Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fatmir Dragidella | -- | 2058 | 2022-07-18 11:04:57 | | | |
| 2 | Vivi Li | Meta information modification | 2058 | 2022-07-18 11:35:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dalipi, Z.S.; Dragidella, F. Calcium and Vitamin D Supplementation for Periodontal Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/25232 (accessed on 12 January 2026).
Dalipi ZS, Dragidella F. Calcium and Vitamin D Supplementation for Periodontal Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/25232. Accessed January 12, 2026.
Dalipi, Zana Sllamniku, Fatmir Dragidella. "Calcium and Vitamin D Supplementation for Periodontal Disease" Encyclopedia, https://encyclopedia.pub/entry/25232 (accessed January 12, 2026).
Dalipi, Z.S., & Dragidella, F. (2022, July 18). Calcium and Vitamin D Supplementation for Periodontal Disease. In Encyclopedia. https://encyclopedia.pub/entry/25232
Dalipi, Zana Sllamniku and Fatmir Dragidella. "Calcium and Vitamin D Supplementation for Periodontal Disease." Encyclopedia. Web. 18 July, 2022.
Copy Citation
Periodontal disease is a complex disease that involves an imbalance between the oral microbiota and an individual’s inflammatory response. Moreover, the inflammatory response contributes to further imbalance; if left untreated, periodontal disease may result in tooth loss. Vitamin D is intricately involved in the regulation of calcium–phosphate homeostasis and bone mineral metabolism; considering that periodontal surgery usually includes regenerative therapy, adequate vitamin D and calcium levels are essential.
vitamin D
calcium
periodontal disease
1. Introduction
Periodontal disease is a complex polymicrobial disease, which involves the disruption of oral homeostasis and is characterized by gum inflammation (gingivitis) that leads to progressive loss of tooth-supporting tissues [1][2]. This disease results from an imbalance between the oral microbiota and an individual’s inflammatory response; it contributes to dysbiosis [3]. The systemic effects of the host response to periodontal disease are suspected to develop along with dysbiosis, resulting in a state of nososymbiocity [4].
Periodontal disease has important systemic effects and can exacerbate other diseases [3][5][6]. These effects are partly mediated by the movement of pro-inflammatory cytokines from oral tissues into systemic circulation. In the liver, these cytokines increase the levels of various proteins (e.g., C-reactive protein, fibrinogen, and serum amyloid A) that reportedly exacerbate atherosclerosis and intrauterine inflammation. Additionally, periodontal bacteria can directly enter systemic circulation during tooth brushing or tooth extraction [7], producing at least 5 min of detectable bacteremia [8].
The major predictors of tooth loss in patients with periodontal disease include the presence of plaque-associated bacteria, older age, poor compliance with dental care, smoking, and diabetes [1][4]. Vitamin D and calcium supplementation can have a positive effect in the management of periodontal disease, when used as an adjunct to non-surgical periodontal treatment; moreover, vitamin D and calcium supplementation may reduce tooth loss and alveolar ridge resorption [9][10].
Although guidelines have been published, there is a lack of consensus among countries regarding non-surgical periodontal therapy. For example, articles discussing guidelines were published by Imrey et al. in 1994 [11] in the USA and by Vandenbulcke et al. in 2008 [12] in Belgium. Imrey et al. [11] indicated that the proposed guidelines were meant to provide a framework for future development (i.e., the authors proposed a method for evaluating adjunct therapies, rather than stand-alone guidelines for direct clinical application), whereas the article by Vandenbulcke et al. [12] specifically described guidelines for non-surgical periodontal therapy in Belgium. Furthermore, the American Academy of Periodontology published limited guidelines (without extensive explanation) in 2001 [13] and the American Dental Association published comprehensive guidelines (with information regarding level of certainty, adverse effects, and strength of recommendation) in 2015 [14]. A recent report by Könönen et al. [15] compared recommendations by the European Federation of Periodontology with guidelines in Nordic countries; the Nordic guidelines were generally in agreement with recommendations by the European Federation of Periodontology. Notably, the European Federation of Periodontology recommendations discussed clinical treatment and risk factor control, while the American Dental Association guidelines focused entirely on clinical treatment; there were also differences in guidance concerning sub-antimicrobial dose doxycycline and non-surgical use of lasers as adjunct therapy. Additionally, the Journal of Clinical Periodontology published a series of articles regarding clinical guidelines for the treatment of periodontal disease in 2020 [16]; some of the systematic review findings differed from the clinical practice guidelines on the same issue (e.g., concerning the use of systemic antimicrobials during periodontal therapy).
