalyst remained stable due to fewer coke formations and a shortage of sintering. During the 72-h testing, the decrease in CO2 conversions was less than 13%, indicating the remarkable stability of Rh/AZT and Ni/AZT [16]. Delparish et al. produced syngas from glycerol by oxidative steam reforming. The impregnation technique was used to prepare 2 wt.% of Rh doped Al2O3 catalysts. The glycerol was completely converted at 550 °C, and maximum hydrogen yield was achieved at 700 °C. Temperature influenced product distribution by increasing the amount of combustible species in the product mixture that were oxidized. The effect became more severe when C/O was lowered from 1.125 to 0.75. The promotion of WGS in the presence of steam was confirmed by reducing S/C from 5 to 4 and then from 4 to 3, which resulted in monotonically decreased H2 and CO2 yields and an increase in CO yield [20]. Danga et al. studied glycerol and ethanol for dry reforming with CaCO3 to produce syngas. A series of metal components, such as nickel, iron, copper, platinum, palladium, ruthenium, and rhodium, were used to promote dry reforming. In these series of metals, nickel became a promising candidate due to its outstanding performance, as well as its low price. Here, 100% glycerol conversion along with approximate 92% syngas selectivity was achieved on 10 wt.% Ni contained in CaCO3catalyst [T = 550 °C, and P = 1 atm.]. The CO2 released from the CaCO3 catalyst eliminated cokes and attained stability. The direct use of CaCO3 in this process was discovered viable, and the CO2 generated from CaCO3 decomposition may be used to modify the H2/CO ratio. Ni was the most promising contender among the active metals, including Ni, Co, Fe, Cu, Rh, Ru, Pt, and Pd, due to its great performance and low price. Over the 10 Ni–CaCO3 catalyst, 100% conversion of glycerol and ethanol, 92% summed H2 and CO selectivity, and an H2/CO ratio of 1.2 could be reached under ideal conditions. Meanwhile, the robustness of this integrated technique was proved over five dry reforming–regeneration cycles. The findings suggest a novel way to use CO2 in the form of carbonates [21].
5. Nickel Catalysts for Glycerol Reforming
The catalyst based on Ni becomes a choice for the reason that the metallic sites of Ni work well enough in water gas shift reaction as well as C–H and C–O cleavage. Nevertheless, the tremendous challenge is how to control catalysts’ deactivation triggered by sintering and deposition of carbon at catalyst active sites under optimum operating conditions [22]. Hence, various protocols [14][23] and supports like Al2O3 [24][25][26], ZrO2 [27][28][29], CeO2 [30][31] were generated.
Yancheshmeh et al. suggested a new and simple method for preparing Ni-modified Al2O4 spinel catalyst from the steam reforming technique of glycerol for the production of hydrogen. Solvothermal preparation method was used for catalyst represented as NiAl-G1, and the coprecipitation preparation method was used for catalyst described as NiAl-G2 as well as NiAl-C (NiAl2O4 catalyst synthesized via the coprecipitation method was designated as NiAl-C). The maximum hydrogen yield was 76.38% achieved with 95.42% maximum glycerol transformation on NiAl-G2 catalyst 630 °C, 1 atm, and 19,600 cm3/gcat/h space velocity reaction conditions. By creating an oxidative environment surrounding nickel active sites, the creation of a well-dispersed CeAlO3 phase slowed the growth of filamentous carbon on the nickel surface and aided the gasification of carbon deposits. During the 16-h SRG process, the catalytic activity was maintained, and the rate of coke generation was as low as 0.0004 gcokegcatalh−1. These findings support Ce/NiAl_G2’s potential as a catalyst for the SRG reaction [31]. Ni added to CaO-modified attapulgite (CaO–ATP) catalyst from reforming of glycerol for hydrogen manufacturing. Using the impregnation technique, Ni-Al2O3, Ni-ATP, and Ni-CaO-ATP catalysts can be synthesized. A maximum hydrogen yield of 85.3% was achieved on Ni-CeO-ATP catalyst with 93.71% glycerol conversion at T = 600 °C and GHSV = 1 h−1 reaction condition. Stability of the catalyst showed that ATP had better resistance to carbon deposition compared to alumina and CaO addition further reduced the production of carbon. Due to its unusual intermediate structure, ATP outperformed Al2O3 as a carrier. When CaO was utilized as an ATP modifier, it promoted the water gas shift reaction, resulting in increased hydrogen yield, hydrogen selectivity, and a reduction in CO production. The stability results revealed that ATP is more resistant to carbon deposition than Al2O3, and the addition of CaO lowered carbon formation even more. As a result, a Ni-based catalyst supported on a CaO-modified attapulgite could be a potential material for efficient glycerin steam reforming to create H2 [32].
Wu et al. explored the mesoporous Ni-Cu/CeO2 for the production of renewable hydrogen via APR of glycerol. The C-C breakage of glycerol benefits from Ni, but Cu can amplify the WGS reaction. Cu metal’s surface is amenable to adsorption of carbonyl radical following glycerol’s decarbonylation and hydroxyl free radical in the solution, which facilitates the reaction. Consequently, the addition of Cu element enhanced the proportion of H2 and CO2 in the overall gas. It was discovered that increasing the catalyst’s Cu concentration improved hydrogen production (125.08 to 195.57 μmol min−1 gcat−1). Additionally, the CO2 in overall gas was absorbed by the addition of CaO and it lowers the activation energy. Hence, 1Ni2Cu/CeO2 + 0.2gCaO catalyst shows H2 production rate increased from 168.97 to 301.92 μmol min−1 gcat−1. Therefore, improved H2 production as a result of the inclusion of Cu and CaO [3].
