Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dorota Jakubczyk | -- | 2219 | 2022-07-21 02:59:06 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jakubczyk, D.; Dussart, F. Selected Fungal Natural Products with Antimicrobial Properties. Encyclopedia. Available online: https://encyclopedia.pub/entry/25369 (accessed on 08 February 2026).
Jakubczyk D, Dussart F. Selected Fungal Natural Products with Antimicrobial Properties. Encyclopedia. Available at: https://encyclopedia.pub/entry/25369. Accessed February 08, 2026.
Jakubczyk, Dorota, Francois Dussart. "Selected Fungal Natural Products with Antimicrobial Properties" Encyclopedia, https://encyclopedia.pub/entry/25369 (accessed February 08, 2026).
Jakubczyk, D., & Dussart, F. (2022, July 21). Selected Fungal Natural Products with Antimicrobial Properties. In Encyclopedia. https://encyclopedia.pub/entry/25369
Jakubczyk, Dorota and Francois Dussart. "Selected Fungal Natural Products with Antimicrobial Properties." Encyclopedia. Web. 21 July, 2022.
Copy Citation
Natural products (NPs) are a very rich source of antimicrobial drugs. They constitute more than two-thirds of clinically used antibiotics and half of anticancer drugs. Fungal natural products and their effects have been known to humankind for hundreds of years. Their later medicinal applications, followed by the discovery of the first class of antibiotics, penicillins and other drugs of fungal origin, such as peptidic natural products, terpenoids or polyketides, have altered the historically negative reputation of fungal “toxins”.
antimicrobial properties
antimicrobial resistance
biosynthesis
ergot alkaloids
fungal metabolites
natural products
peptides
polyketides
terpenoids
1. Introduction
Natural products (NPs) are a very rich source of antimicrobial drugs. They constitute more than two-thirds of clinically used antibiotics and half of anticancer drugs [1]. Molecules biosynthesised by fungi are a diverse and useful group of NPs. Plant endophytic and pathogenic fungi produce many secondary metabolites that play important roles in virulence and competition against other microbes. Due to their broad-spectrum activity, some of these NPs can also exhibit high biocidal activity against human pathogenic microbes. In recent years, marine fungi have emerged as a novel source of fungal NPs and may be a potential game changer in drug discovery; however, marine fungi still constitute an underrepresented resource of diverse NPs.
Fungal NPs have an important place in human history. For instance, ergot alkaloids (EAs) which are produced by the filamentous fungi of the genus Claviceps, have been referenced in ancient historical texts. References to grain diseases have been found in the Bible, in the Old Testament (850–550 BC). In the Middle Ages, the first reported ergotism epidemic was recorded in 944–1000 AD when almost half the population of the Aquitaine region of France (about 60,000 people) died of ergot poisoning [2][3]. The gangrenous form of the disease (medically known under the name Ergotismus gangraenosus) was commonly known as ‘‘ergotism’’, ‘‘holy fire’’ or ‘‘St. Anthony’s fire’’. Symptoms include delirium, hallucinations, muscle spasms, convulsions and gangrene of the limbs. The gruesome history of ergots has overshadowed their beneficial medicinal properties. However, the use of ergots as medicinal compounds was first documented in 1582, as they were administered for ‘‘quickening childbirth’’. Further research and screening of ergot analogues for oxytocic drugs that stimulate uterine contractions to hasten childbirth resulted, in 1938, in the synthesis of lysergic acid diethylamide (LSD) 5 (Figure 1B), a hallucinogenic compound that has become infamous for its use as an illicit “recreational drug” [4]. Currently, EAs are the inspiration for numerous semi-synthetic derivatives, such as cabergoline 6 or ergotamine 8 (Figure 1B,D, respectively) that have been applied in a wide range of medicinal treatments, such as the treatment of migraines, Parkinson’s disease, reduction of tumour growth, and other lesser-known synergistic antimicrobial activities.