The host inflammatory response is involved in periodontal disease; therefore, adjunctive methods to mechanical non-surgical periodontal treatment are needed in some patients. In addition to vitamin D and calcium, multiple newer non-surgical modalities for periodontal disease have been investigated. These non-surgical modalities include probiotics, prebiotics/synbiotics, statins, pro-resolving mediators, omega-6 and -3 therapy, ozone therapy, and epigenetic therapy [17], as well as photodynamic therapy (specifically as an adjunct therapy) [18].
Although studies have evaluated the potential for vitamin D and calcium to prevent alveolar bone loss and improve periodontal disease, only two randomized controlled trials (RCTs) have been published to date [19][20]. Furthermore, sex differences have been reported in vitamin D and calcium status [21] and supplementation; previous studies have not addressed these differences [22][23][24]. In the literature, the effects of vitamin D and calcium supplementation in men and women have been described together without consideration of any sex differences, despite evidence that the potential to develop periodontal disease is affected by pregnancy-related hormonal changes that impact oral health [25]. Moreover, periodontal disease has been associated with preterm birth [26] and/or low birth weight [27][28], which is associated with an increase in neonatal mortality rate [29].
2. Theory Supporting Vitamin D and Calcium Supplementation as Non-Surgical Treatment for Periodontal Disease
2.1. Key Physiological Roles of Vitamin D and Calcium
Vitamin D is a key factor in the regulation of calcium–phosphate homeostasis and mineral bone metabolism [30]. This vitamin increases absorption of calcium in the intestine and decreases parathyroid hormone secretion, thereby reducing systemic bone resorption (Figure 1). Vitamin D also stimulates osteoclasts and alkaline phosphatase activity, optimizes bone remodeling, and affects bone mass by increasing bone matrix protein production [30]. Thus, vitamin D has important roles in bone metabolism. Because periodontal surgery usually involves bone regeneration [31], adequate vitamin D levels are essential.