Liu et al. studied MgO supported Ni-Co bimetallic catalyst prepared by sol-gel method followed by calcination. The resulted catalyst examined the APR of glycerol for hydrogen production. After the addition of MgO support, H2 production activity improves by 1.5 times compared to pure Ni-Co bimetallic catalyst. Due to the in-situ adsorption and removal of CO2, the WGS reaction was further stimulated with the addition of CaO, and the methanation reaction was inhibited, resulting in the catalyst having a high activity of hydrogen production. The stability test also showed that the gelatinous MgO supports had an impact on the catalyst’s activity and stability [4].
Veiga et al. studied crude glycerol steam reforming with oxalic acid to synthesize Ni-doped La-Zr catalysts. Ni-doped La-Zr catalyst was prepared with the coprecipitation method at different calcination temperatures. Ni-doped La-Zr catalysts calcined at 850 °C achieved 90% hydrogen yield and 99.9% glycerol conversion. The catalyst prepared at 850 °C calcination and operated at 650 °C reaction showed higher catalytic stability, activity, and better resistance to carbon formation. The greatest performance seen for Ni-850 catalysts is mostly due to the development of oxygen vacancies caused by nickel substitution into the La-Zr lattice during calcination, favoring the oxidation of carbon deposits throughout the process [33].
Charisiou et al. investigated Ni supported over Y2O3–ZrO2 catalyst for the glycerol steam reforming. The catalyst synthesized through the impregnation technique was Ni modified Zr and Ni modified YZr. The maximum transformation was 90% achieved at 700 °C, and the maximum H2 selectivity was 82% achieved at 450 °C over Ni modified YZr catalyst. The research on spent catalyst revealed the less carbon deposition on Ni/Zr (Ni modified Zr) catalyst as it was more graphitic in nature and higher carbon deposition on Ni/YZr spent catalyst. The addition of Y2O3 stabilized the ZrO2 tetragonal phase, resulted in more easily reducible NiO nanoparticles, increased the O2 storage capacity of the support and the medium strength acid sides of the catalyst, and, despite having a higher concentration of basic sites, the Ni/YZr presented more stable monodentate carbonates. Allyl alcohol, acetaldehyde, acetone, acrolein, acetic acid, and acetol were the primary liquid products detected for both catalysts. Although higher amounts of carbon were deposited on the Ni/YZr spent catalytic sample (0.70 gcoke/gcatal compared to 0.51 gcoke/gcatal for the Ni/Zr catalyst), the addition of Y2O3 on to the ZrO2 support resulted in structures with lower crystallinity (amorphous carbon) that are more easily oxidized during the reaction. The coke deposits found on spent Ni/Zr catalysts, on the other hand, were graphitic structures with little flaws, resulting in the deadly encapsulation of Ni particles [29].
6. Cobalt Catalysts for Glycerol Reforming
Cobalt (Co) played an extensive role in the synthesis of hydrogen as many researchers represented this glycerol steam reforming. Ghungrud and Vaidya studied the synthesis of hydrogen by sorption–enhanced steam reforming technique (SESGR) on a Co-promoted hydrotalcites catalyst. 92.8% maximum glycerol conversion and 89.7% hydrogen yield were achieved over Co-Ca-HTlc catalyst represented as HM2 at T = 823 K, GHSV = 5600 mL g−1 h−1. Ca, Co, and Zn promoted hydrotalcite catalysts were manufactured through the precipitation method. HM2 contained Cu showed the best performance for the dehydrogenation process, and it was known that basic sites promoted the dehydrogenation process. At 550 °C, Cu-bearing materials showed a long pre-breakthrough duration (40 min) and high H2 purity (93.1 mol%). They were also better adsorbents, removing 1.1 mol CO2/kg sorbent at 550 °C. Over 20 cycles of adsorption and regeneration, the cyclic stability of materials was examined. The potential of customized HTlc-materials for better H2 production from the SESGR process is explored in this work [34].
A feasible reaction pathway for the SESGR process was provided based on the product composition (Figure 2). Glycerol undergoes dehydration hydrogenation reactions upon adsorption, resulting in acetaldehyde. The adsorbed acetaldehyde was then further hydration-dehydrogenated into acetic acid. Over Co metal, acetic acid undergoes C-C bond breakage, yielding H2, CO2, CO, and CH4. The basic catalyst promotes rapid dehydrogenation, which prevents the generation of olefins and carbon deposition. CO and CH4 are converted to H2 and CO2 by WGS reaction and steam reforming processes, with trace amounts of CO remaining [34].