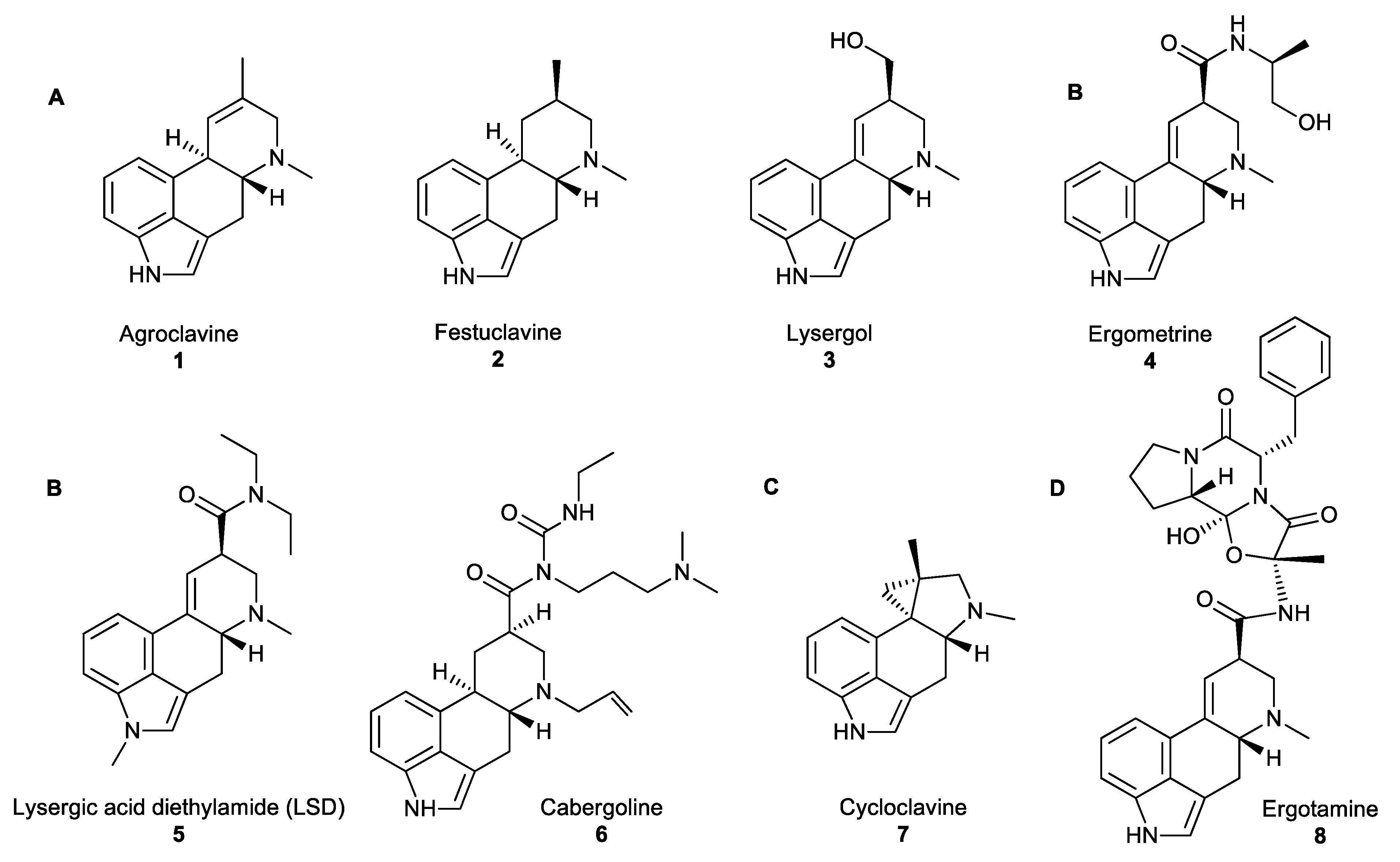
Figure 1. (A) Examples of clavines. (B) Simple lysergic acid derivatives. (C) The unusual ergoline scaffold of cycloclavine. (D) Ergopeptides consisting of D-lysergic acid with a cyclic tripeptide moiety [5].
