Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carla Fiorentini | -- | 3665 | 2022-07-11 15:16:50 | | | |
| 2 | Jessie Wu | + 34 word(s) | 3699 | 2022-07-12 04:29:52 | | | | |
| 3 | Jessie Wu | Meta information modification | 3699 | 2022-07-12 04:30:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pizzo, F.; Maroccia, Z.; Benvenuti, A.; Ferri, I.H.; Fiorentini, C. Role of the Microbiota in Lung Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/25012 (accessed on 07 February 2026).
Pizzo F, Maroccia Z, Benvenuti A, Ferri IH, Fiorentini C. Role of the Microbiota in Lung Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/25012. Accessed February 07, 2026.
Pizzo, Federica, Zaira Maroccia, Alessandro Benvenuti, Ivano Hammarberg Ferri, Carla Fiorentini. "Role of the Microbiota in Lung Cancer" Encyclopedia, https://encyclopedia.pub/entry/25012 (accessed February 07, 2026).
Pizzo, F., Maroccia, Z., Benvenuti, A., Ferri, I.H., & Fiorentini, C. (2022, July 11). Role of the Microbiota in Lung Cancer. In Encyclopedia. https://encyclopedia.pub/entry/25012
Pizzo, Federica, et al. "Role of the Microbiota in Lung Cancer." Encyclopedia. Web. 11 July, 2022.
Copy Citation
The microbiota is increasingly recognized as a critical player in cancer onset, progression, and response to chemotherapy treatment. In recent years, several preclinical and clinical studies have evidenced the involvement of microbiota in lung cancer, one of the world’s deadliest cancers.
lung
carcinoma
immune system
microbiota
1. Microbiota in Lung Cancer
Accordingly to recent estimates, lung cancer is the principal cause of mortality due to cancer and the second most common cancer type [1][2]. It is evaluated that 90% of all lung cancer cases can be attributed to smoking, with tobacco smoke, air pollution, and other carcinogens established risk factors. However, the exact mechanisms are not well understood. As the mucosa site with the largest surface area in the body and an essential interface with the external environment, the lung represents a unique opportunity for exposure to microbes and environmental challenges. Although traditionally thought to be sterile, the lung is home to a wide range of microbes. The prevalence of microbes is dictated by the immigration of new bacteria, mechanical and immune elimination, and replicative success in local environmental conditions. The lung microbiota appears to be dysregulated in lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis [3][4][5][6], and cancer (Figure 1).