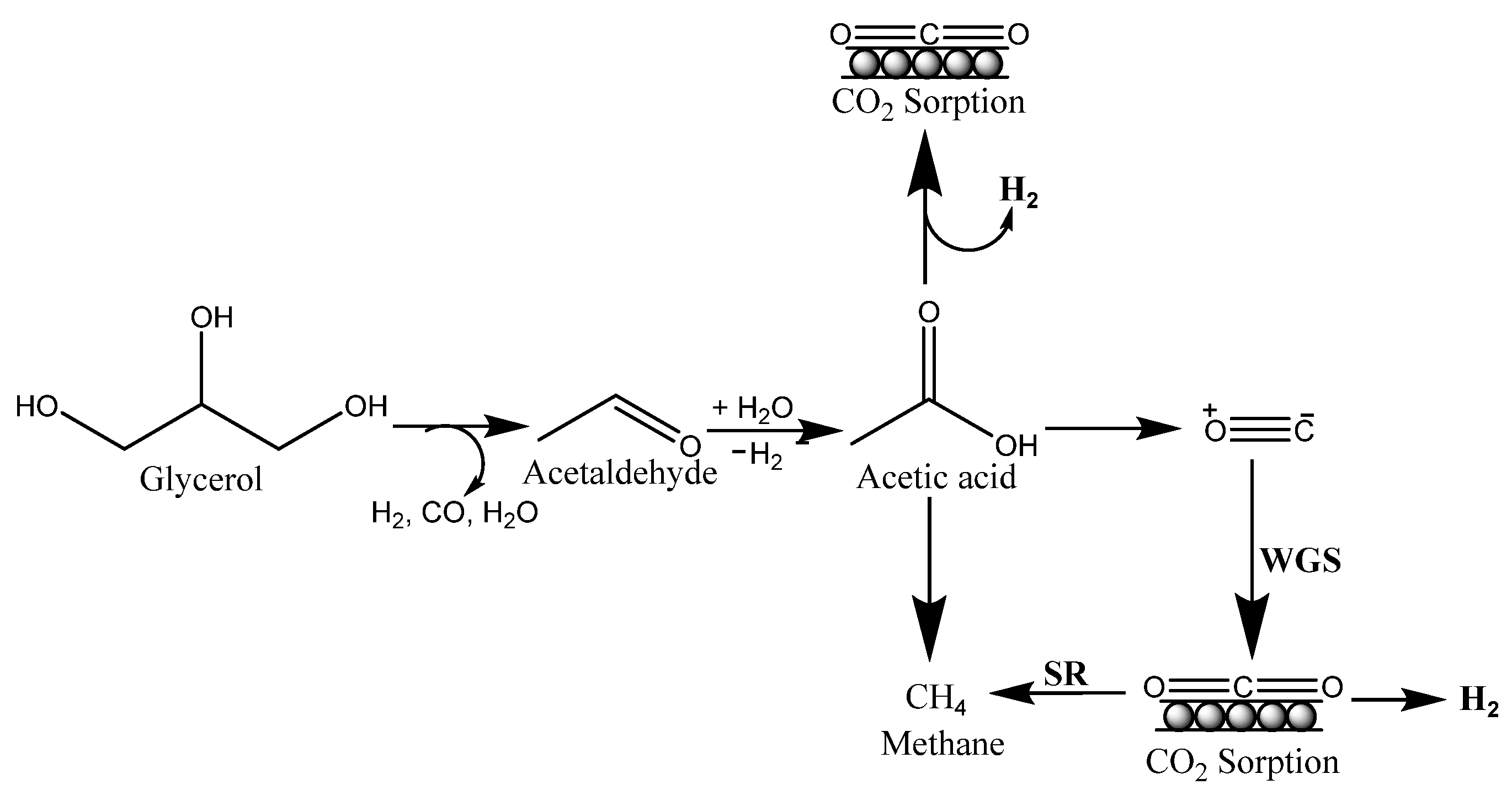
Figure 2. Plausible reaction mechanism for SESGR.
Zhou et al. investigated NiCo modified CNTs bimetallic catalysts with an appropriate distribution of Ni, as well as Co, metal for synthesizing hydrogen through the impregnation technique in which metal precursors were injected into the cave of CNTs by ultra-radiations. At 450 °C and 1 atm, 100% glycerol transformation was achieved with 91.4% H2 selectivity over Ni(i)Co(i)/CNTs catalyst. Ni(i)Co(i)/CNTs exhibited the best stability and catalyst performance. Although the Ni and Co oxides were only partially reduced, the location of metal species had a significant impact on reducibility. The synergetic effect between Ni and Co species was found in Ni(i)Co(i)/CNTs and Ni(o)Co(o)/CNTs, where they had the same distribution on the internal or external wall. Sintering of active sites was markedly simpler on the external wall of CNTs, causing the catalyst to deactivate quickly and become less reactive for the water gas shift process. The result of this study revealed that there is a new and straightforward method for assembling bimetallic catalyst systems. The Ni-based bimetallic catalysts might be used to reform mono/multi-alcohols such as ethanol, ethylene glycol, sorbitol, and other organic wastes from second-generation biorefineries, as well as biogas and other organic wastes [11].
Al-Salihi et al. investigated Co-Ni-MgO-based SBA-15 nano-catalysts for the glycerol steam reforming for hydrogen manufacturing. The one pot hydrothermal method was used to create different mesoporous catalysts, such as Co-SBA-15, Ni-SBA-15, Co-MgO-SBA-15, Ni-MgO-SBA-15, and Co-Ni-SBA-15 and bimetallic 10 wt.% Co-5 wt.% Ni modified SBA-15 catalyst synthesized by the impregnation method. A glycerol conversion rate of 100% along with 82.8% hydrogen selectivity was achieved over 10 wt.% Co-5 wt.% Ni modified SBA-15 catalyst at 650 °C. At a low temperature (450 °C), the one-pot catalysts showed 50% glycerol conversion compared to 70% for the impregnated catalyst, while both yielded 62% H2 selectivity. Cobalt-based SBA-15 catalysts have superior GSR activity and stability than nickel-based catalysts in monometallic catalysts. The addition of MgO to Co-SBA-15 improved glycerol conversion (up to 99%) and catalyst stability. The addition of MgO to Ni-SBA-15 reduced the quantity of carbon deposition on the catalysts by as much as 66%, according to TGA-TPO analyses of spent catalysts. It’s worth noting that under the experimental conditions, all of the catalysts examined at the optimum temperature of 650 °C demonstrated a remarkable hydrogen selectivity of 70% [10].
Duo et al. developed chemical looping steam reforming process for glycerol conversion to hydrogen over Al-MCM-41 and SBA-15 well-order mesoporous OCs containing NiO and CeO2 nanoparticles [7]. It was discovered that the NiO and CeO2 in the OCs were reduced by glycerol, and the reduced OCs were in charge of producing hydrogen. It was shown remarkable stability in 60 redox cycles (180 h), and the carbon conversion and hydrogen selectivity were both over 60% and 85%, respectively. Oxygen transport was enhanced by heavily loading mesoporous MCM-41 and SBA-15 with NiO and CeO2, which also produced outstanding redox CLSR cycles and long-term stability [7].