2. Selected Examples of Antimicrobial Natural Products from Fungi
2.1. Ergot Alkaloids: Fungal Natural Products Derived from Amino Acids
All naturally occurring EAs share a common tetracyclic scaffold, the so-called ‘‘ergoline scaffold”, derived from L-tryptophan. EAs are divided into three major classes based on the substituents decorating this scaffold: clavines (festuclavine and agroclavine derivatives), simple lysergic acid derivatives and ergopeptides (Figure 1A,B,D, accordingly) [6]. Clavines include the partially or fully saturated ring species D, such as agroclavine 1, festuclavine 2 or lysergol 3 (Figure 1A). Simple lysergic acid derivatives consist of the basic D-lysergic acid structure as an alkyl amide (Figure 1B), and ergopeptides also based on D-lysergic acid and a cyclic tripeptide moiety (Figure 1D). Cycloclavine 7 is a newly characterised ergot alkaloid which has been reproduced in vitro, and has an unusual ring system, where ring D has been transformed into a new five- and three-membered ring fusion [7].
EAs are produced by fungi occupying distinct ecological niches. Clavicipitaceous species, such as Claviceps purpurea and Neotyphodium lolii from the order Eurotiales are plant pathogenic and symbiotic fungi, respectively. Aspergillus fumigatus from the same order, Eurotiales, is an opportunistic pathogen of mammals which also produces EAs, such as festuclavine 2 [8][9]. Cycloclavine 7 is biosynthesised in nature by Aspergillus japonicus, which is frequently responsible for the post-harvest decay of fresh fruit (apples, pears, peaches, citrus, grapes, figs, strawberries, tomatoes or melons) and some vegetables (especially onions, garlic, and yams) [10].
The biosynthetic pathways of EAs have been well studied and are described in depth elsewhere [5][11]; however, a brief overview of EAs biosynthesis is given here. First, the prenylation of L-tryptophan by dimethylallyl pyrophosphate (DMAPP) yields 4-(γ,γ-dimethylallyl)tryptophan (DMAT) and is followed by the N-methylation of DMAT to 4-dimethyl-L-abrine (N-Me-DMAT). Subsequently, a series of successive oxidation steps catalyses the intramolecular cyclization of the prenyl and indole moieties to form ring C in tricyclic chanoclavine-I, which, in turn, is oxidised to form chanoclavine-I-aldehyde. At this branch point, chanoclavine-I-aldehyde undergoes intramolecular cyclization to form either ring D of tetracyclic agroclavine 1 (C. purpurea, N. lolii) or festuclavine 2 (A. fumigatus). Subsequently festuclavine 2 is further biotransformed into fumigaclavines. The new branch of this pathway is an unusual oxidation of the cyclic iminium form of chanoclavine-I-aldehyde catalysed by non-heme iron and α-ketoglutarate dependent oxidase EasH, to yield a unique cyclopropyl ring moiety which is fused to a five-membered ring, in cycloclavine 7. The structure of EasH and possible mechanism of cycloclavine 7 formation has been published recently [12][13].
Ergot-derived medicines, such as ergometrine 4, were used to facilitate obstetric deliveries or to treat postpartum haemorrhage. The high bioactivity of EAs is correlated with the ability of these compounds to act as agonists or antagonists toward neuroreceptors. Although some EAs, such as the hallucinogenic compound LSD 5 have been used as recreational drugs, most EAs were associated with medicinal applications, including treatments against migraine and tumour (ergotamine 8), Parkinson’s disease or restless leg syndrome (cabergoline 6; Figure 1B). However, their synergistic antimicrobial activity is a less commonly known fact. Lysergol 3 is a synthetic EA that exhibits a synergistic antibiotic pharmaceutical activity as a bioactive enhancer and a bioavailability facilitator for broad-spectrum antibiotics. This property facilitates the absorption of antibiotics across the cell membrane in animal cells resulting in increased action against Gram-positive and -negative bacteria [6]. With the recommended dosage of lysergol of 10 μg/mL, the improved activity of antimicrobial effect is in the range of 2–12 folds, against a wide spectrum of both Gram-positive and -negative bacteria including Escherichia. coli, Bacillus subtilis, Mycobacterium smegmatis and other similar microorganisms.