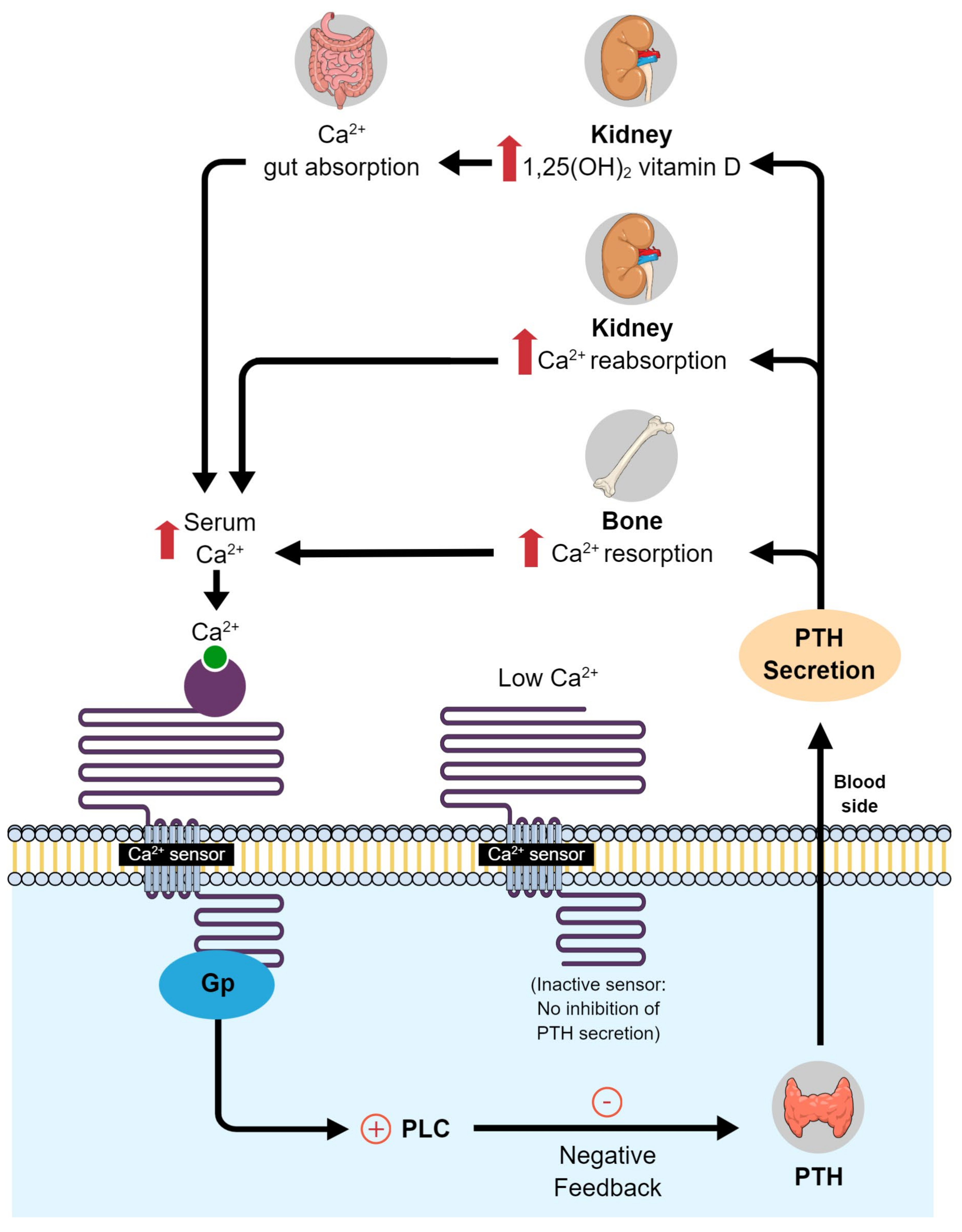
Figure 1. Calcium and vitamin D metabolism in the body. PLC, phospholipase C; PTH, parathyroid hormone.
2.2. Relationships of Serum Vitamin D and Calcium Levels with Systemic and Periodontal Inflammation
Various benefits of vitamin D and calcium supplementation have been reported [32][33][34]. For example, Meghil et al. [33] supplemented 23 patients with 25-hydroxyvitamin D (OH)D (25[OH]D), 4000 IU orally, for 12 weeks. The authors measured serum 25(OH)D levels before and after supplementation; they found a two-fold increase after supplementation, compared with baseline levels. In the same study, the authors observed decreased blood levels of inflammatory markers and salivary pro-inflammatory cytokines. Furthermore, analysis of peripheral blood mononuclear cells revealed that the levels of antimicrobial autophagy-related proteins were higher in participants who had received supplementation. However, the authors reported that supplementation did not have a significant effect on clinical parameters, presumably because the patients did not exhibit vitamin D deficiency at the onset of supplementation. Bonnet et al. [35] analyzed Canadian Health Measures Survey data for 5604 participants aged 13–79 years to evaluate the association between 25(OH)D levels and periodontal disease. Periodontal disease was evaluated by the gingival index and loss of attachment. The authors concluded that there was modest evidence to support a relationship between 25(OH)D levels and periodontal disease; specifically, patients with lower 25(OH)D levels exhibited greater loss of attachment and a more severe gingival index. In addition to its effects on bone metabolism, a study of gingivitis severity indicated that vitamin D has a dose-dependent anti-inflammatory effect [20].
