Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhiqiang Tao | -- | 3011 | 2022-07-19 11:25:02 | | | |
| 2 | Catherine Yang | Meta information modification | 3011 | 2022-07-20 03:18:03 | | | | |
| 3 | Catherine Yang | -12 word(s) | 2999 | 2022-07-22 05:31:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tao, Z.; Yan, P.; Zhang, X.; Wang, D.; Wang, Y.; Ma, X.; Yang, Y.; Liu, X.; Chang, X.; Sui, P.; et al. Abscisic Acid-Induced Heat-Tolerance Responses in Wheat and Maize. Encyclopedia. Available online: https://encyclopedia.pub/entry/25268 (accessed on 11 January 2026).
Tao Z, Yan P, Zhang X, Wang D, Wang Y, Ma X, et al. Abscisic Acid-Induced Heat-Tolerance Responses in Wheat and Maize. Encyclopedia. Available at: https://encyclopedia.pub/entry/25268. Accessed January 11, 2026.
Tao, Zhiqiang, Peng Yan, Xuepeng Zhang, Demei Wang, Yanjie Wang, Xinglin Ma, Yushuang Yang, Xiwei Liu, Xuhong Chang, Peng Sui, et al. "Abscisic Acid-Induced Heat-Tolerance Responses in Wheat and Maize" Encyclopedia, https://encyclopedia.pub/entry/25268 (accessed January 11, 2026).
Tao, Z., Yan, P., Zhang, X., Wang, D., Wang, Y., Ma, X., Yang, Y., Liu, X., Chang, X., Sui, P., & Chen, Y. (2022, July 19). Abscisic Acid-Induced Heat-Tolerance Responses in Wheat and Maize. In Encyclopedia. https://encyclopedia.pub/entry/25268
Tao, Zhiqiang, et al. "Abscisic Acid-Induced Heat-Tolerance Responses in Wheat and Maize." Encyclopedia. Web. 19 July, 2022.
Copy Citation
Abscisic acid (ABA) plays a physiological role in regulating the heat tolerance of plants and maintaining crop productivity under high-temperature stress. Appropriate cultivation techniques can regulate endogenous ABA and help farmers improve food production under high-temperature stress. High-temperature stress stimulates ABA, which reduces stomatal opening and promotes root growth. The root system absorbs water to maintain the water status, thus allowing the plant to maintain physiological activities under high-temperature stress.
Abscisic acid
heat stress
crop management
wheat
1. Introduction
Abscisic acid (ABA) is an important hormone in crops and plays a role as a signaling molecule in biosynthesis, catabolism, transport and signal transduction pathways. ABA synthesis in the root plays a major role in regulating root growth and the absorption of soil water [1]. ABA also plays a key role in establishing a hydrophobic barrier to prevent water loss [2]. Under high-temperature stress and low-water status, ABA synthesized in leaf vascular cells can be transferred to guard cells to induce stomatal closure, reducing water loss through transpiration [3]. This reduces the amount of CO2 that enters the blade, limiting photosynthesis but reducing water loss; this allows the plant to maintain physiological functions, which improves heat resistance and reduces production losses [4][5][6]. In addition, in response to high-temperature stress, ABA can enhance glucose metabolism and provide energy for heat resistance [7]. ABA can also increase the supply of assimilates to ears, enhancing the fertility of pollen, increasing kernel number, and improving grain filling [8][9][10]. ABA induces the production of heat-shock proteins (HSPs), which accumulate in the cell membrane and protect the membrane against thermal denaturation and damage from reactive oxygen species (ROS) [11][12]. ABA also induces the production of energy in the form of adenosine triphosphate (ATP), which is needed for HSP accumulation [13].
2. The Mechanism of How Cultivation Techniques Respond to High Temperature in Wheat and Maize
2.1. Cultivars
One approach for improving production under heat stress is to select cultivars with specific characteristics. For example, heat cultivars adapted to high temperatures have the following characteristics: (ⅰ) The heading time is advanced. Wheat varieties with early heading were selected in North and South China, France and Australia to facilitate adaptation to high-temperature stress during the grain-filling period caused by climate warming [14][15]. (ii) Organs have a strong antioxidant capacity. When wheat is subjected to high-temperature stress, leaves and non-leaf organs (flag leaf sheath, internodes under the ear, glumes and kernels) have strong and sustained antioxidant activity, and the antioxidant capacity of non-leaf organs is higher than that of leaves [16]. (ⅲ) Starch-synthesis-related enzyme activity is high. The activities of ADP-glucose-pyrophosphorylase, a starch branching enzyme and sucrose synthase in wheat seeds, were significantly positively correlated with the contents of amylopectin and total starch in grains at the late stage of grain filling (22–26 d after flowering) [17]. Increasing the activity of enzymes involved in the synthesis of these sugars or starches can improve heat resistance, increase the starch content in the grains, and reduce the effect of high temperature on yield [18]. (ⅳ) The capacity for storage protein accumulation is high, which can compensate for the loss of yield caused by the decrease in starch accumulation [19][20][21]. One study found that the content of protein and its components was increased by high-temperature stress at the grain-filling stage [22] and that the activity of enzymes related to starch synthesis decreased, but the levels of starch precursors remained relatively stable.
