Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ji Li | -- | 1966 | 2022-07-12 13:53:35 | | | |
| 2 | Catherine Yang | Meta information modification | 1966 | 2022-07-13 03:18:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zoungrana, L.I.; Krause-Hauch, M.; Wang, H.; Fatmi, M.K.; Bates, L.; Li, Z.; Kulkarni, P.; Ren, D.; Li, J. Nrf2 in Neurogenesis and Disease Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/25060 (accessed on 14 January 2026).
Zoungrana LI, Krause-Hauch M, Wang H, Fatmi MK, Bates L, Li Z, et al. Nrf2 in Neurogenesis and Disease Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/25060. Accessed January 14, 2026.
Zoungrana, Linda Ines, Meredith Krause-Hauch, Hao Wang, Mohammad Kasim Fatmi, Lauryn Bates, Zehui Li, Parth Kulkarni, Di Ren, Ji Li. "Nrf2 in Neurogenesis and Disease Development" Encyclopedia, https://encyclopedia.pub/entry/25060 (accessed January 14, 2026).
Zoungrana, L.I., Krause-Hauch, M., Wang, H., Fatmi, M.K., Bates, L., Li, Z., Kulkarni, P., Ren, D., & Li, J. (2022, July 12). Nrf2 in Neurogenesis and Disease Development. In Encyclopedia. https://encyclopedia.pub/entry/25060
Zoungrana, Linda Ines, et al. "Nrf2 in Neurogenesis and Disease Development." Encyclopedia. Web. 12 July, 2022.
Copy Citation
Neurogenesis occurs in the brain during embryonic development and throughout adulthood. Neurogenesis occurs in the hippocampus and under normal conditions and persists in two regions of the brain—the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles. The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) is a major regulator of metabolism, protein quality control, and antioxidative defense, and is linked to neurogenesis.
Nrf2
neurogenesis
neurodegenerative diseases
1. Nrf2 Mechanism Pathway
One of the key regulators of cellular oxidation is nuclear factor erythroid 2-related factor 2 (Nrf2) which is a key transcriptional factor that regulates different genes in cells under both normal and stress conditions [1][2]. Nrf2 maintains cellular homeostasis by activating the expression of cytoprotective, antioxidant, and anti-inflammatory genes [2]. Nrf2 is a basic leucine zipper (bZip) transcription factor from the cap ‘n’ collar (CNC) family which includes p45 NF-E2, NRF1, NRF3, CNC homolog 1 (Bach 1) and Bach 2, broad-complex, tramtrack, and bric-a-brac (BTB) [1][3]. One difference between the members of this family of transcription factors is that Nrf1 through 3 acts as a transcriptional activator, while Bach 1 and 2 serve as transcriptional repressors, and play a role in regulating Nrf2 [1]. In the nucleus, Nrf2 can heterodimerize with small MAF or JUN proteins. When Nrf2 heterodimerizes with small MAF (sMaf), it enhances nucleotide sequences in the DNA called antioxidant response elements (ARE) or electrophile response elements (EpRE) [1][2]. Brain-derived neurotrophic factors show that Nrf2 cellular function includes metabolic regulation, protein quality control, and antioxidative defense by initiating the transcription of cytoprotective genes, including anti-inflammatory interleukin (IL)-10, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and iron exporter ferroportin 1 [1][2]. The Nrf2 molecular structure consists of seven Nrf2-erythroid-derived CNC homology (ECH; Neh) domains (Neh1-7), that are critical in Nrf2 repression and activity [1]. For instance, Kelch-like ECH associated protein 1 (Keap1) is a negative regulator of Nrf2 under unstressed cellular conditions [1]. Moreover, Neh6 is important for Nrf2 degradation in stressed cells independent of Keap1 while on the other hand Neh3 interacts with other transcription factors through DNA binding and dimerization [1]. Nrf2 is regulated in both the nucleus and the cytosol [1]. Glycogen synthase kinase 3 (GSK3) mediated proteasomal degradation of Nrf2 in the nucleus while Keap1-mediated proteasomal degradation of Nrf2 occurs in the cytosol with RBX1 and Cul3 [1]. Nrf2 transcription factor is activated by Aryl hydrocarbon receptor (AhR), nuclear factor (NF)-κB (NF-κB), p53, myocyte-specific enhancer factor 2 D (MEF2D), breast cancer 1 (BRCA1), proliferator-activated receptor (PPAR)α or PPARγ, c-Jun, and c-Myc. All can activate Nrf2 transcription, while the mechanistic target of rapamycin complex 1 (mTORC1), AMP-activated protein kinase (AMPK), as well as GSK3, all play a role in Nrf2 stability and activity by directly or indirectly interacting with Nrf2 [1]. With this interconnection of Nrf2 with other genes and proteins, it is no surprise that Nrf2 might be linked to neurogenesis and the development of neurodegenerative diseases. A summary of the Nrf2 signaling pathways is presented in Figure 1. Although the NRF2 pathway plays a protective role against regulating oxygen reactive species (ROS), some studies have suggested that both the suppression of the activity of the Nrf2 transcription factor and priming are necessary for the upregulation of antioxidant molecules. However, there are contradictions among the literature regarding cell-generated memory mechanisms of activation [4][5].