2.3. Vitamin D and Calcium Supplementation in the General Population
Today, factors that complicate achieving adequate internal levels of vitamin D and calcium are the lower levels of sun exposure and calcium intake, compared with previous generations [36]. A specific threshold of serum 25(OH)D for vitamin D deficiency has not been established [37][38]; moreover, it has been difficult to establish recommendations for optimal levels [39]. The Endocrine Society Task Force on Vitamin D has defined <75 nmol/L as the threshold for vitamin D deficiency, while other groups use thresholds of <25 or <30 nmol/L because these levels are associated with increased risks of osteomalacia and rickets [39][40]. However, even for the lowest threshold of 25(OH)D (i.e., <25 nmol/L), there is consensus in both low- and high-income countries regarding the need to address vitamin D deficiency [37]. It is challenging to address this issue for a few reasons, which are as follows: environmental and individual factors affect 25(OH)D generation from sun exposure; naturally rich sources of vitamin D are scarce and may be consumed infrequently; and nutritional surveillance data indicate that vitamin D intake is generally lower than the recommended level [37].
The sources of vitamin D are sun exposure, fatty fish, and oral supplementation [41]. There is reportedly limited knowledge in the general population regarding the health benefits of vitamin D. For example, several studies revealed that participants were unsure about which foods are good sources of vitamin D [24][41][42]. Participants in another study [42] were confused by unclear messaging from health professionals regarding the risks and benefits of sun exposure. Additionally, a study of Canadian university students found that only 14% knew the correct amount of time required in the sun (10–30 min of mid-day sun several times per week) to generate vitamin D in the skin [43].
Although sun exposure is the main source of vitamin D, there are multiple barriers to sun exposure; these include climate, living in high-rise buildings, limited access to outdoor public spaces, low physical activity, and cultural customs (e.g., wearing clothing that covers most or all of the skin) [24]. Thus, there is a critical need for knowledge regarding the importance of supplementation. However, there have been mixed results regarding attitudes towards vitamin D supplementation [24][41][44][45][46]. Supplementation was considered harmful/helpful, and/or ineffective, depending on the study. Several factors are associated with the barriers to vitamin D and calcium supplementation, including the unpalatability of combined tablets [43].
3. Effects of Vitamin D and Calcium Supplementation on Periodontal Disease
The effects of vitamin D and calcium supplementation on periodontal disease have not been fully determined (Figure 2). Most published studies concerning vitamin D and periodontal disease are observational, with cross-sectional or case–control designs; thus, they have limitations compared with studies such as RCTs. Studies concerning calcium and periodontal disease are also generally observational; nearly all have had a cross-sectional design [47]. Furthermore, the required dose of vitamin D varies according to population (e.g., healthy adults vs patients with illness) and season (e.g., higher doses/greater supplementation is needed in winter). The required dose also depends on an individual’s pre-supplementation levels of 25(OH)D. Vitamin D supplementation at appropriate doses is generally considered safe, although doses above the recommended amounts can be harmful. The effects of calcium supplementation on periodontal disease may be influenced by the presence of sufficient vitamin D and protein [47].