The summary of some more reported literature related to glycerol reforming over various catalysts to produce hydrogen is represented in Table 1. Noble metal catalysts, such as Pd, Ru, Ir, Rh, and Pt, are known for their high catalytic activity, which results in good performance and coke resistance. Other catalysts, such as Ni, Co, and Cu, on the other hand, are less expensive and more readily available and have a desirable catalytic activity for boosting hydrogen production. Their stability, on the other hand, is undesirable since the coking process frequently deactivates them. As a result, developing a highly active and stable catalyst requires the employment of metals as catalysts in combination with the proper selection of support (based on porosity, redox characteristics, and thermal stability, among other factors). Alumina and silica are the most common catalytic supports in research, but some catalysts can also be supported on active carbon, nanotubes, mesoporous carbon, nanoparticles, and other materials. Due to its high specific surface and thermal stability, alumina is the preferred and most used support for this purpose. However, carbon deposition tends to render it inactive. The optimal bimetallic catalyst must possess excellent thermal strength, mechanical resistance, and redox characteristics to produce H2 with high selectivity.
Table 1. Hydrogen production from glycerol reforming using various catalytic systems under optimal reaction conditions.
| S. No. | Catalyst | Reaction Condition/Reactor | X (%) | S (%) | Y (%) | References |
|---|---|---|---|---|---|---|
| 1 | Co-Ni-MgO-based SBA-15 | T = 650 °C | 100 | 82.8 | 82.8 | [10] |
| (one-pot hydrothermal and impregnation method) | P = 1 atm | |||||
| (Fixed bed reactor) | ||||||
| 2 | Pt and/or Ni supported over γ-alumina | T = 250 °C | 100 | - | - | [11] |
| (Sol–gel method) | P = 30 bar | |||||
| WHSV = 2.6 h−1 | ||||||
| 3 | Pt and Ni supported SiO2 | T = 350 °C | 100 | 70 | 70 | [12] |
| (Ion exchange method) | P = 1 atm | |||||
| Feed flowrate = 0.5 cm3 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 4 | Ru doped Al2O3 | T = 500 °C, | 89.1 | - | - | [15] |
| P = 0.1 MPa | ||||||
| Cat. Wt. = 0.1 g | ||||||
| W/FA0 = 1.98 | ||||||
| 5 | CeZr-CoRh | T = 650 °C, | 100 | 86 | 86 | [18] |
| P = 0.1 MPa | ||||||
| Cat. Wt. = 55 mg | ||||||
| WHSV = 8.4 gglygcat−1 | ||||||
| 6 | Rh modified MeAl2O4 at Al | T = 723 K | 100 | - | - | [19] |
| WHSV = 34,000 mL g−1 h−1 | ||||||
| 7 | Rh/Al2O3 | T = 823 K | 100 | - | - | [20] |
| (Impregnation technique) | WHSV = 4.8 × 103 N mL/gcat min | |||||
| 8 | Ni/CaCO3 | T = 550 °C | 100 | 92 | 92 | [21] |
| P = 1 atm | ||||||
| 9 | Rh/γ—Al2O3@CeO2, MgO, La2O3 | T = 600 °C | 90 | 78 | 78 | [24] |
| (Co-precipitation method) | P = 1 atm | |||||
| GHSV = 5000 | ||||||
| N mL g−1 hr−1 | ||||||
| (Fixed bed reactor) | ||||||
| 10 | Ni supported over Y2O3–ZrO2 | T = 700 °C | 90 | 91 | 82 | [29] |
| (Impregnation technique) | ||||||
| 11 | Ni | T = 630 °C | 95.42 | 80 | 76.4 | [31] |
| modified Al2O4 | P = 1 atm press., WHSV = 19,600 cm3/gcat/h | |||||
| (Coprecipitation method) | ||||||
| 12 | Ni added to CaO—modified attapulgite | T = 600 °C | 93.7 | 91 | 85.3 | [32] |
| (Impregnation method) | Cat. Wt. = 0.5 g | |||||
| GHSV = 1 h−1 | ||||||
| 13 | Co-promoted hydrotalcite | T = 550 °C | 92.8 | 96.7 | 89.7 | [34] |
| (Precipitation method) | GHSV = 5600 mL g−1 h−1 | |||||
| 14 | NiCo modified CNTs bimetallic catalysts | T = 450 °C | 100 | 91.4 | 91.4 | [35] |
| (Impregnation method) | P = 1 atm | |||||
| (Fixed bed reactor) | ||||||
| 15 | Pt/Al2O3 | T = 200–240 °C | 84 | 16.8 | 14.1 | [36] |
| P = 16–33.5 bar | ||||||
| (Batch reactor) | ||||||
| 16 | Pt/Al2O3 | T = 240 °C | 34 | 36.8 | 12.5 | [37] |
| P = 42 bar | ||||||
| (Batch reactor) | ||||||
| 17 | Pt/SiO2 | T = 300–400 °C | 96.8 | 90 | 89.7 | [38] |
| (Sol-gel method) | P = 1 atm | |||||
| (Microchannel reactor) | ||||||
| 18 | Pt/γ—Al2O3, SiO2 & silica—alumina | T = 225 °C | 11.9 | 73 | 8.9 | [39] |
| (Impregnation method) | P = 29 bar | |||||
| WHSV = 4.2 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 19 | Pt/ZrO2 | T = 350 °C | 100 | 69 | 6.9 | [40] |
| (Impregnation method) | P = 29 bar | |||||