2.2. Fungal Polyketides
The polyketide pathway constitutes one of the major biosynthetic pathways leading to the production of fungal NPs. Polyketides are polymers synthesised from simple carboxylic acid derivatives (e.g., acetyl-CoA, malonyl-CoA, and methylmalonyl-CoA) into linear chains by iterative Claisen condensation, followed, in some cases, by reductive modification of the resulting β-keto groups. These compounds are synthesised in fungi (and other organisms) by enzymes called polyketide synthases (PKSs). Polyketides are extremely diverse and include compounds such as polyesters, polyphenols, macrolides (macrocyclic esters), polyenes and enediynes.
Strobilurins are an important group of polyketide-derived fungal NPs which have yielded one of the major classes of fungicides currently in use to protect agricultural crops from fungal diseases. The discovery of these compounds occurred after the observation that Strobilurus tenacellus and Oudemansiella mucida, two agaricomycetes growing on decaying wood in European forests, were able to defend themselves against other fungi. Their antifungal activity was associated with the production of the compounds strobilurin A 9 and oudemansin A 10 in S. tenacellus and O. mucida, respectively (Figure 2) [14][15]. These two compounds inhibit the transfer of electrons between complexes II and III of the electron transport chain in the mitochondria, resulting in impaired cell respiration and ATP synthesis [16]. Despite strobilurin A 9 and oudemansin A 10 exhibiting high antifungal activity, these NPs are quickly degraded by light, rendering them unsuitable for use in crop protection. Many attempts were made to modify the chemical structures of natural strobilurins to increase photo-stability while maintaining antifungal activity [17]. After several years of research, azoxystrobin 11 was synthesised and became the first photo-stable strobilurin-derived active ingredient registered for use in crop protection (Figure 2) [18], paving the way for the synthesis of a multitude of fungicides belonging to the quinone outside inhibitor (QoI) class of fungicides. However, resistance to QoI fungicides arose after a few years of use in fields. The single point mutation which confers resistance to QoI leads to the substitution of the amino acid glycine for alanine at position 143 (G143A). This mutation is now widespread in many fungal species, including Zymoseptoria tritici, Botrytis cinerea and Cercospora beticola, the agents responsible for Septoria leaf blotch in wheat, Botrytis grey mould and Cercospora leaf spot in beets, respectively [19][20][21]. Despite numerous resistance issues, QoIs are still used to control some of the most devastating rust fungi, such as Puccinia striiformis and Phakopsora pachyrhizi, the causative agents of the yellow rust of cereals and soybean rust diseases, respectively [22].