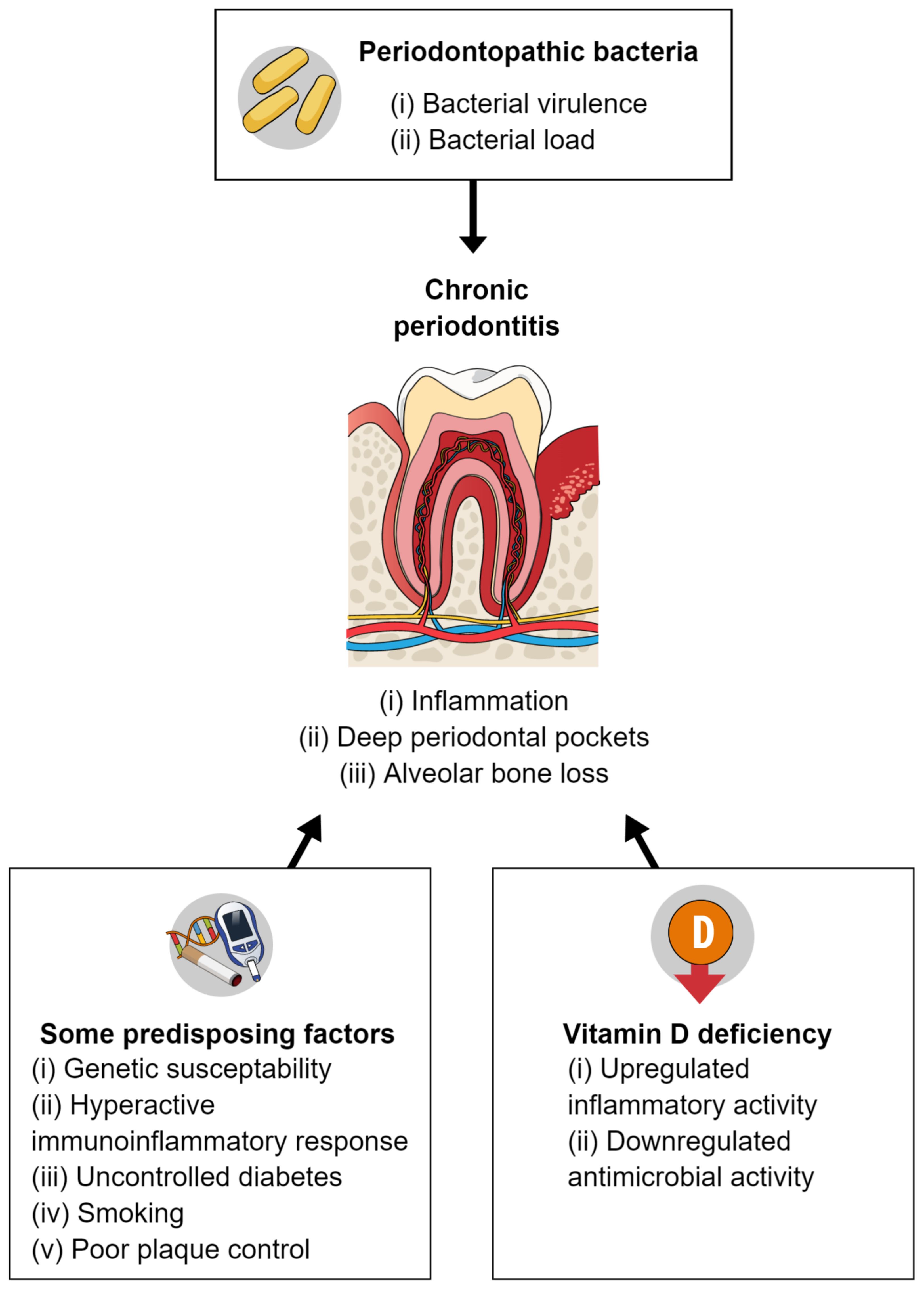
Figure 2. Pathogenesis of periodontal disease and a possible relationship with vitamin D deficiency.
Pinto et al. [48] conducted a systematic review to explore whether patients with lower vitamin D levels have an increased risk of periodontal disease; they also explored whether treatment outcomes are improved during supplementation or in patients with elevated levels of serum vitamin D. The authors identified 27 studies, including 13 cross-sectional, 6 case–control, 5 cohort, 2 RCTs, and 1 case series. Among the cross-sectional studies analyzed, 65% indicated that low vitamin D levels were significantly associated with periodontal disease. However, the findings highlighted the need for additional rigorous studies with standardized definitions for periodontal disease and internal vitamin D levels. Additionally, the findings of the only two published RCTs [19][20] to date suggest that vitamin D supplementation can aid in the prevention of tooth loss and gingival bleeding. One of the RCTs [19] evaluated vitamin D and calcium supplementation in adults aged ≥65 years over a 3-year period; 13% of the supplemented patients lost teeth, compared with 23% of the non-supplemented patients. In the other RCT, Hiremath et al. [20] reported a dose-dependent anti-inflammatory effect of vitamin D after supplementation at a dose of 2000 IU/day, 1000 IU/day, or 500 IU/day. All three groups showed significant (p < 0.0001) improvement in gingivitis based on gingival scores; improvement occurred more quickly at the higher doses. However, the extent of this improvement in gingivitis is unclear. A recent study by Gao et al. [34] evaluated attachment loss and probing depth in patients who received 2000 IU/day vs 1000 IU/day vitamin D or vs placebo. Although the authors found a statistically significant difference between vitamin D supplementation and placebo in favor of supplementation, they concluded that the magnitude of the effect was modest and had limited clinical relevance.