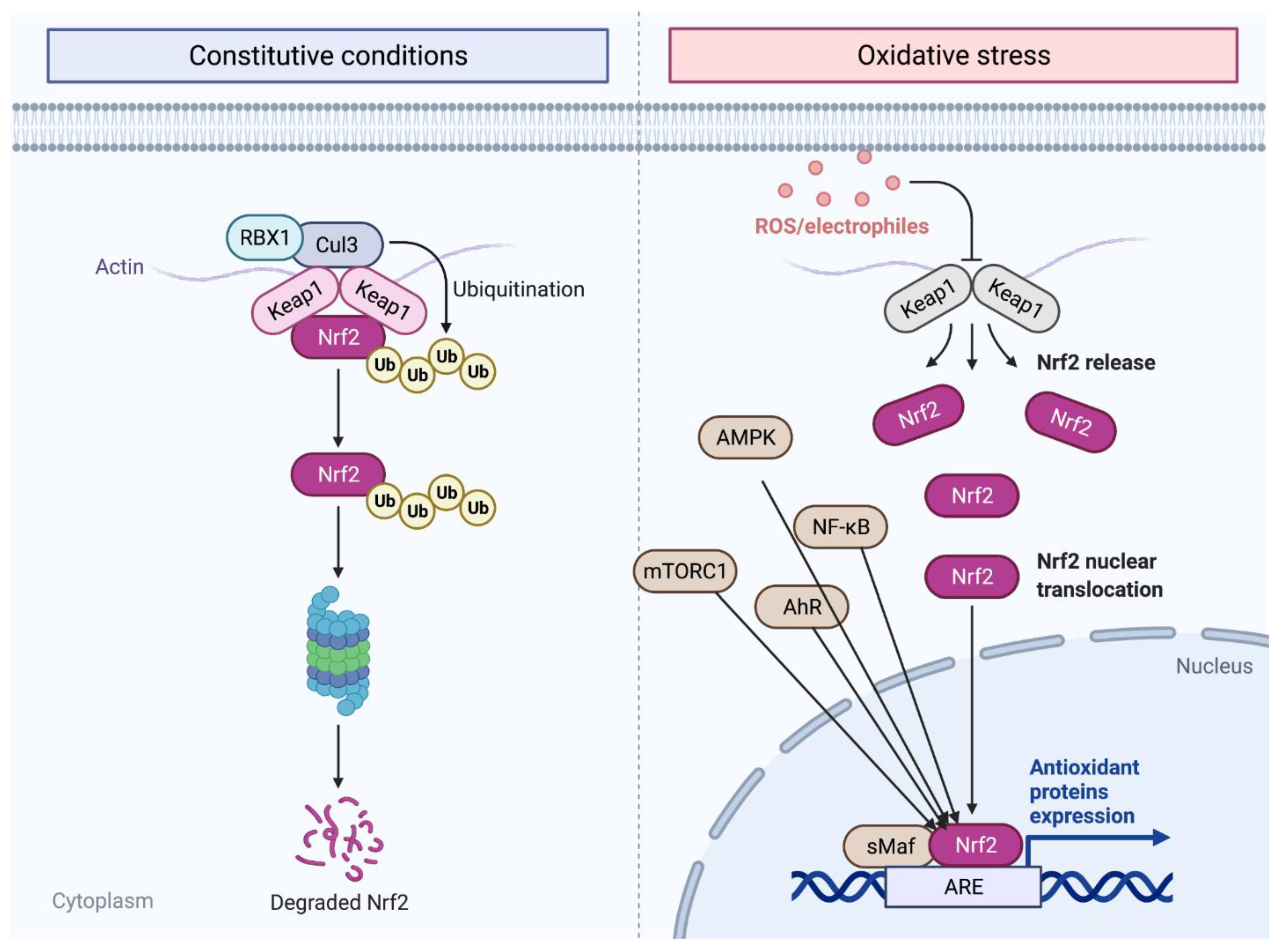
Figure 1. Nrf2 mechanism pathway. Nrf2 is released in the cytoplasm then migrates into the nucleus where it is transcripted during oxidative stress and degraded when it is no longer needed through ubiquitination (see text for more information). The figure was prepared by software provided by Biorender.com (accessed 3 March 2022).
2. Nrf2 in Neurogenesis
As previously mentioned, Nrf2 controls cellular homeostasis linked to multiple stressors by regulating other genes involved in the anti-inflammatory response, metabolic reprogramming of tumor cells, antioxidant defense, autophagy, etc. [1][2]. The expression of those genes promote self-renewal, cell survival, differentiation, cell growth, proliferation, and increased lifespan [6]; all of which are important factors to the function of stem cells [6]. Some studies have explored the Nrf2 association in neurogenesis and found that the decline in Nrf2 expression during aging was linked to the reduction of neural stem cells in the SVZ [6][7]. Other studies have looked into the role of Nrf2 in the homeostasis of the neurogenic niche in the SGZ and reported that the overexpression of Nrf2 enhances neuronal differentiation. They have also found that an upregulation of Nrf2 improves amyloid β-mediated neural stem cell death, and genetic change in Nrf2 expression can either rejuvenate or suppress the neural stem cell niche in the SGZ [2][6][8].
One of the main characteristics of Alzheimer’s disease (AD) development is the extracellular deposits of amyloid β (Aβ) and the increase in oxidative stress associated with Aβ toxicity; so understanding the mechanism of Nrf2 in this process could lead to a new therapeutic approach in AD [8][9]. Regulation of oxidative stress also has an impact on neural stem cell