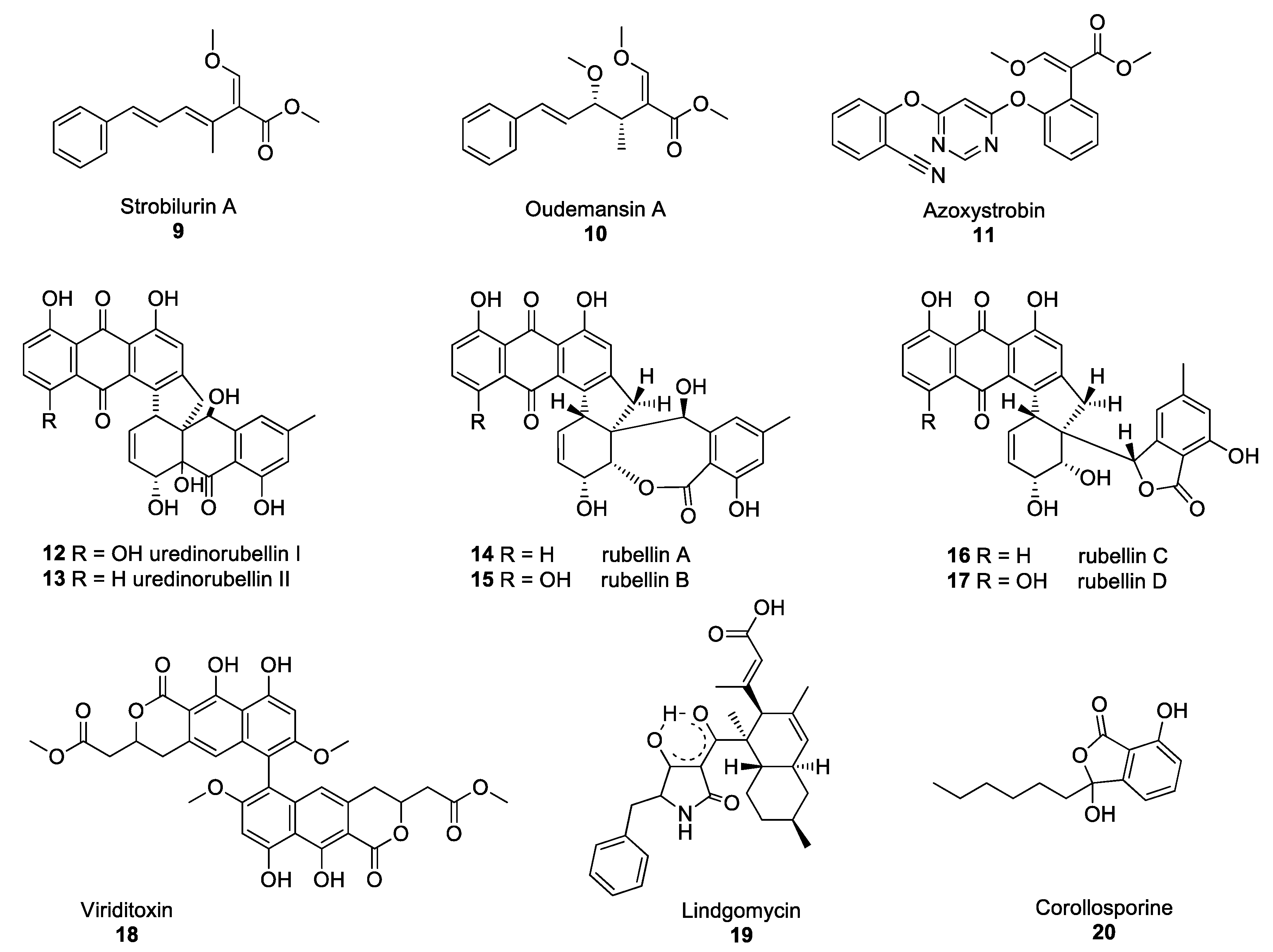
Figure 2. Structures of the selected fungal polyketides: Strobilurin A 9, oudemansin A 10, azoxystrobin 11, uredinorubellins I and II (12–13) rubellins A–D (14–17), viriditoxin 18, lindgomycin 19, corollosporine 20.
Some of the polyketide NPs synthesised by pathogenic fungi exhibit dimeric structures. Such is the case of lesser-known compounds produced by the Torrubiella species. The Torrubiella species are arthropod-pathogenic fungi that parasitise spiders, scale-insects and hoppers and are known to synthesise derivatives of uredinorubellin I 12 and II 13 (Figure 2). These compounds exhibited photodynamic activity, influencing cell viability in three mammalian cell lines, such as HIG82, HT29 and J774A.1, as well as antibacterial activity against Staphylococcus aureus [23]. The genus Torrubiella which belongs to the Clavicipitaceae family, is related to the genus Ramularia which also contains fungi that produce polyketide NPs with antimicrobial activity.