Van der Putten et al. [49] performed a systematic review of studies that evaluated the associations of deficiencies in vitamin B complex, vitamin C, vitamin D, calcium, and magnesium with periodontal disease in older adults. The authors found no conclusive evidence to support associations of such deficiencies with periodontal disease in older adults. Amarasena et al. [50] evaluated the progression of periodontal disease by measuring attachment loss in 266 Japanese non-institutionalized participants aged 70 years. The authors measured serum calcium, albumin, random blood sugar, immunoglobulins (IgG, IgA, and IgM), sex, smoking habits, education, gingival bleeding, and the number of teeth present at baseline. Serum calcium was the only variable significantly associated with the progression of periodontal disease. In a systematic review, Perić et al. [51] found that some data from the analyzed studies supported a “perio-protective” role for vitamin D; however, they did not evaluate calcium in that review.
References
- Helal, O.; Göstemeyer, G.; Krois, J.; Fawzy El Sayed, K.; Graetz, C.; Schwendicke, F. Predictors for tooth loss in periodontitis patients: Systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 699–712.
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490.
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44.
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 12, 745–759.
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038.
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80.
- Mougeot, F.K.; Saunders, S.E.; Brennan, M.T.; Lockhart, P.B. Associations between bacteremia from oral sources and distant-site infections: Tooth brushing versus single tooth extraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 430–435.
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125.
- Garcia, M.N.; Hildebolt, C.F.; Miley, D.D.; Dixon, D.A.; Couture, R.A.; Spearie, C.L.; Langenwalter, E.M.; Shannon, W.D.; Deych, E.; Mueller, C.; et al. One-year effects of vitamin D and calcium supplementation on chronic periodontitis. J. Periodontol. 2011, 82, 25–32.
- Assaf, M.; Aboelsaad, N. The effectiveness of vitamin D supplementation in chronic periodontitis patients: A randomized controlled clinical trial. Egypt. Dent. J. 2019, 65, 1311–1321.
- Imrey, P.B.; Chilton, N.W.; Pihlstrom, B.L.; Proskin, H.M.; Kingman, A.; Listgarten, M.A.; Zimmerman, S.O.; Ciancio, S.G.; Cohen, M.E.; D’Agostin, R.B. Proposed guidelines for American Dental Association acceptance of products for professional, non-surgical treatment of adult periodontitis. Task Force on Design and Analysis in Dental and Oral Research. J. Periodontal. Res. 1994, 29, 348–360.
- Vandenbulcke, W.; Cosyn, J.; De Bruyn, H. Guidelines for non-surgical treatment of chronic periodontitis in Belgium. Rev. Belg. Med. Dent. 2008, 63, 86–90.
- Greenwell, H.; Committee on Research, Science and Therapy; American Academy of Periodontology. Position paper: Guidelines for periodontal therapy. J. Periodontol. 2001, 72, 1624–1628.
- American Dental Association. Nonsurgical Treatment of Chronic Periodontitis Clinical Practice Guideline. 2015. Available online: https://www.ada.org/resources/research/science-and-research-institute/evidence-based-dental-research/nonsurgical-treatment-of-periodontitis-guideline (accessed on 28 February 2022).