Figure 1 illustrates how wheat and maize cultivars adapt to high temperature. Under high-temperature stress, ABA promotes root growth, and roots absorb water to maintain the water status of the plant. Roots also absorb nitrogen to supply the requirement to produce storage proteins in the grains. ABA also regulates stomatal opening and closing, maintains photosynthesis, and promotes starch and sugar synthesis to meet the demand of the panicle for photosynthates and energy. ABA induces the accumulation of the molecular chaperones HSPs, which improve the antioxidant capacity of chloroplasts and maintain photosynthesis. Storage proteins also compensate for the loss of starch in the grains. A sufficient amount of photosynthates and energy guarantees pollination and a high kernel number per ear, increases grain filling and kernel weight, and weakens the inhibitory effect of high temperature on yield.
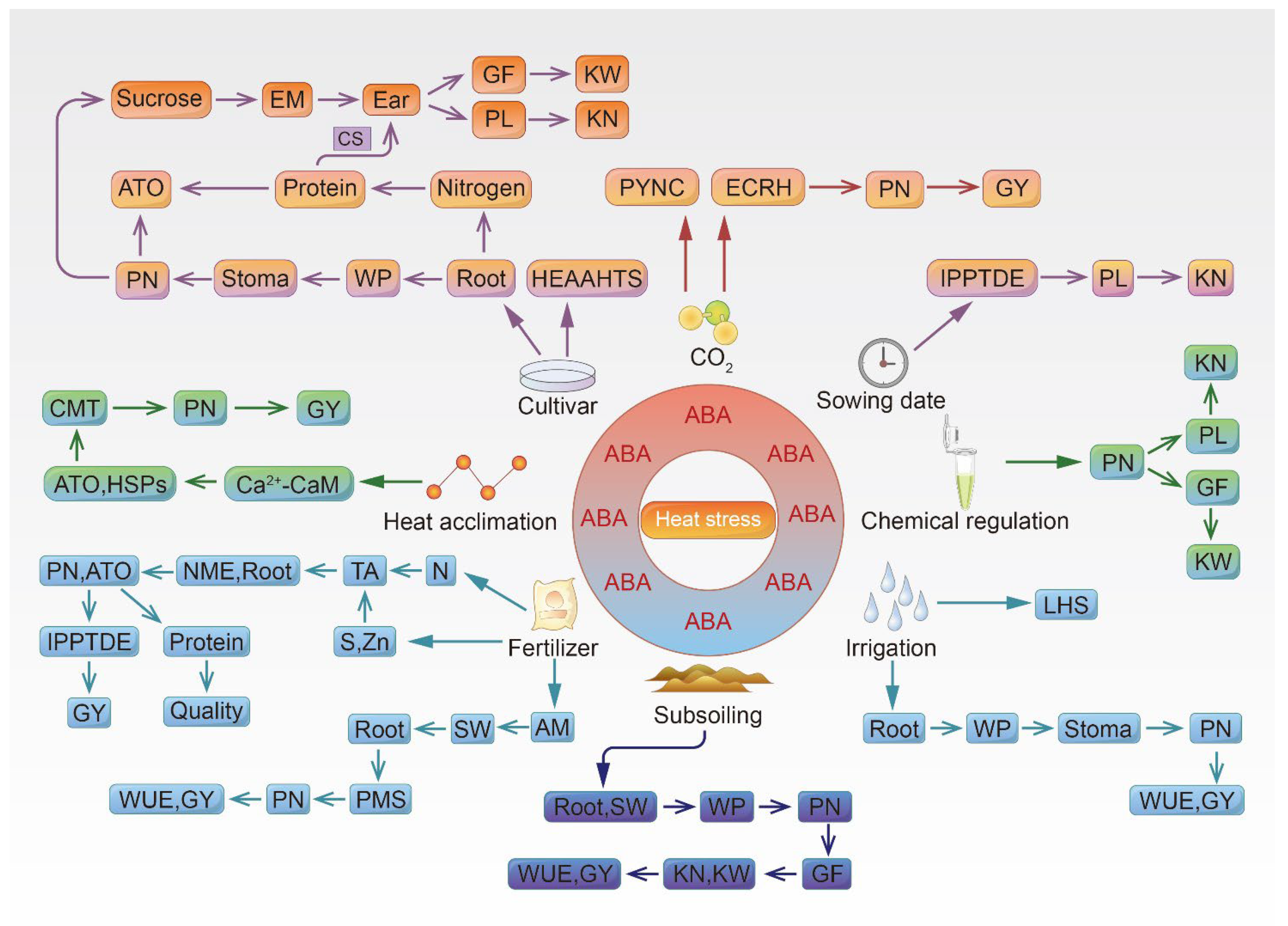
Figure 1. Diagram of how cultivation and farming techniques facilitate the adaptation of wheat and maize to high-temperature stress. AM, arbuscular mycorrhizal fungi; ATO, antioxidant ability; CMT, cell-membrane thermostability; CS, compensation for the loss of starch; ECRH, equilibrium of cell redox homeostasis; EM, energy and matter; GF, grain filling; GS, glutamine synthetase; GY, grain yield; HEAAHTS, heading early to avoid or adapt to high-temperature stress; IPPTDE, improve the production of photosynthates and promote transfer and distribution to ears; KN, kernel number per ear; KW, kernel weight; LHS, lower heat sensitivity; NME, nitrogen metabolism enzyme; PL, pollination; PMS, plant moisture status; PN, photosynthesis; PYNC, protein yield negatively correlated with CO2 concentration; SW, soil water-holding capacity; TA, topdressing before and after anthesis; WP, water status of the plant; WUE, water use efficiency.
2.2. Sowing Date
The sowing date of wheat or maize can be adjusted to affect the amount of light and heat the crop is exposed to, which affects the ABA contents in the stem, leaves, and ears of the plant. Optimizing ABA content can improve the production of photosynthates and promote the transport and distribution of photosynthates to ears, which ensures pollination and seed setting and increases