| (Fixed bed reactor) | ||||||
| 20 | Pt/Al2O3, Puralox, Catapal B | T = 250 °C | 57 | - | 85 | [41] |
| (Impregnation method) | Cat. Wt. = 300 mg | |||||
| Feed flowrate = 0.5 mL/min | ||||||
| (Fixed bed reactor) | ||||||
| 21 | Pt—Re/C | T = 225 °C | 89.4 | 26.1 | 23 | [42] |
| (Impregnation method) | P = 420 psi | |||||
| WHSV = 5 hr−1 | ||||||
| (Fixed bed reactor) | ||||||
| 22 | 10 Ni/Al2O3/5 CeO2 | T = 550–800 °C | 100 | 88.6 | 86 | [43] |
| (Impregnation method) | P = 1 atm | |||||
| WHSV = 10 hr−1 | ||||||
| (Fixed bed reactor) | ||||||
| 23 | Ru-Mg-Al hydrotalcite- mixed oxides | T = 400–700 °C | 45 | 90 | 40.5 | [44] |
| (Impregnation method) | P = 1 atm | |||||
| GHSV = 69,000 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 24 | Ru/Al2O3 | T = 400–600 °C | 100 | 80 | 80 | [45] |
| (Wet co–impregnation method) | P = 1 atm | |||||
| Cat. Wt. = 200 mg | ||||||
| W/F = 1.05 mg min mL−1 | ||||||
| (Fixed bed reactor) | ||||||
| 25 | Ru/C | T = 500 oC | 94.6 | 99.8 | 94.5 | [46] |
| (Impregnation method) | P = 1 atm | |||||
| (Fixed bed reactor) | ||||||
| 26 | Mg(Al)O/Ru | T = 450–650 °C | 97 | 96 | 90 | [47] |
| (Impregnation method) | P = 1 atm | |||||
| (Fixed bed reactor) | ||||||
| 27 | C/Pt and Pt/Ru | T = 225 °C | 24 | - | - | [48] |
| (Impregnation method) | P = 1 bar | |||||
| (Fixed bed reactor) | ||||||
| 28 | Rh/ZrO2, Rh/CeO2 | T = 650–750 °C | 79 | 30.6 | 24.2 | [49] |
| (Impregnation method) | Cat. Wt. = 20 mg | |||||
| (Fixed bed reactor) | ||||||
| 29 | Rh/Al2O3 | T = 250 °C | 82 | 85.2 | 69.9 | [50] |
| (Impregnation method) | P = 50 bar | |||||
| Cat. Wt. = 250 mg | ||||||
| WHSV = 2.45 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 30 | Pt and Pt-Rh/α-Al2O3 | T = 230 oC | 67 | 1.67 | 1.12 | [51] |
| (Impregnation method) | Cat. Wt. = 300 mg | |||||
| (Batch reactor) | ||||||
| 31 | Rh and Ni/γ—Al2O3 | T = 450-800 oC | 69 | 50 | 34.5 | [52] |
| (Impregnation method) | P = 1 atm | |||||
| GHSV = 5000 to 30,000 mLglyh−1 mLcat−1 | ||||||
| 32 | Ni-La-Ti oxide | T = 500–650 °C | 99.7 | 90.3 | 90 | [53] |
| (Coprecipitation method) | P = 1 bar | |||||
| Cat. Wt. = 0.1 g | ||||||
| WHSV = 3 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 33 | NiAl2O4 | T = 600 °C | 99 | 98.9 | 98 | [54] |
| (Impregnation method) | Feed flowrate = 2.5 mL/h | |||||
| (Fixed bed reactor) | ||||||
| 34 | La-promoted Ni/Al2O3 | T = 650–850 °C | 96 | 98.9 | 95 | [55] |
| P = 1 atm | ||||||
| WHSV = 3.6 × 104 mL−1 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 35 | La1-xCaxNiO3perovskite-oxides | T = 550 °C | 100 | 80 | 80 | [56] |
| (Impregnation method) | P = 1 atm | |||||
| Cat. Wt. = 200 mg | ||||||
| WHSV = 2.5 hr−1 | ||||||
| (Fixed bed reactor) | ||||||
| 36 | Ni and Ni—Co/alumina | T = 300–700 °C | - | - | 83 | [57] |
| (Impregnation method) | P = 1 atm | |||||
| WHSV = 10 hr−1 | ||||||
| (Fixed bed reactor) | ||||||
| 37 | Ni/α—Al2O3 and α—Al2O3modified by ZrO2 and CeO2 | T = 600 °C | 90 | 95.6 | 86 | [58] |
| (Impregnation method) | P = 1 atm | |||||
| Feed flow rate = 0.022 cm3 min−1 | ||||||
| (Fixed bed reactor) | ||||||
| 38 | LaNiO3 and LaCoO3 | T = 700 °C | 100 | 98 | 98 | [59] |
| P = 1 atm | ||||||
| (Fixed bed reactor) | ||||||
| 39 | 0.25 CoAl, 0.625 CoAl | T = 260 °C | 98 | 29 | 10 | [60] |
| Co3O4 | P = 5 MPa | |||||
| WHSV = 24.5 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 40 | Co-Ce/Hap | T = 750 °C | 99 | 79 | 7.2 | [61] |
| (Impregnation method) | P = 1 atm | |||||
| (Fixed bed reactor) | ||||||
| 41 | Co/SBA-15 with Zr, Ce and La | T = 500–600 °C | 100 | 72 | 72 | [62] |
| (Impregnation method) | P = 1 atm | |||||
| WHSV = 7.7 h−1 | ||||||
| 42 | Lax-Cey-CoO3 | T = 700 °C | 100 | 68 | 68 | [63] |
| (Co—precipitation method) | P = 1 atm | |||||
| (Fixed bed reactor) | ||||||
| 43 | Co/Al2O3 | T = 450–650 oC | 65 | 80 | 52 | [64] |
| (Impregnation method) | P = 1 atm | |||||
| GHSV = 5 × 104 mL gcat−1 h−1 | ||||||
| (Fixed bed reactor) | ||||||
| 44 | Ceria/Ir, Co and Ni | T = 250–600 °C | 100 | 85 | 85 | [65] |
| (deposition—precipitation method) | P = 1 atm | |||||
| GHSV = 11,000 mL gcat−1 h−1 | ||||||
| (Fixed bed reactor) |
X = Conversion, S = Selectivity, Y = Yield.