- Könönen, E.; Klausen, B.; Verket, A.; Derk, J. Non-Surgical Periodontal Therapy: Recommendations by the European Federation of Periodontology and Guidelines in Nordic Countries. Available online: https://www.tannlegetidende.no/journal/2022/1/m-657/Recommendations_by_the_European_Federation_of_Periodontology_and_guidelines_in_Nordic_countries (accessed on 28 February 2022).
- Journal of Clinical Periodontology. Clinical Guidelines for the Treatment of Periodontitis. Available online: https://onlinelibrary.wiley.com/doi/toc/10.1111/(ISSN)1600-051x.Clinical-Guidelines-for-the-treatment-of-Periodontitis (accessed on 28 February 2022).
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilhom, R.M.; Sanz, M.; Messora, M.R. New tendencies in non-surgical periodontal therapy. Braz. Oral Res. 2021, 24, e095.
- Meqa, K.; Disha, M.; Dragidella, F.; Sllamniku-Dalipi, Z. Non-surgical periodontal treatment supplemented with photodynamic therapy. J. Int. Dent. Med. Res. 2016, 9, 139.
- Krall, E.A.; Wehler, C.; Garcia, R.I.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 15, 452–456.
- Hiremath, V.P.; Rao, C.B.; Naik, V.; Prasad, K.V. Anti-inflammatory effect of vitamin D on gingivitis: A dose-response randomised control trial. Oral Health Prev. Dent. 2013, 11, 61–69.
- Ishikawa, M.; Yokomichi, H.; Yokoyama, T. Difference and variance in nutrient intake by age for older adults living alone in Japan: Comparison of dietary reference intakes for the Japanese population. Nutrients 2021, 13, 1431.
- Dixon, D.; Hildebolt, C.F.; Miley, D.D.; Garcia, M.N.; Pilgram, T.K.; Couture, R.; Anderson Spearie, C.; Civitelli, R. Calcium and vitamin D use among adults in periodontal disease maintenance programmes. Br. Dent. J. 2009, 206, 627–631.
- Lee, S.; Teschemaker, A.R.; Daniel, M.; Maneno, M.K.; Johnson, A.A.; Wutoh, A.K.; Lee, E. Calcium and vitamin D use among older adults in U.S.: Results from national survey. J. Nutr. Health Aging 2016, 20, 300–305.
- Aljefree, N.; Lee, P.; Ahmed, F. Exploring knowledge and attitudes about vitamin D among adults in Saudi Arabia: A qualitative study. Healthcare 2017, 5, 76.
- Ortiz-Sánchez, B.J.; Legorreta-Herrera, M.; Rodriguez-Sosa, M. Influence of gestational hormones on the bacteria-induced cytokine response in periodontitis. Mediat. Inflamm. 2021, 2021, 5834608.
- Govindaraju, P.; Venugopal, S.; Shivakumar, M.A.; Sethuraman, S.; Ramaiah, S.K.; Mukundan, S. Maternal periodontal disease and preterm birth: A case-control study. J. Indian Soc. Periodontol. 2015, 19, 512–515.
- Chambrone, L.; Guglielmetti, M.R.; Pannuti, C.M.; Chambrone, L.A. Evidence grade associating periodontitis to preterm birth and/or low birth weight: I. A systematic review of prospective cohort studies. J. Clin. Periodontol. 2011, 38, 795–808.
- Teshome, A.; Yitayeh, A. Relationship between periodontal disease and preterm low birth weight: Systematic review. Pan Afr. Med. J. 2016, 24, 215.
- Katz, J.; Lee, A.C.; Kozuki, N.; Lawn, J.E.; Cousens, S.; Blencowe, H.; Ezzati, M.; Bhutta, Z.A.; Marchant, T.; Willey, B.A.; et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. Lancet 2013, 382, 417–425.
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244.
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216.
- Perayil, J.; Menon, K.S.; Kurup, S.; Thomas, A.E.; Fenol, A.; Vyloppillil, R.; Bhaskar, A.; Megha, S. Influence of vitamin D & calcium supplementation in the management of periodontitis. J. Clin. Diagn. Res. 2015, 9, ZC35–ZC38.