kernel number per ear (Figure 1). There is a linear relationship between wheat yield and grain number per unit area, and floret fertility is greatly affected under high-temperature stress. In one study conducted in Taian City, Shandong Province, China, wheat cultivars sown on 15 October or 22 October were compared with those sown on 1 October or 8 October. Cultivars sown at the later date had higher ABA contents in the stem and lower ABA contents in the panicle, which promoted the transport of carbon compounds and water-soluble carbohydrates to the panicle and led to an increased number of florets and grains per panicle [23].
2.3. Chemical Regulation
Plant growth regulators that are commonly applied to wheat and maize to promote resistance to high-temperature stress are 2,4-dichloroformamide cyclopropane acid (2,4-D) and monopotassium phosphate (KH2PO4). These chemicals improve photosynthesis, reduce the length of the tip of a maize cob, increase kernel number per ear, and increase the grain-filling rate and kernel weight under high-temperature stress (Figure 1). When 2,4-D was sprayed on the leaves at the ninth-expanded-leaf stage of spring maize grown under heat stress, the ear leaf had a significantly increased chlorophyll content and ABA content and an enhanced net photosynthesis rate at the grain-filling stage compared with plants that were not sprayed. Spraying also improved the grain-filling rate, grain volume, and grain dry weight and decreased the bare tip length, which increased the grain yield by 8.5% [24].
In wheat plants sprayed with KH2PO4, compared with those not sprayed with KH2PO4, the ABA content 4–20 d after flowering and the ethylene evolution rate 4–16 d after flowering were significantly higher in inferior grains (the grains in the middle spikelets (spikelets 4–12) most distal from the bottom of the spike), and the ratio of ABA/ethylene was also higher. Moreover, the maximum and mean grain-filling rates and grain weight were significantly and positively correlated with the ratio of ABA/ethylene in the inferior grains. As the inferior grain weight was significantly and positively correlated with the ear kernel weight, this is the main reason why there was an increase in kernel weight in wheat sprayed with KH2PO4 [25].