The knowledge of various catalysts, such as platinum, ruthenium, rhodium, nickel and cobalt-based catalysts for glycerol reforming was reviewed and cataloged. Today, researchers and engineers are exploring new technologies, highly novel and tolerant catalysts, improving reactor systems and activation methods, and coordinating the chemical and biological catalysts to improve the weaknesses involved with each catalyst. The modern improvement in technology and economical use of crude glycerol in hydrogen fuel production clearly demonstrate that crude glycerol may play a critical part in the bio-refining industry’s fulfillment.
References
- Zakaria, Z.Y.; Amin, N.A.S.; Linnekoski, J. A perspective on catalytic conversion of glycerol to olefins. Biomass Bioenergy 2013, 55, 370–385.
- Anitha, M.; Kamarudina, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130.
- Wu, K.; Dou, B.; Zhang, H.; Liu, D.; Chen, H.; Xu, Y. Aqueous phase reforming of biodiesel byproduct glycerol over mesoporous Ni-Cu/CeO2 for renewable hydrogen production. Fuel 2022, 308, 122014.
- Liu, D.; Dou, B.; Zhang, H.; Zhao, L.; Wu, K.; Zeng, P.; Chen, H.; Xu, Y. Comparison of gelatinous and calcined magnesia supported Ni or/and Co-based catalysts for aqueous phase reforming of glycerol. Renew. Energy 2022, 186, 656–666.
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236.
- Su, H.; Yan, M.; Wang, S. Recent advances in supercritical water gasification of biowaste catalyzed by transition metal-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2022, 154, 111831.
- Dou, B.; Zhao, L.; Zhang, H.; Wu, K.; Zhang, H. Renewable hydrogen production from chemical looping steam reforming of biodiesel byproduct glycerol by mesoporous oxygen carriers. Chem. Eng. J. 2021, 416, 127612.
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593.
- Markocic, E.; Kramberger, B.; Van Bennekom, J.G.; Heeres, H.J.; Vos, J.; Knez, Z. Glycerol reforming in supercritical water; a short review. Renew. Sustain. Energy Rev. 2013, 23, 40–48.
- Al-Salihi, S.; Abrokwah, R.; Dade, W.; Deshmane, V.; Hossain, T.; Kuila, D. Renewable hydrogen from glycerol steam reforming using Co-Ni-MgO based SBA-15 nanocatalysts. Int. J. Hydrogen Energy 2020, 45, 14183–14198.
- El Doukkali, M.; Iriondo, A.; Cambra, J.F.; Gandarias, I.; Jalowiecki-Duhamel, L.; Dumeignil, F.; Arias, P.L. Deactivation study of the Pt and/or Ni-based -Al2O3 catalysts used in the aqueous phase reforming of glycerol for H2 production. Appl. Catal. A Gen. 2014, 472, 80–91.
- Pompeo, F.; Santori, G.F.; Nichio, N.N. Hydrogen production by glycerol steam reforming with Pt/SiO2 and Ni/SiO2 catalysts. Catal. Today 2011, 172, 183–188.
- Huang, X.; Dang, C.; Yu, H.; Wang, H.; Peng, F. Morphology Effect of Ir/La2O2CO3 Nanorods with Selectively Exposed Facets in Catalytic Steam Reforming of Glycerol. ACS Catal. 2015, 5, 1155–1163.
- Kim, J.; Kim, J.; Lee, D. Glycerol steam reforming on Ru catalysts supported on core-shell metal–ceramic microcomposites developed by a microwave-induced hydrothermal method. Appl. Catal. A Gen. 2015, 449, 197–204.
- Sundari, R.; Vaidya, P.D. Reaction Kinetics of Glycerol Steam Reforming Using a Ru/Al2O3 Catalyst. Energy Fuels 2012, 26, 4195–4204.
- Baca, S.; Sayb, Z.; Kocak, Y.; Ercanb, K.; Harfouched, M.; Ozensoy, E.; Avci, A.K. Exceptionally active and stable catalysts for CO2reforming of glycerol to syngas. Appl. Catal. B Environ. 2019, 256, 117808.
- Vaidya, P.D.; Rodrigues, A.E. Glycerol Reforming for Hydrogen Production: A Review. Chem. Eng. Technol. 2009, 32, 1463–1469.
- Martinez, L.M.T.; Araque, M.; Vargas, J.C.; Roger, A.C. Effect of Ce/Zr ratio in CeZr-CoRh catalysts on the hydrogen production by glycerol steam reforming. Appl. Catal. A Gen. 2013, 132–133, 499–510.
- Lee, J.; Doohwan, K. Synthesis and Properties of Core–Shell Metal–Ceramic Microstructures and their Application as Heterogeneous Catalysts. ChemCatChem 2014, 6, 2642–2647.
- Delparish, A.; Koc, S.; Caglayan, B.S.; Avc, A.K. Oxidative steam reforming of glycerol to synthesis gas in a microchannel reactor. Catal. Today 2019, 323, 200–208.