Ramularia collo-cygni is an ascomycete fungus responsible for the important plant disease Ramularia leaf spot (RLS) [24]. RLS is primarily a disease of barley but the fungus can infect other grain crops, such as wheat and oats as well as wild grasses. R. collo-cygni produces a range of secondary metabolites, including rubellin anthraquinones 14–17 (Figure 2). Rubellins are non-host-specific phytotoxins with photodynamic properties [25]. Miethbauer et al. showed that rubellins are biosynthesised via a polyketide pathway, by demonstrating the incorporation of both [1-13C]-acetate and [2-13C]-acetate into the rubellins during their formation [26]. McGrann and co-workers have recently sequenced and analysed the genome of R. collo-cygni and found that it contains the genetic architecture to synthesise a wide range of secondary metabolites, including rubellins [27]. In a later study, it was suggested that the co-expression of genes coding for PKSs and hybrid PKS/nonribosomal peptide synthetases (NRPSs) may be associated with the competitive colonisation of the host plant and early symptom development [28]. However, no exact determination of the biosynthetic pathway of rubellins has been elucidated yet and the role of these metabolites remains unclear. Despite the phytotoxic properties of rubellins, these compounds may have potential pharmaceutical applications. Miethbauer et al. have observed initial activities against Gram-positive bacteria, including MDR strains, such as B. subtilis (ATCC) 6633, S. aureus (SG) 511, S. aureus 134/94 (MRSA), Enterococcus faecalis 1528 (VRE) or Mycobacterium vaccae (IMET) 10670 [29]. Rubellins also exhibit antimicrobial, antiproliferative, cytotoxic and tau aggregation inhibitory activity in vitro tests [29]. Minimal inhibitory concentrations (MICs) were determined with and without illumination, showing a light-dependent increase in the antibacterial activity of compounds 14–17 (except against M. vaccae), with rubellin D 17 being the most active.
PKSs are not only involved in the biosynthesis of anthraquinone derivatives, such as rubellins, but also that of other dimers, such as viriditoxin 18 or lindgomycin 19 (Figure 2). Viriditoxin 18 belongs to the group of xanthoradones produced by Penicillium radicum FKI-3765-2. Xanthoradones exhibit activity against methicillin-resistant S. aureus (MRSA) by inhibiting FtsZ, the bacterial tubulin homolog which is crucial in septum formation [30].
Lindgomycin 19, an unusual antibiotic polyketide, contains two distinct structural domains, a bicyclic hydrocarbon and a tetramic acid that are connected by a carbonyl functional group. Naturally occurring tetramic acid derivatives, originating from a variety of marine and terrestrial fungi (Arctic fungus of the Lindgomycetaceae family), have attracted great interest due to the breadth of the spectrum of their biological activities, as well as their challenging structural complexity [31][32]. The majority of the compounds isolated to date have exhibited mostly antibiotic or antiviral activity. Lindgomycin 19 revealed good antibiotic activity against a number of Gram-positive bacteria (IC50: 2–6 μM), as well as the yeast Candida albicans and the plant pathogenic fungus Z. tritici (IC50: 5–10 μM) [33]. This compound also showed antibiotic activity against an MRSA strain with IC50 values of 5.1 μM. Another example of polyketide-derived NP from marine fungi is corollosporine 20 (Figure 2). Corollosporine 20 is an antibacterial phthalide derivative produced by the fungus Corollospora maritima which was isolated from driftwood found near the island of Heligoland, Germany [34].
2.3. Peptidic Fungal Natural Products
Two distinct pathways in fungi are responsible for the production of peptidic NPs. Enzymes in the nonribosomal peptide (NRP) pathway produce the majority of peptide metabolites. These are highly specific, multimodular enzymes called NRPSs, which utilise both proteinogenic and non-proteinogenic amino acids to synthesise the peptidic backbones. The genes encoding these enzymes are usually located within a biosynthetic cluster, comprising several genes that are co-regulated. The other pathway is that of ribosomally synthesised and post-translationally modified peptides (RiPPs); very large peptidic NPs with molecular weights typically around 1000 Da are synthesised through this pathway [35].