differentiation. Nrf2 has an impact on neural stem cell survival, differentiation, and neurogenesis by ROS [2]. ROS, which is produced in the endoplasmic reticulum (ER), membrane-bound NADPH oxidase, and cellular mitochondria, is the main oxidative stressor that is constantly produced by cells during metabolic functions [2][10]. The amounts of ROS produced has been associated with cellular survival, proliferation, and differentiation [2][10]. The overproduction of ROS in the mitochondria of neural stem cell in the presence of glucose alters endogenous antioxidant homeostasis leading to oxidative stress [2][11]. A study of neural stem cell lines (i.e., C17.2) shows that high glucose-mediated oxidative stress induces endoplasmic reticulum (ER) stress, inhibiting C17.2 cell differentiation into glia or neuron cells [2][11]. Modulating physiological ROS signaling and stimulating Nrf2-dependent developmental genes, in primarily the mitochondria as well as the ER, determines the molecular metabolism and the fate of NSC is crucial [2][12]. Studies conducted on the Nrf2 knockout rate in different ages show that Nrf2 expression regulates the glial differentiation of NSC in the dentate gyrus as well as neurogenesis-related hippocampal behaviors [2][13]. Semkova et al. presented in their study that both Nrf2 proteasomal activity and pathway response to oxidative stress was crucial to developing the nervous system, showing the importance of Nrf2 in neurogenesis [14]. However, the stress that could be encountered during neurogenesis has not been fully explored, which can present limitations on Nrf2 mechanistic pathways associated with neurogenesis. Nrf2 is an important factor in NSC proliferation, differentiation, and survival, and could provide a further understanding of the fundamental aspects of NSC biology.