- Danga, C.; Wu, S.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. Syngas production by dry reforming of the mixture of glycerol and ethanol with CaCO3. J. Energy Chem. 2020, 43, 90–97.
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213.
- Rezendea, S.M.D.; Franchini, C.A.; Dieuzeide, M.L.; de Farias, A.M.D.; Amadeo, N.; Fraga, M.A. Glycerol steam reforming over layered double hydroxide-supported Pt catalysts. Chem. Eng. J. 2015, 272, 108–118.
- Charisiou, N.D.; Italiano, C.; Pino, L.; Sebastian, V.; Vita, A.; Goula, M.A. Hydrogen production via steam reforming of glycerol over Rh/γ-Al2O3 catalysts modified with CeO2, MgO or La2O3. Renew. Energy 2020, 162, 908–925.
- Wu, G.; Zhang, C.; Li, S.; Han, Z.; Wang, T.; Ma, X.; Gong, J. Hydrogen Production via Glycerol Steam Reforming over Ni/Al2O3: Influence of Nickel Precursors. ACS Sustain. Chem. Eng. 2013, 1, 1052–1062.
- Sánchez, N.; Encinar, J.M.; González, J.F. Sorption Enhanced Steam Reforming of Glycerol: Use of La-Modified Ni/Al2O3 as Catalyst. Ind. Eng. Chem. Res. 2016, 55, 3736–3741.
- Ruppert, R.A.M.; Niewiadomski, M.; Grams, J.; Kwapiński, W. Optimization of Ni/ZrO2 catalytic performance in thermochemical cellulose conversion for enhanced hydrogen production. Appl. Catal. B Environ. 2014, 145, 85–90.
- Yan, Z.; Liu, S.; Zhang, Y.; Wang, T.; Luo, S.; Chu, W.; Jing, F. The role of Zr in NiZrAl oxides catalyst and the evaluation on steam reforming of glycerol for hydrogen product. Catal. Today 2019, 319, 229–238.
- Charisiou, C.N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3—ZrO2 catalyst for hydrogen production through the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460.
- Xiong, Y.; Wang, B.; Yan, J.; Hong, J.; Wang, L.; Zhang, Y.; Li, J.; Jing, F.; Chu, W. Plasma assisted preparation of nickel-based catalysts supported on CeO2 with different morphologies for hydrogen production by glycerol steam reforming. Powder Technol. 2019, 345, 324–332.
- Yancheshmeh, M.S.; Alizadeh, O.S.; Aissaoui, M.; Iliuta, M.C. A novel synthesis of NiAl2O4 spinel from a Ni-Al mixed-metal alkoxide as a highly efficient catalyst for hydrogen production by glycerol steam reforming. Appl. Catal. B Environ. 2020, 265, 118535.
- Feng, P.; Huang, K.; Xu, Q.; Qi, W.; Xin, S.; Wei, T.; Liao, L.; Yan, Y. Ni supported on the CaO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 8223–8233.
- Veiga, S.; Faccio, R.; Romero, M.; Bussi, J. Utilization of waste crude glycerol for hydrogen production via steam reforming over Ni–La–Zr catalysts. Biomass Bioenerg. 2020, 135, 105508.
- Ghungrud, S.A.; Vaidya, P.D. Sorption-enhanced reaction process for glycerol-tohydrogen conversion over cobalt catalyst supported on promoted hydrotalcites. Int. J. Hydrogen Energy 2020, 45, 9440–9450.
- Zhou, H.; Liu, S.; Jing, F.; Luo, S.Z.; Shen, J.; Pang, Y.; Chu, W. Synergetic Bimetallic NiCo/CNT Catalyst for Hydrogen Production by Glycerol Steam Reforming: Effects of Metal Species Distribution. Ind. Eng. Chem. Res. 2020, 59, 17259–17268.
- Seretis, A.; Tsiakaras, P. Aqueous phase reforming (APR) of glycerol over platinum supported on Al2O3 catalyst. Renew. Energy 2016, 85, 1116–1126.
- Callison, J.; Subramanian, N.D.; Rogers, S.M.; Chutia, A.; Gianoliod, D.; Catlow, C.R.A.; Wells, P.P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628.
- Touri, A.E.; Taghizadeh, M. Hydrogen Production via Glycerol Reforming over Pt/SiO2Nanocatalyst in a Spiral-Shaped Microchannel Reactor. Int. J. Chem. React. 2016, 14, 1059–1068.
- Ciftci, A.; Peng, B.; Jentys, A.; Lercher, J.A.; Hensen, E.J.M. Support effects in the aqueous phase reforming of glycerol over supported platinum catalysts. Appl. Catal. A Gen. 2012, 431–432, 113–119.
- Pompeo, F.; Santori, G.; Nichio, N.N. Hydrogen and/or syngas from steam reforming of glycerol. Study of platinum catalysts. Int. J. Hydrogen Energy 2010, 35, 8912–8920.
- Lehnert, K.; Claus, P. Influence of Pt particle size and support type on the aqueous-phase reforming of glycerol. Catal. Commun. 2008, 9, 2543–2546.
- King, D.L.; Zhang, L.; Xia, G.; Karim, A.M.; Heldebrant, D.J.; Wang, X.; Peterson, T.; Wang, Y. Aqueous phase reforming of glycerol for hydrogen production over Pt–Re supported on carbon. Appl. Catal. B Environ. 2010, 99, 206–213.
- Demsash, H.D.; Kondamudi, K.V.K.; Upadhyayula, S.; Mohan, R. Ruthenium doped nickel-alumina-ceria catalyst in glycerol steam reforming. Fuel Process. Technol. 2018, 169, 150–156.