2.4. Terpenoid Compounds
Terpenoids are a large class of natural products related to terpenes, and are made of condensed isoprene units. Terpenoids are often considered mostly as plant NPs; however, fungal species are also known to produce terpene-derived metabolites. The screening of marine fungi for natural products with potential pharmaceutical applications has only recently begun, but has the potential to yield several new drugs.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335.
- Schiff, P.L. Ergot and its alkaloids. Am. J. Pharm. Educ. 2006, 70, 98.
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: West Sussex, UK, 2002; ISBN 0471496413.
- Mukherjee, J.; Menge, M. Progress and prospects of ergot alkaloid research. In New Products and New Areas of Bioprocess Engineering; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–20.
- Bräse, S. Privileged Scaffolds in Medicinal Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2016; ISBN 978-1-78262-030-3.
- Schardl, C.L.; Panaccione, D.G.; Tudzynski, P. Ergot alkaloids–biology and molecular biology. Alkaloids Chem. Biol. 2006, 63, 45–86.
- Jakubczyk, D.; Caputi, L.; Hatsch, A.; Nielsen, C.A.F.; Diefenbacher, M.; Klein, J.; Molt, A.; Schröder, H.; Cheng, J.Z.; Naesby, M.; et al. Discovery and Reconstitution of the Cycloclavine Biosynthetic Pathway—Enzymatic Formation of a Cyclopropyl Group. Angew. Chem. Int. Ed. 2015, 54, 5117–5121.
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947.
- Brookman, J.L.; Denning, D.W. Molecular genetics in Aspergillus fumigatus. Curr. Opin. Microbiol. 2000, 3, 468–474.
- Perrone, G.; Susca, A.; Cozzi, G.; Ehrlich, K.; Varga, J.; Frisvad, J.C.; Meijer, M.; Noonim, P.; Mahakarnchanakul, W.; Samson, R.A. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2007, 59, 53–66.
- Florea, S.; Panaccione, D.G.; Schardl, C.L. Ergot Alkaloids of the Family Clavicipitaceae. Phytopathology 2017, 107, 504–518.
- Jakubczyk, D.; Caputi, L.; Stevenson, C.E.M.; Lawson, D.M.; O’Connor, S.E. Structural characterization of EasH (Aspergillus japonicus)—an oxidase involved in cycloclavine biosynthesis. Chem. Commun. 2016, 52, 14306–14309.
- Yan, L.; Liu, Y. Insights into the Mechanism and Enantioselectivity in the Biosynthesis of Ergot Alkaloid Cycloclavine Catalyzed by Aj_EasH from Aspergillus japonicus. Inorg. Chem. 2019.
- Anke, T.; Oberwinkler, F.; Steglich, W.; Schramm, G. The strobilurins—New antifungal antibiotics from the basidiomycete strobilurus tenacellus (Pers. ex Fr.) Sing. J. Antibiot. (Tokyo) 1977, 30, 806–810.
- Anke, T.; Hecht, H.; Schramm, G.; Steglich, W. Antibiotics from basidiomycetes. IX Oudemansin, an antifungal antibiotic from Oudemansiella mucida (Schrader ex Fr.) Hoehnel (agaricales). J. Antibiot. (Tokyo) 1979, 32, 1112–1117.
- Brandt, U.; Haase, U.; Schaegger, H.; von Jagow, G. Species specificity and mechanism of action of strobilurins. Dechema Monogr. 1993, 129, 27–38.
- Clough, J. The strobilurins, oudemansins, and myxothiazols, fungicidal derivatives of β-methoxyacrylic acid. Nat. Prod. Rep. 1993, 10, 565–574.