3. Nrf2 in AD
AD is one of the most common neurodegenerative diseases and its medical management is still a challenge [15]. AD pathology is associated with an aggregate of Aβ plaques as well as intracellular aggregations of neurofibrillary tangles (NFTs), composed of hyperphosphorylated microtubule-associated τ and p-tau [16]. Aβ plaques develop and spread from the basal neocortex regions of the brain to the hippocampus, amygdala, and basal ganglia [16]. In patients with advanced AD, Aβ was found further in the cerebellar cortex and throughout the mesencephalon, while in the critical stage, it spread to the hippocampus and neocortex [16]. The abnormal concentration of Aβ causes a τ-tangle to form, which is mostly found in the locus coeruleus and transentorhinal and entorhinal are as of the brain [16].
In the aged population, Nrf2 expression decreases. This phenomenon is also observed in AD patients [17]. The correlation between low Nrf2 and AD can be explained due to Nrf2 playing a role in inflammation, oxidative stress, and influencing autophagy directly or indirectly [18][19][20]. Rojo et al. (2017) and Joshi et al. (2015) demonstrated a decline in Nrf2 in AD animal models and aggregate-like pathology enhancing Nrf2 correlation to AD [20][21]. Some of the Nrf2 target genes, Heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase I (NQO1), and glutamate-cysteine ligase catalytic subunit (GCLC) have been observed in AD brains [19][22]. For instance, NQO1, which protects the plasma membrane from free radicals and lipid peroxidation, is upregulated in the AD frontal cortex region [19]. Bahn et al. (2019) in their study showed that high expression of NQO1 expression in 3xTg-AD mice leads to Aβ immunoreactivity [19][23]. Furthermore, Rojo et al. (2017) observed an increase in Aβ and p-tau expression in Nrf2-deficient mice [21]. Other studies implicate the link between Nrf2 and chaperone-mediated autophagy [19]. Pajares et al. (2018) identified Nrf2 binding sequences in lysosomal-associated membrane protein 2A (LAMP2) in both mouse models and humans. Moreover, an overexpression and under expression of Nrf2 is linked to an increase or reduction in LAMP2 [19][24]. Nrf2 deletion has been associated with an increase in intracellular Aβ42 and Aβ40 [19][20]. Bahn et al.’s study of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) revealed that Nrf2 inhibits BACE1, a rate-limiting enzyme for Aβ peptides in AD model mouse, by binding to the are promoter of BACE1 [19][23]. Finally, Nrf2 affects p-tau by inducing nuclear dot protein 52 (NDP52), an autophagy-associated protein linked in p-tau degradation and binding in its are promoter [19][25]. This suggests that Nrf2 could facilitate tau clearance [19][25]. Further study of the regulatory mechanism of Nrf2 in AD could provide a better understanding of the disease development.
4. Nrf2 in PD
PD is one of the most common neurodegenerative diseases that affect individual movements. The pathogenesis of PD is characterized by the degeneration of dopamine neurons in the midbrain leading to the loss of dopamine neurotransmitters, the primary motor neurons [19][26]. This results in symptoms such as ataxia, bradykinesia, and rigidity [26]. In addition to the loss of dopamine, the presence of protein inclusions such as Lewy bodies have been observed in PD [26]. Further, the upregulation of free radical-generating enzymes is another attribute that has been observed in PD [19][27]. Guo et al. observed that Nrf2 activity was reduced in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, which enhances the PD phenotype and MPTP has been associated with iron deposit and astroglia HO-1 expression [19][28][29]. Furthermore, studies have shown that NQO1 expression in PD links Nrf2 to its neuroprotective effect [19][30]. An increase in proinflammatory cytokines released by activated microglia was observed in the cerebrospinal fluid of PD patients [19][31]. Another study by Rojo et al. has shown that Nrf2 decreases microglial activation in PD progression [32].