- Dahdah, E.; Aouad, S.; Gennequin, C.J.E.; Nsouli, B.; Aboukais, A.; Abi-Aad, E. Glycerol steam reforming over Ru-Mg-Al hydrotalcite-derived mixed oxides: Role of the preparation method in catalytic activity. Int. J. Hydrogen Energy 2018, 43, 19864–19872.
- Kousi, K.; Kondarides, D.I.; Verykios, X.E.; Papadopoulou, C. Glycerol steam reforming over modified Ru/Al2O3 catalysts. Appl. Catal. A Gen. 2017, 542, 201–211.
- Gallegos-Suárez, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Efficient hydrogen production from glycerol by steam reforming with carbon supported ruthenium catalysts. Carbon 2016, 96, 578–587.
- Galloa, A.; Pirovano, C.; Ferrini, P.; Marelli, M.; Psaroc, R.; Santangelo, S.; Faggio, G.; Santo, V.D. Influence of reaction parameters on the activity of ruthenium based catalysts for glycerol steam reforming. Appl. Catal. B Environ. 2012, 121–122, 40–49.
- Simonetti, D.A.; Kunkes, E.L.; Dumesic, J.A. Gas-phase conversion of glycerol to synthesis gas over carbon-supported platinum and platinum–rhenium catalysts. J. Catal. 2007, 247, 298–306.
- Bulutoglu, P.S.; Say, Z.; Bac, S.; Ozensoy, E.; Avci, A.K. Dry Reforming of Glycerol Over Rh-Based Ceria and Zirconia Catalysts: New Insights on Catalyst Activity and Stability. Appl. Catal. A Gen. 2018, 564, 157–171.
- Larimi, A.; Khorasheh, F. Renewable hydrogen production over Pt/Al2O3 nano-catalysts: Effect of M-promoting (M=Pd, Rh, Re, Ru, Ir, Cr). Int. J. Hydrogen Energy 2019, 44, 8243–8251.
- Oliveira, E.V.; Seixas, A.C.M.; Jordão, E. Performance of Pt and Pt-Rh Catalyst in the Hydrogen Production from Glycerol. Can. J. Chem. Eng. 2017, 95, 2018–2023.
- Chiodo, V.; Freni, S.; Galvagno, A.; Mondelloa, N.; Frusteri, F. Catalytic features of Rh and Ni supported catalysts in the steam reforming of glycerol to produce hydrogen. Appl. Catal. A Gen. 2010, 381, 1–7.
- Veiga, S.; Faccio, R.; Segobia, D.; Apesteguia, C.; Bussi, J. Hydrogen production by crude glycerol steam reforming over NieLaeTi mixed oxide catalysts. Int. J. Hydrogen Energy 2017, 42, 30525–30534.
- Suffredini, D.F.P.; Thyssen, V.V.; de Almeida, P.M.M.; Gomes, R.S.; Borges, M.C.; de Farias, A.M.D.; Assaf, E.M.; Fraga, M.A.; Brandão, S.T. Renewable hydrogen from glycerol reforming over nickel aluminate-based catalysts. Catal. Today 2016, 289, 96–104.
- Siew, K.W.; Lee, H.C.; Gimbun, J.; Chin, S.Y.; Khan, M.R.; Taufiq-Yap, Y.H.; Cheng, C.K. Syngas production from glycerol-dry (CO2) reforming over La-promoted Ni/Al2O3 catalyst. Renew. Energy 2015, 74, 441–447.
- Wu, G.; Li, S.; Zhang, C.; Wang, T.; Gong, J. Glycerol steam reforming over perovskite-derived nickel-based catalysts. Appl. Catal. B Environ. 2014, 144, 277–285.
- Sanchez, E.A.; Comelli, R.A. Hydrogen production by glycerol steam-reforming over nickel and nickel-cobalt impregnated on alumina. Int. J. Hydrogen Energy 2014, 39, 8650–8655.
- Buffoni, I.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Nickel catalysts applied in steam reforming of glycerol for hydrogen production. Catal. Commun. 2009, 10, 1656–1660.
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, M.S.; van Steen, S.E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 425, 60–67.
- Reynoso, A.J.; Ayastuy, J.L.; Iriarte-Velasco, U.; Gutierrez-Ortiz, M.A.; Chemical Technologies for the Environmental Sustainability Group. Cobalt aluminate spinel-derived catalysts for glycerol aqueous phase reforming. Appl. Catal. B Environ. 2018, 239, 86–101.
- Dobosz, J.; Cichy, M.; Zawadzki, M.; Borowieck, T. Glycerol steam reforming over calcium hydroxyapatite supported cobalt and cobalt-cerium catalysts. J. Energy Chem. 2018, 27, 404–412.
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with Zr, Ce and La. J. Energy Chem. 2016, 26, 42–48.
- Surendar, M.; Sagar, T.V.; Babu, B.H.; Lingaiah, B.; Rao, K.S.R.; Prasad, P.S.S. Glycerol steam reforming over La-Ce-Co mixed oxide-derived cobalt catalysts. ACS Catal. 2015, 5, 45184–45193.
- Cheng, C.K.; Foo, S.Y.; Adesina, A.A. H2-rich synthesis gas production over Co/Al2O3 catalyst via glycerol steam reforming. Catal. Commun. 2010, 12, 292–298.
- Zhang, B.; Tang, X.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production from steam reforming of ethanol and glycerol over ceria-supported metal catalysts. Int. J. Hydrogen Energy 2007, 32, 2367–2373.
More
Information
Subjects:
Chemistry, Applied; Chemistry, Inorganic & Nuclear
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
3 times
(View History)
Update Date:
25 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