2.4. Fertilization
2.4.1. Nitrogen Fertilization
The rate of nitrogen application and whether soil water is sufficient can affect the degree of heat tolerance [26][27]. During the production of wheat in Shijiazhuang city in the North China Plain, infrared heaters were used to increase the ambient temperature. Compared with fields without nitrogen fertilizer, in fields receiving an application of nitrogen fertilizer (240 kg ha−1), the soil temperature in the 5–40 cm soil layer increased by 0.2–0.3 °C, the soil volumetric water content in the 0–60 cm soil layer decreased by 0–3.4%, and the yield increased significantly (p < 0.05), but the reduction in yield under heat stress was larger for crops receiving nitrogen fertilizer than for those that did not. This is mainly because, in the nitrogen fertilizer treatment, evapotranspiration increased with increasing air temperature and soil temperature, which caused a decrease in the soil water content and a significant decrease in the number of panicles [27]. This shows that nitrogen fertilizer helps improve crop productivity under heat stress when there is adequate soil moisture. In addition, the results of several studies suggest that farmers should control the total amount of nitrogen fertilization. In Catalonia, in northeastern Spain, wheat was subjected to high-temperature stress during the grain-filling period, and the amount of nitrogen applied to the field increased from 68 kg ha−1 to 376 kg ha−1; yield loss, which ranged from 10–25%, increased with increasing nitrogen applied [28].
The timing of fertilizer application can affect the degree of heat tolerance. Under high-temperature stress, compared with plants treated with all nitrogen fertilizer applied at once as a basal fertilizer, plants treated with half of the nitrogen fertilizer applied as a basal fertilizer and the other half applied as a topdressing at the jointing stage or booting stage had increased glutamine synthetase, catalase and peroxidase activity in the flag leaf, increased photosynthetic rate and stomatal conductance, higher translocation of dry matter from the vegetative organs before flowering to grain, improved heat tolerance, and significantly increased kernel weight and grain yield [29].
2.4.2. Sulfur Fertilization
Sulfur (S) is a mineral nutrient essential for plant growth. It is the fourth major nutrient after nitrogen, phosphorus and potassium. Ninety percent of the S in plants is used to synthesize S-containing amino acids. In recent years, the use of S in industrial production processes has decreased, resulting in reduced atmospheric acid deposition and the use of high-concentration phosphate fertilizers with less S; therefore, S has become a major constraint on crop production [30][31]. When oil-seed rape plants treated with low S (8.7 µM SO42−) or high S (500 µM SO42−) during the seed-filling stage were subjected to high-temperature stress (33 °C/day, 19 °C/night vs. control temperature 20 °C/day, 15 °C/night), high-temperature stress and low S decreased the number of seeds per plant; decreased the ABA content in grains; decreased the (raffinose + stachyose)/sucrose ratio, linoleic acid/linolenic acid ratio, and S-poor seed-storage-protein (12S albumins)/S-rich seed storage protein (2S albumins) ratio. This led to impaired synthesis of methionine and cysteine. The balance of protein production was maintained, but the grain yield and quality were reduced [32].
Supplementary application of S fertilizer can improve the heat resistance of crops and improve grain yield and quality. For example, it was found that high-temperature stress significantly increased the contents of total protein, albumin, alcohol soluble protein, gluten, cysteine and methionine in wheat grains (p < 0.05) but decreased grain yield, grain weight, globulin content, total starch accumulation and overall quality. However, supplementary application of S fertilizer at the heading stage had a significant positive effect on the activities of nitrate reductase and other nitrogen-metabolism enzymes, glucose metabolism and photosynthesis [31]. S application improved nitrate reductase and glutamine synthetase activities in the flag leaf, grain yield, grain weight, and the contents of cysteine, methionine, total protein, albumin, alcohol-soluble protein, gluten and globulin and improved the overall quality [33]. These results show that the application of S fertilizer alleviates the negative effects of high-temperature stress on grain yield, starch content and grain quality.
2.4.3. Zinc Fertilization
Foliar application of zinc (Zn) fertilizer or related nutrient compounds has an obvious effect on the resistance of wheat and corn to abiotic stress. Treating soil with 15 mg Zn kg–1 was found to increase the grain yield, protein yield, grain weight, and total protein, albumin, gliadin and gluten contents, as well as the activities of nitrate reductase and glutamine synthetase in flag leaves after 20 days of high-temperature stress at the filling stage of wheat, but decreased the globulin content; the negative effects of high-temperature stress on grain yield, protein content, protein component content and comprehensive quality were also reduced [34]. The addition of chitosan oligosaccharides and marine polysaccharides to nutritional compound preparations composed of Zn sulfate, potassium dihydrogen phosphate and urea was found to effectively enhance root activity, increase the chlorophyll content and betaine content of the flag leaf, delay leaf senescence, improve grain filling, and increase the 1000-kernel weight and the harvest index in wheat; in general, the harm caused by heat stress during grain filling in wheat was reduced, and the yield was stabilized [35]. In the dry land of western Henan, China, increased Zn fertilizer application was found to enhance root stability under drought stress, improve the development of wheat roots and alleviate the effect of drought stress on yield reduction [36]. Applying Zn fertilizer to maize in Henan Province increased the leaf area, chlorophyll content, net photosynthetic rate, total amount of dry matter per plant, accumulation of dry matter in each organ, and transport of dry matter to seeds, and enhanced drought resistance and yield [37].