Moreover, studies have shown that the PI3K/AKT/GSK3β signaling axis is involved in PD neuroprotection, and a decline in AKT is associated with sporadic PD [19]. In addition, elevated expression of GSK3β expression and activity has been reported in PD, and inhibition of GSK3β is known to increase antioxidant genes as well as Nrf2 activity [19]. In summary, Nrf2 has potential neuroprotective effects in PD.
5. Nrf2 in HD
HD is a rare hereditary neuronal disease contrary to AD and PD, which are caused by mutations or protein misfolding. HD autosomal hereditary disease is caused by CAG trinucleotide repeat expansion in the huntingtin (HTT) gene leading to an expansion of polyglutamine repeats in the huntingtin protein (mtHtt) [33][34]. This mutation causes progressive degeneration of nerve cells in the brain and leads to a lack of movement coordination along with motor impairments [33]. Even though there is a lot to learn about HD, studies have shown that cellular antioxidants, as well as mitochondrial dysfunction, play a role in HD pathology connecting Nrf2 to HD [33]. Enhanced lipid peroxidation has been observed in HD mice. Impairment in this antioxidant mechanism can lead to the inhibition of Nrf2 activity by mtHtt [33]. mtHtt interacts with the CBP/p300 dimer, preventing its acetylation and function, which then blocks Nrf2 cellular localization and stability [33][35]. The mechanism by which Nrf2 affects the formation and aggregation of huntingtin protein is not fully understood. However, studies have found that Nrf2 activation in certain parts of the brain have a neuroprotective effect and extends the lifespan in HD animal models [33][36]. Furthermore, Saito et al. (2020) observed that Nrf2 induced the expression of p62 autophagy-related proteins and microtubule-associated protein 1A/1B-light chain 3 (LC3) [37]. Both aid in the rapid clearance of toxic of mtHtt aggregates by forming a shell around it [37].
The deposit of toxic mtHtt aggregate, astrocyte, and microglia activation all contribute to HD pathology and progression. This deposit triggers the release of cytokines and pro-inflammatory mediators [33]. The connection between NF-κB and the Nrf2 pathway is well documented and shows the activated Nrf2 upregulation of HO-1. This upregulation decreases the expression of pro-inflammatory cytokines and reduces inflammatory mediator levels [33][38][39]. The Nrf2/ARE pathway assists in the neuroinflammation caused by mtHtt accumulation [33][38][39]. The interaction between NF-κB and the Nrf2 pathway could be the target of new treatment therapies for HD.
References
- Zang, H.; Mathew, R.O.; Cui, T. The Dark Side of Nrf2 in the Heart. Front. Physiol. 2020, 11, 722.
- Kahroba, H.; Ramezani, B.; Maadi, H.; Sadeghi, M.R.; Jaberie, H.; Ramezani, F. The Role of Nrf2 in Neural Stem/Progenitors Cells: From Maintaining Stemness and Self-Renewal to Promoting Differentiation Capability and Facilitating Therapeutic Application in Neurodegenerative Disease. Ageing Res. Rev. 2021, 65, 101211.
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-Regulation in Brain Health and Disease: Implication of Cerebral Inflammation. Neuropharmacology 2014, 79, 298–306.
- Bischoff, L.J.M.; Kuijper, I.A.; Schimming, J.P.; Wolters, L.; Braak, B.T.; Langenberg, J.P.; Noort, D.; Beltman, J.B.; van de Water, B. A Systematic Analysis of Nrf2 Pathway Activation Dynamics during Repeated Xenobiotic Exposure. Arch. Toxicol. 2019, 93, 435–451.
- Shah, S.Z.A.; Zhao, D.; Hussain, T.; Sabir, N.; Mangi, M.H.; Yang, L. P62-Keap1-NRF2-ARE Pathway: A Contentious Player for Selective Targeting of Autophagy, Oxidative Stress and Mitochondrial Dysfunction in Prion Diseases. Front. Mol. Neurosci. 2018, 11, 310.
- Robledinos-Antón, N.; Rojo, A.I.; Ferreiro, E.; Núñez, Á.; Krause, K.-H.; Jaquet, V.; Cuadrado, A. Transcription Factor NRF2 Controls the Fate of Neural Stem Cells in the Subgranular Zone of the Hippocampus. Redox Biol. 2017, 13, 393–401.