- Meghil, M.M.; Hutchens, L.; Raed, A.; Multani, N.A.; Rajendran, M.; Zhu, H.; Looney, S.; Elashiry, M.; Arce, R.M.; Peacock, M.E.; et al. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019, 25, 1403–1413.
- Gao, W.; Tang, H.; Wang, D.; Zhou, X.; Song, Y.; Wang, Z. Effect of short-term vitamin D supplementation after nonsurgical periodontal treatment: A randomized, double-masked, placebo-controlled clinical trial. J. Periodontal Res. 2020, 55, 354–362.
- Bonnet, C.; Rabbani, R.; Moffatt, M.E.K.; Kelekis-Cholakis, A.; Schroth, R.J. The relation between periodontal disease and vitamin D. J. Can. Dent. Assoc. 2019, 84, j4.
- Hildebolt, C.F. Effect of vitamin D and calcium on periodontitis. J. Periodontol. 2005, 76, 1576–1587.
- Cashman, K.D. Vitamin D deficiency: Defining, prevalence, causes, and strategies of addressing. Calcif. Tissue Int. 2020, 106, 14–29.
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513.
- EFSA. Panel on Dietetic Products. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547.
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415.
- Deschasaux, M.; Souberbielle, J.C.; Partula, V.; Lécuyer, L.; Gonzalez, R.; Srour, B.; Guinot, C.; Malvy, D.; Latino-Martel, P.; Druesne-Pecollo, N. What do people know and believe about vitamin D? Nutrients 2016, 8, 718.
- Kotta, S.; Gadhvi, D.; Jakeways, N.; Saeed, M.; Sohanpal, R.; Hull, S.; Famakin, O.; Martineau, A.; Griffiths, C. “Test me and treat me”—Attitudes to vitamin D deficiency and supplementation: A qualitative study. BMJ Open 2015, 5, e007401.
- Boland, S.; Irwin, J.D.; Johnson, A.M. A survey of university students’ vitamin D-related knowledge. J. Nutr. Educ. Behav. 2015, 47, 99–103.
- Kung, A.W.; Lee, K.K. Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: A population survey in Hong Kong. BMC Public Health 2006, 6, 226.
- Vu, L.H.; van der Pols, J.C.; Whiteman, D.C.; Kimlin, M.G.; Neale, R.E. Knowledge and attitudes about vitamin D and impact on sun protection practices among urban office workers in Brisbane, Australia. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1784–1789.
- Habib, S.S.; Alhalabi, H.B.; Alharbi, K.S.; Alghamdi, O.S.; Alghamdi, A.I.; Ajarem, M.A.; Alqarni, M.A. Knowledge attitude and practices of university students to Vitamin D and Vitamin D supplements during times of low sun exposure and post lockdown. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7297–7305.
- Varela-López, A.; Giampieri, F.; Bullón, P.; Battino, M.; Quiles, J.L. A systematic review on the implication of minerals in the onset, severity and treatment of periodontal disease. Molecules 2016, 21, 1183.
- Pinto, J.P.N.S.; Goergen, J.; Muniz, F.W.M.G.; Haas, A.N. Vitamin D levels and risk for periodontal disease: A systematic review. J. Periodontal Res. 2018, 53, 298–305.
- Van der Putten, G.J.; Vanobbergen, J.; De Visschere, L.; Schols, J.; de Baat, C. Association of some specific nutrient deficiencies with periodontal disease in elderly people: A systematic literature review. Nutrition 2009, 25, 717–722.
- Amarasena, N.; Yoshihara, A.; Hirotomi, T.; Takano, N.; Miyazaki, H. Association between serum calcium and periodontal disease progression in non-institutionalized elderly. Gerodontology 2008, 25, 245–250.
- Perić, M.; Cavalier, E.; Toma, S.; Lasserre, J.F. Serum vitamin D levels and chronic periodontitis in adult, Caucasian population-a systematic review. J. Periodontal Res. 2018, 53, 645–656.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
19 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