2.4.4. Arbuscular Mycorrhizal Fungi Fertilization
Arbuscular mycorrhizal fungi application can increase heat tolerance. Maize roots inoculated with arbuscular mycorrhizal fungi under heat stress can improve heat resistance by improving photosynthesis and water status. Arbuscular mycorrhizal fungi help optimize the soil water-holding capacity, which improves the plant moisture status (relative water content in maize leaves) and then indirectly increases stomatal conductance and increases photosynthesis parameters, including the net photosynthetic rate, maximal fluorescence, maximum quantum efficiency of PSII photochemistry and potential photochemical efficiency, and concentrations of chlorophyll a and chlorophyll b [38][39].
2.5. Carbon Dioxide
The stimulation of grain yield by elevated CO2 in wheat depends on high temperatures. It also relies on the availability of nitrogen nutrient resources. A study of 11 wheat varieties in four continents and eight countries found that, for nitrogen application rates up to 200 kg ha−1, increasing the CO2 concentration can increase grain yield. However, for nitrogen application rates >200 kg ha−1, when the CO2 concentration was increased, grain yield stagnated or even decreased. For all nitrogen application rates, increasing CO2 significantly reduced grain protein yield by 7% on average. This suggests that, in an environment where extreme temperatures are common because of global warming, studying the interaction between nitrogen fertilizer and CO2 is an effective way to simultaneously improve wheat yield and quality. Plant responses to increased CO2 and higher temperatures are regulated by ABA and redox homeostasis networks [40]. Increased CO2 concentrations balance cellular redox homeostasis and increase malondialdehyde content and electrolyte leakage but also significantly increase the antioxidant capacity of plants, improving the Fv/Fm (the ratio of variable to maximum fluorescence after dark adaptation, representing the maximum quantum yield of photosystem II) of plants subjected to high-temperature stress [41]. ABA is indirectly involved in the mitigation of high-temperature stress induced by increased CO2 concentrations by increasing the antioxidant capacity of the plants.
2.6. Irrigation
Irrigation has become an important method for adapting global crop production to climate change. In India, the heat sensitivity of irrigated wheat was found to be only one-quarter of the heat sensitivity of wheat grown under rain-fed conditions. However, as the negative effects of climate change continue and additional constraints on expanding irrigation are imposed, increasing production through irrigation in a warming climate will be a serious challenge [42].
In China, which has limited water resources, scientists and agricultural production operators are improving irrigation technologies to cope with the negative impacts of climate change on crop production. In North China, the use of micro-sprinkling hoses (5–10 mm) during the grain-filling stage of wheat at 10:00 on days with forecasted high temperatures can significantly reduce the canopy temperature and increase the relative humidity of the canopy, the water potential of the flag leaf and the photosynthetic rate of the plant population. Furthermore, the earlier the time of micro-sprinkling, the higher the increase in kernel weight and grain yield [43]. In semiarid regions of China, it was found that supplementary irrigation of 30 mm during the flowering stage of wheat could prevent future yield losses due to the increase in CO2 concentrations and temperatures caused by climate change. Higher supplemental irrigation levels of 60 and 90 mm were found to increase wheat yields by 3.8 and 10.1%, respectively. Thus, supplementary irrigation (30–90 mm) may play a key role in maintaining rainfed spring wheat yields in regions affected by global climate change [44].
2.7. Subsoiling
To cope with high-temperature stress during the spring maize grain-filling period in the North China Plain, subsoiling is performed before planting. Subsoiling can directly increase the root length density and soil moisture in the 0–80 cm soil profile, indirectly alleviate the inhibitory effects of high temperature on leaf photosynthetic rate and plant water status, increase the ABA-induced activity of superoxide dismutase, decrease the content of malondialdehyde, increase heat tolerance, improve the filling rate, prolong the grain linear-filling stage, and increase grain number per spike and yield [45][46].