- Corenblum, M.J.; Ray, S.; Remley, Q.W.; Long, M.; Harder, B.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. Reduced Nrf2 Expression Mediates the Decline in Neural Stem Cell Function during a Critical Middle-Age Period. Aging Cell 2016, 15, 725–736.
- Kärkkäinen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.-L.; Yamamoto, M.; et al. Nrf2 Regulates Neurogenesis and Protects Neural Progenitor Cells Against Aβ Toxicity. Stem Cells 2014, 32, 1904–1916.
- Yanker, B.A. New Clues to Alzheimer’s Disease: Unraveling the Roles of Amyloid and Tau. Nat. Med. 1996, 2, 850–852.
- Hu, Q.; Khanna, P.; Wong, B.S.E.; Heng, Z.S.L.; Subhramanyam, C.S.; Thanga, L.Z.; Tan, S.W.S.; Baeg, G.H. Oxidative Stress Promotes Exit from the Stem Cell State and Spontaneous Neuronal Differentiation. Oncotarget 2017, 9, 4223–4238.
- Chen, X.; Shen, W.-B.; Yang, P.; Dong, D.; Sun, W.; Yang, P. High Glucose Inhibits Neural Stem Cell Differentiation Through Oxidative Stress and Endoplasmic Reticulum Stress. Stem Cells Dev. 2018, 27, 745–755.
- Khacho, M.; Clark, A.; Svoboda, D.S.; Azzi, J.; MacLaurin, J.G.; Meghaizel, C.; Sesaki, H.; Lagace, D.C.; Germain, M.; Harper, M.-E.; et al. Mitochondrial Dynamics Impacts Stem Cell Identity and Fate Decisions by Regulating a Nuclear Transcriptional Program. Cell Stem Cell 2016, 19, 232–247.
- Ray, S.; Corenblum, M.J.; Anandhan, A.; Reed, A.; Ortiz, F.O.; Zhang, D.D.; Barnes, C.A.; Madhavan, L. A Role for Nrf2 Expression in Defining the Aging of Hippocampal Neural Stem Cells. Cell Transplant. 2018, 27, 589–606.
- Semkova, V.; Haupt, S.; Segschneider, M.; Bell, C.; Ingelman-Sundberg, M.; Hajo, M.; Weykopf, B.; Muthukottiappan, P.; Till, A.; Brüstle, O. Dynamics of Metabolic Pathways and Stress Response Patterns during Human Neural Stem Cell Proliferation and Differentiation. Cells 2022, 11, 1388.
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321.
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s Disease: Pathogenesis, Diagnostics, and Therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554.
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative Stress Response and Nrf2 Signaling in Aging. Free Radic. Biol. Med. 2015, 88, 314–336.
- Riley, B.E.; Kaiser, S.E.; Kopito, R.R. Autophagy Inhibition Engages Nrf2-P62 Ub-Associated Signaling. Autophagy 2011, 7, 338–340.
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2022, 15, 787258.
- Joshi, G.; Gan, K.A.; Johnson, D.A.; Johnson, J.A. Increased Alzheimer’s Disease–like Pathology in the APP/PS1ΔE9 Mouse Model Lacking Nrf2 through Modulation of Autophagy. Neurobiol. Aging 2015, 36, 664–679.
- Rojo, A.I.; PajAREs, M.; Rada, P.; Nuñez, A.; Nevado-Holgado, A.J.; Killik, R.; Van Leuven, F.; Ribe, E.; Lovestone, S.; Yamamoto, M.; et al. NRF2 Deficiency Replicates Transcriptomic Changes in Alzheimer’s Patients and Worsens APP and TAU Pathology. Redox Biol. 2017, 13, 444–451.
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Zazueta, C.; Königsberg, M. Nrf2: Molecular and Epigenetic Regulation during Aging. Ageing Res. Rev. 2018, 47, 31–40.
- Bahn, G.; Park, J.-S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE Pathway Negatively Regulates BACE1 Expression and Ameliorates Cognitive Deficits in Mouse Alzheimer’s Models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523.