- Godwin, J.; Anthony, V.; Clough, J.; Godfrey, C. ICIA5504: A novel, broad spectrum, systemic beta-methoxyacrylate fungicide. In Proceedings of the Brighton Crop Protection Conference—Pests and Diseases; British Crop Protection Council: Farnham, Surrey, UK, 23–26 November 1992; pp. 435–442.
- Torriani, S.F.F.; Brunner, P.C.; McDonald, B.A.; Sierotzki, H. QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag. Sci. 2009, 65, 155–162.
- Leroux, P.; Gredt, M.; Leroch, M.; Walker, A.S. Exploring mechanisms of resistance to respiratory inhibitors in field strains of botrytis cinerea, the causal agent of gray mold. Appl. Environ. Microbiol. 2010, 76, 6615–6630.
- Piszczek, J.; Pieczul, K.; Kiniec, A. First report of G143A strobilurin resistance in Cercospora beticola in sugar beet (Beta vulgaris) in Poland. J. Plant Dis. Prot. 2018, 125, 99–101.
- Oliver, R.P. A reassessment of the risk of rust fungi developing resistance to fungicides. Pest Manag. Sci. 2014, 70, 1641–1645.
- Isaka, M.; Palasarn, S.; Tobwor, P.; Boonruangprapa, T.; Tasanathai, K. Bioactive anthraquinone dimers from the leafhopper pathogenic fungus Torrubiella sp. BCC 28517. J. Antibiot. (Tokyo) 2012, 65, 571.
- Miethbauer, S.; Heiser, I.; Liebermann, B. The phytopathogenic fungus Ramularia collo-cygni produces biologically active rubellins on infected barley leaves. J. Phytopathol. 2003, 151, 665–668.
- Heiser, I.; Heß, M.; Schmidtke, K.-U.; Vogler, U.; Miethbauer, S.; Liebermann, B. Fatty acid peroxidation by rubellin B, C and D, phytotoxins produced by Ramularia collo-cygni (Sutton et Waller). Physiol. Mol. Plant Pathol. 2004, 64, 135–143.
- Miethbauer, S.; Haase, S.; Schmidtke, K.-U.; Günther, W.; Heiser, I.; Liebermann, B. Biosynthesis of photodynamically active rubellins and structure elucidation of new anthraquinone derivatives produced by Ramularia collo-cygni. Phytochemistry 2006, 67, 1206–1213.
- McGrann, G.R.D.; Andongabo, A.; Sjokvist, E.; Trivedi, U.; Dussart, F.; Kaczmarek, M.; Mackenzie, A.; Fountaine, J.M.; Taylor, J.M.G.; Paterson, L.J.; et al. The genome of the emerging barley pathogen Ramularia collo-cygni. BMC Genom. 2016, 17, 584.
- Dussart, F.; Douglas, R.; Sjökvist, E.; Hoebe, P.N.; Spoel, S.H.; McGrann, G.R.D. Genome-Based Discovery of Polyketide-Derived Secondary Metabolism Pathways in the Barley Pathogen Ramularia collo-cygni. Mol. Plant-Microbe Interact. 2018, 31, 962–975.
- Miethbauer, S.; Gaube, F.; Mollmann, U.; Dahse, H.-M.; Schmidtke, M.; Gareis, M.; Pickhardt, M.; Liebermann, B. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungus Ramularia collo-cygni. Planta Med. 2009, 75, 1523–1525.
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448.
- Royles, B.J.L. Naturally Occurring Tetramic Acids: Structure, Isolation, and Synthesis. Chem. Rev. 1995, 95, 1981–2001.
- Marfori, E.C.; Bamba, T.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Biosynthetic studies of the tetramic acid antibiotic trichosetin. Tetrahedron 2002, 58, 6655–6658.
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19.
- Liberra, K.; Jansen, R.; Lindequist, U. Corollosporine, a new phthalide derivative from the marine fungus Corollospora maritima Werderm. 1069. Pharmazie 1998, 53, 578–581.
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
767
Revision:
1 time
(View History)
Update Date:
21 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