2.8. Heat Acclimation
Heat acclimation is also an effective way to increase the heat tolerance of crops. Crops that have been acclimated through high temperatures can maintain low respiratory costs and exhibit no or slight reductions in photosynthesis under high-temperature stress, which allows the crop to maintain net carbon gain [47]. In one study of heat-tolerant and heat-sensitive varieties of wheat seedlings, the thermal death times at 50 °C ranged from 8–26 min. After acclimation for 3 days at 34 °C, the thermal lethal times of heat-tolerant and heat-sensitive varieties at 50 °C increased to 87–110 min and 35–55 min, respectively. The reason for this increase is that heat acclimation increases the activity and stability of superoxide dismutase and catalase, improves the stability of the leaf cell membrane, and stimulates HSPs, which increase protein stability; this enhances the antioxidant capacity of the plants under high-temperature stress, prolonging the thermal death time [48][49]. Of these traits, the thermal stability of the cell membrane is considered an ideal physiological index for evaluating heat resistance [50].
The heat response of maize seedlings is initiated through the intracellular entry of extracellular Ca2+ and the regulation of intracellular CaM after heat acclimation. Heat acclimation enhances the activity of antioxidant enzymes such as superoxide dismutase, catalase and ascorbic acid reductase, which are induced by ABA, and lowers heat stress-induced lipid peroxidation [51]. The heat-acclimation process in wheat is also accompanied by the accumulation of CaM. Moreover, the accumulation of CaM is affected by the concentrations of Ca2+; Ca2+-CaM signaling can regulate the heat-shock response through HSP70 [52].
References
- Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Peirats-Llobet, M.; Pizzio, G.A.; Fernandez, M.A.; De Winne, N.; De Jaeger, G.; Dietrich, D.; Bennett, M.J.; et al. PYRABACTIN RESISTANCE1-LIKE8 Plays an Important Role for the Regulation of Abscisic Acid Signaling in Root. Plant Physiol. 2013, 161, 931–941.
- Barberon, M.; Vermeer, J.E.M.; De Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E.; et al. Adaptation of Root Function by Nutrient-Induced Plasticity of Endodermal Differentiation. Cell 2016, 164, 447–459.
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522.
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174.
- Umezawa, T.; Okamoto, M.; Kushiro, T.; Nambara, E.; Oono, Y.; Seki, M.; Kobayashi, M.; Koshiba, T.; Kamiya, Y.; Shinozaki, K. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. Plant J. 2006, 46, 171–182.
- Yaaran, A.; Negin, B.; Moshelion, M. Role of guard-cell ABA in determining steady-state stomatal aperture and prompt vapor-pressure-deficit response. Plant Sci. 2019, 281, 31–40.
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955.
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Xu, G.W.; Zhu, Q.S. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 2004, 135, 1621–1629.
- Zhu, Y.; Dun, X.L.; Zhou, Z.F.; Xia, S.Q.; Yi, B.; Wen, J.; Shen, J.X.; Ma, C.Z.; Tu, J.X.; Fu, T.D. A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Mol. Biol. 2010, 72, 111–123.
- Yadav, M.R.; Choudhary, M.; Singh, J.; Lal, M.K.; Jha, P.K.; Udawat, P.; Gupta, N.K.; Rajput, V.D.; Garg, N.K.; Maheshwari, C.; et al. Impacts, Tolerance, Adaptation, and Mitigation of Heat Stress on Wheat under Changing Climates. Int. J. Mol. Sci. 2022, 23, 2838.
- Hu, X.L.; Jiang, M.Y.; Zhang, J.H.; Zhang, A.Y.; Lin, F.; Tan, M.P. Calcium-calmodulin is required for abscisic acid-induced antioxidant defense and functions both upstream and downstream of H2O2 production in leaves of maize (Zea mays) plants. New Phytol. 2007, 173, 27–38.
- Rezaul, I.M.; Feng, B.H.; Chen, T.T.; Fu, W.M.; Zhang, C.X.; Tao, L.X.; Fu, G.F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019, 165, 644–663.
- Xue, G.P.; Sadat, S.; Drenth, J.; McIntyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557.
- Zheng, B.Y.; Chenu, K.; Dreccer, M.F.; Chapman, S.C. Breeding for the future: What are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties? Glob. Chang. Biol. 2012, 18, 2899–2914.
- Liu, B.; Liu, L.; Tian, L.; Cao, W.; Zhu, Y.; Asseng, S. Post-heading heat stress and yield impact in winter wheat of China. Glob. Chang. Biol. 2014, 20, 372–381.
- Zhang, Y.H.; Yang, Y.M.; Cao, L.; Hao, Y.F.; Huang, Q.; Li, J.P.; Yao, D.X.; Wang, Z.M. Effect of high temperature on photosynthetic capability and antioxidant enzyme activity of flag leaf and non-leaf organs in wheat. Acta Agron. Sin. 2015, 41, 136–144, (In Chinese with English Abstract).