- Pajares, M.; Rojo, A.I.; Arias, E.; Díaz-Carretero, A.; Cuervo, A.M.; Cuadrado, A. Transcription Factor NFE2L2/NRF2 Modulates Chaperone-Mediated Autophagy through the Regulation of LAMP2A. Autophagy 2018, 14, 1310–1322.
- Jo, C.; Gundemir, S.; Pritchard, S.; Jin, Y.N.; Rahman, I.; Johnson, G.V.W. Nrf2 Reduces Levels of Phosphorylated Tau Protein by Inducing Autophagy Adaptor Protein NDP52. Nat. Commun. 2014, 5, 3496.
- MacMahon Copas, A.N.; McComish, S.F.; Fletcher, J.M.; Caldwell, M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay Between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021, 12, 771.
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription Factor NFE2L2/NRF2 Is a Regulator of Macroautophagy Genes. Autophagy 2016, 12, 1902–1916.
- Guo, X.; Han, C.; Ma, K.; Xia, Y.; Wan, F.; Yin, S.; Kou, L.; Sun, Y.; Wu, J.; Hu, J.; et al. Hydralazine Protects Nigrostriatal Dopaminergic Neurons From MPP+ and MPTP Induced Neurotoxicity: Roles of Nrf2-ARE Signaling Pathway. Front. Neurol. 2019, 10, 271.
- Youdim, M.B.H.; Stephenson, G.; Shachar, D.B. Ironing Iron Out in Parkinson’s Disease and Other Neurodegenerative Diseases with Iron Chelators: A Lesson from 6-Hydroxydopamine and Iron Chelators, Desferal and VK-28. Ann. N. Y. Acad. Sci. 2004, 1012, 306–325.
- Lastres-Becker, I.; García-Yagüe, A.J.; Scannevin, R.H.; CasAREjos, M.J.; Kügler, S.; Rábano, A.; Cuadrado, A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 25, 61–77.
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219.
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 Regulates Microglial Dynamics and Neuroinflammation in Experimental Parkinson’s Disease. Glia 2010, 58, 588–598.
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592.
- Tabrizi, S.J.; Leavitt, B.R.; Landwehrmeyer, G.B.; Wild, E.J.; Saft, C.; Barker, R.A.; Blair, N.F.; Craufurd, D.; Priller, J.; Rickards, H.; et al. Targeting Huntingtin Expression in Patients with Huntington’s Disease. N. Engl. J. Med. 2019, 380, 2307–2316.
- Ganner, A.; Pfeiffer, Z.-C.; Wingendorf, L.; Kreis, S.; Klein, M.; Walz, G.; Neumann-Haefelin, E. The Acetyltransferase P300 Regulates NRF2 Stability and Localization. Biochem. Biophys. Res. Commun. 2020, 524, 895–902.
- Tsvetkov, A.S.; Arrasate, M.; Barmada, S.; Ando, D.M.; Sharma, P.; Shaby, B.A.; Finkbeiner, S. Proteostasis of Polyglutamine Varies among Neurons and Predicts Neurodegeneration. Nat. Chem. Biol. 2013, 9, 586–592.
- Saito, Y.; Yako, T.; Otsu, W.; Nakamura, S.; Inoue, Y.; Muramatsu, A.; Nakagami, Y.; Shimazawa, M.; Hara, H. A Triterpenoid Nrf2 Activator, RS9, Promotes LC3-Associated Phagocytosis of Photoreceptor Outer Segments in a P62-Independent Manner. Free Radic. Biol. Med. 2020, 152, 235–247.
- Dowling, R.J.O.; Topisirovic, I.; Fonseca, B.D.; Sonenberg, N. Dissecting the Role of MTOR: Lessons from MTOR Inhibitors. Biochim. Biophys. Acta BBA Proteins Proteom. 2010, 1804, 433–439.
- Luo, J.-F.; Shen, X.-Y.; Lio, C.K.; Dai, Y.; Cheng, C.-S.; Liu, J.-X.; Yao, Y.-D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911.
More
Information
Subjects:
Physiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
13 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