- Hu, Y.Y.; Lu, H.F.; Liu, W.X.; Kang, J.; Ma, G.; Meng, S.S.; Chu, Y.Y.; Wang, C.Y. Effects of high temperature and water deficiency during grain filling on activities of key starch synthesis enzymes and starch accumulation in wheat. Acta Agron. Sin. 2018, 44, 591–600, (In Chinese with English Abstract).
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.P. Heat stress effect on source-sink relationships and metabolome dynamics in wheat. J. Exp. Bot. 2020, 71, 543–554.
- Arena, S.; D’Ambrosio, C.; Vitale, M.; Mazzeo, F.; Mamone, G.; Di Stasio, L.; Maccaferri, M.; Curci, P.L.; Sonnante, G.; Zambrano, N.; et al. Differential representation of albumins and globulins during grain development in durum wheat and its possible functional consequences. J. Proteom. 2017, 162, 86–98.
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705.
- Wang, X.M.; Hou, L.J.; Lu, Y.Z.; Wu, B.J.; Gong, X.; Liu, M.S.; Wang, J.; Sun, Q.X.; Vierling, E.; Xu, S.B. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545.
- Zhang, Y.F.; Wang, C.Y.; Ma, D.Y.; Lu, H.F.; Zhu, Y.J.; Xie, Y.X.; Guo, T.C. Effects of waterlogging, high temperature and their interaction after anthesis on grain protein components and flour color in wheat. Acta Agron. Sin. 2014, 40, 1102–1108.
- Zhu, Y.G.; Chu, J.P.; Dai, X.L.; He, M.R. Delayed sowing increases grain number by enhancing spike competition capacity for assimilates in winter wheat. Eur. J. Agron. 2019, 104, 49–62.
- Tao, Q.; Zhou, Y.Y.; Guo, Q.; Liu, Y.R.; Yu, S.; Yu, C.X.; Zhang, M.C.; Li, Z.H.; Duan, L.S. A novel plant growth regulator alleviates high-temperature stress in maize. Agron. J. 2018, 110, 2350–2359.
- Lv, X.K.; Han, J.; Liao, Y.C.; Liu, Y. Effect of phosphorus and potassium foliage application post-anthesis on grain filling and hormonal changes of wheat. Field Crops Res. 2017, 214, 83–93.
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037.
- Liu, L.T.; Hu, C.S.; Olesen, J.E.; Ju, Z.Q.; Yang, P.P.; Zhang, Y.M. Warming and nitrogen fertilization effects on winter wheat yields in northern China varied between four years. Field Crops Res. 2013, 151, 56–64.
- Elia, M.; Slafer, G.A.; Savin, R. Yield and grain weight responses to post-anthesis increases in maximum temperature under field grown wheat as modified by nitrogen supply. Field Crops Res. 2018, 221, 228–237.
- Jiang, W.W.; Yin, Y.P.; Wang, Z.L.; Li, Y.; Yang, W.B.; Peng, D.L.; Yang, D.Q.; Cui, Z.Y.; Lu, K.L.; Li, Y.X. Effects of postponed application of nitrogen fertilizer on yield and physiological characteristics of flag leaf in wheat under post-anthesis heat stress. Acta Agron. Sin. 2014, 40, 942–949, (In Chinese with English Abstract).
- Xie, Y.X.; Zhang, H.; Zhu, Y.J.; Zhao, L.; Yang, J.H.; Cha, F.N.; Liu, C.; Wang, C.Y.; Guo, T.C. Grain yield and water use of winter wheat as affected by water and sulfur supply in the North China Plain. J. Integr. Agric. 2017, 16, 614–625.
- Zörb, C.; Dorothee, S.; Gödde, V.; Niehaus, K.; Mühling, K.H. Metabolite profiling of wheat flag leaf and grains during grain filling phase as affected by sulfur fertilisation. Funct. Plant Biol. 2012, 39, 156–166.
- D’Hooghe, P.; Dubousset, L.; Gallardo, K.; Kopriva, S.; Avice, J.-C.; Trouverie, J. Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur- limited Brassica napus L. Mol. Cell. Proteom. 2014, 13, 1165–1183.
- Tao, Z.Q.; Chang, X.H.; Wang, D.M.; Wang, Y.J.; Ma, S.K.; Yang, Y.S.; Zhao, G.C. Effects of sulfur fertilization and short-term high temperature on wheat grain production and wheat flour proteins. Crop J. 2018, 6, 413–425.
- Tao, Z.Q.; Wang, D.M.; Chang, X.H.; Wang, Y.J.; Yang, Y.S.; Zhao, G.C. Effects of zinc fertilizer and short-term high temperature stress on wheat grain production and wheat flour proteins. J. Integr. Agric. 2018, 17, 1979–1990.
- Liu, W.H.; Li, S.N.; Hou, G.G.; Yang, J.H.; Duan, J.Z.; Zhu, Y.J. Effects of foliar-spraying of different nutritional mixtures on stress tolerance to dry-hot wind and yield in winter wheat. J. Plant Nutr. Fertilizer 2019, 25, 1600–1606.
- Zhang, J.; Liang, Z.K.; Wang, X.P.; Hu, L.H.; Li, Y.J. Effects of zinc fertilizer on root growth and yield of winter wheat under drought stress. Acta Agric. Boreali-Sin. 2019, 34, 126–136, (In Chinese with English Abstract).
- Wang, Z.Q.; Zhang, L.T.; Peng, L.X.; Zhang, Z.W.; Hu, Y.B.; Lin, T.B. Effect of zinc fertilizer on yield formation of maize under water stresses. J. Henan Agric. Univ. 2014, 48, 674–679, (In Chinese with English Abstract).
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199.
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934.
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721.
- Li, X.; Ahammed, G.J.; Zhang, Y.Q.; Zhang, G.Q.; Sun, Z.H.; Zhou, J.; Zhou, Y.H.; Xia, X.J.; Yu, J.Q.; Shi, K. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol. 2015, 17, 81–89.
- Zaveri, E.; Lobell, D.B. The role of irrigation in changing wheat yields and heat sensitivity in India. Nat. Commun. 2019, 10, 4144.
- Wang, D.; Xu, X.X.; Zhang, H.B.; Lin, X.; Zhao, Y. Effects of irrigation with micro-sprinkling hoses on canopy temperature and humidity at filling stage and grain weight of wheat. Acta Agron. Sin. 2015, 41, 1564–1574, (In Chinese with English Abstract).
- Xiao, G.J.; Liu, W.X.; Xu, Q.; Sun, Z.J.; Wang, J. Effects of temperature increase and elevated CO2 concentration, with supplemental irrigation, on the yield of rain-fed spring wheat in a semiarid region of China. Agric. Water Manag. 2005, 74, 243–255.
- Tao, Z.Q.; Sui, P.; Chen, Y.Q.; Li, C.; Nie, Z.J.; Yuan, S.F.; Shi, J.T.; Gao, W.S. Subsoiling and Ridge Tillage Alleviate the High Temperature Stress in Spring Maize in the North China Plain. J. Integr. Agric. 2013, 12, 2179–2188.
- Tao, Z.Q. Technologic Solutions of High Temperature Stress in Spring Maize during the Filling Stage in the North China Plain. Ph.D. Thesis, China Agriculture University, Beijing, China, 2013. (In Chinese with English Abstract).
- Rashid, F.A.A.; Crisp, P.A.; Zhang, Y.; Berkowitz, O.; Pogson, B.J.; Day, D.A.; Masle, J.; Dewar, R.C.; Whelan, J.; Atkin, O.K.; et al. Molecular and physiological responses during thermal acclimation of leaf photosynthesis and respiration in rice. Plant Cell Environ. 2020, 43, 594–610.
- Zhou, R.G.; Fan, Z.H.; Li, X.Z.; Wang, Z.W.; Han, W. The effect of heat acclimation on celluar membrane thermostability in wheat. Acta Agric. Boreali-Sin. 1993, 8, 33–37, (In Chinese with English Abstract).
- Zhou, R.G.; Fan, Z.H.; Li, X.Z.; Wang, Z.W.; Han, W. The effect of heat acclimation on membrane thermostability and relative enzyme activity. Acta Agron. Sin. 1995, 21, 568–572, (In Chinese with English Abstract).
- Chen, X.Y.; Li, Y.J.; Gao, Z.Y.; Tian, S.M. Relationship of acquired heat tolerance and performance of heat tolerance in wheat. Acta Agric. Boreali-Sin. 2003, 18, 52–55, (In Chinese with English Abstract).
- Gong, M.; Li, Y.J.; Chen, S.Z. Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J. Plant Physiol. 1998, 153, 488–496.
- Li, X.Z.; Zhou, R.G.; Fan, Z.H.; Bai, J. A Study on relationship between heat acclimation and calmodulin level in wheat. Acta Agric. Boreali-Sin. 2000, 15, 20–23, (In Chinese with English Abstract).
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
870
Revisions:
3 times
(View History)
Update Date:
22 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