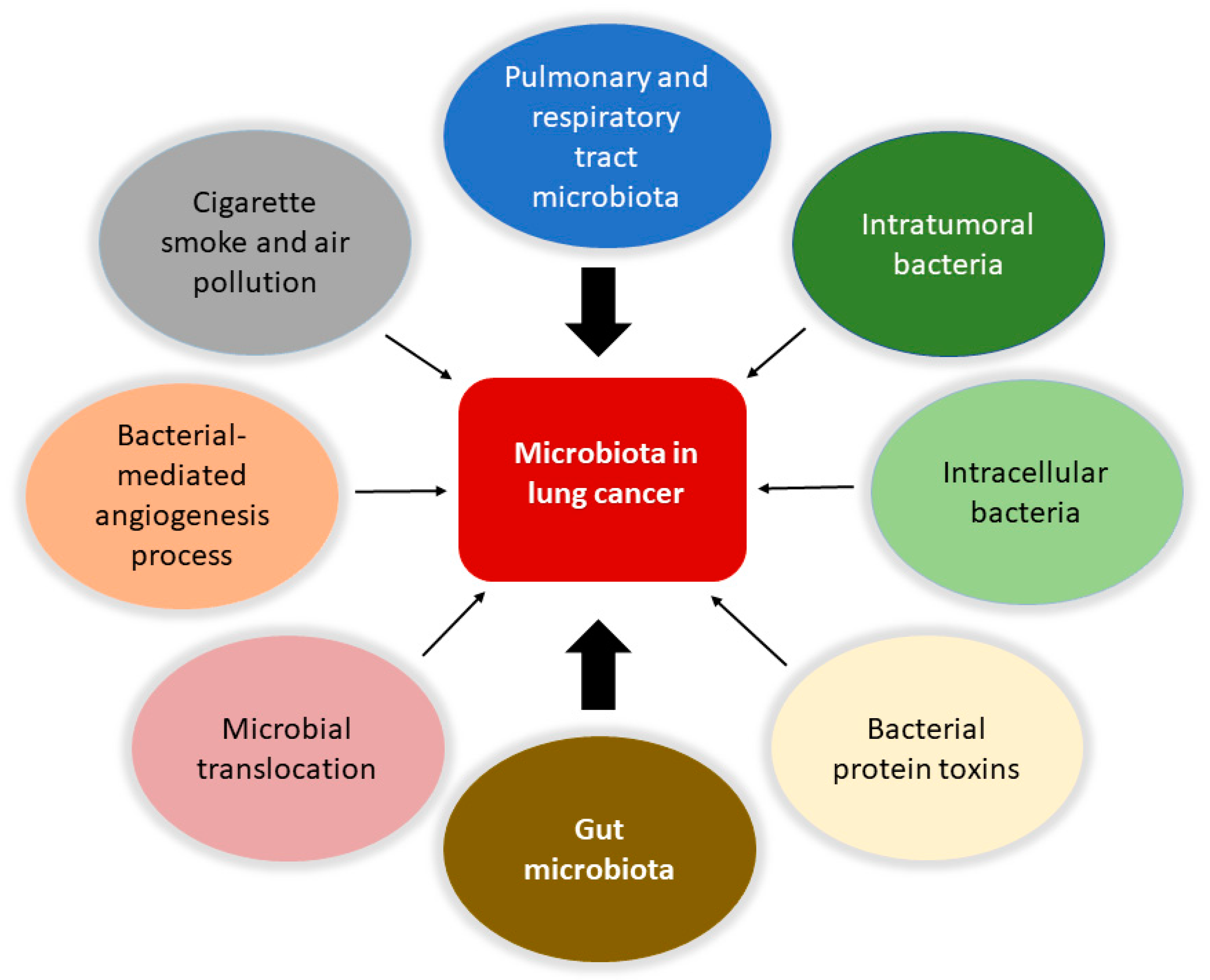
Figure 1. Involvement of the microbiota in lung cancer. The microbiota is involved in the biology of lung cancer at different levels and through various mechanisms. Protagonists are the bacteria in the upper respiratory and pulmonary tract and those that constitute the tumor microenvironment with intratumoral and intracellular localization. The intestinal microbiota plays a central role in modulating the responses of the immune system and the inflammatory state of the body, together with the bacteria involved in translocation phenomena in the bloodstream. It is also possible that certain bacterial toxins that can activate oncogenic pathways may lead to transformation in the lung. Furthermore, pollution and cigarette smoke are directly responsible for dysbiotic changes in the lung. Finally, the microbiota can affect metastasis processes by increasing the expression of vascular endothelial growth factors and promoting inflammation.
Compared to the gastrointestinal tract, the lung microbiota is poorly understood. A study investigating female, never-smoker, lung cancer patients, demonstrated a correlation between gut microbiota and TNM stage and primary tumor size [7]. In detail, there is a significant positive correlation between the relative abundance of Faecalibacterium and T category (TNM) and primary tumor size, and a negative correlation between the relative abundances of Fusicatenibacter saccharivorans and Bacteroides and T category and primary tumor size. EGFR WT (wild-type) seems to be correlated to a higher relative abundance of Bifidobacterium and Faecalibacterium and a lower relative abundance of Blautia, compared to EGFR mutated patients. It is currently impossible to establish whether these gut microbiota changes are prior to cancer development or follow the disease. It is worth remembering that the effect of these changes is potentially due to the influence on the immune system by bacterial biochemical metabolites or molecules, as is the case of Bacteroides that can upregulate T cells in the tumor microenvironment, suppressing tumor proliferation, or the case of Faecalibacterium, that can activate T regulatory cells playing a role in cancer progression.
It has also been reported that Gram-negative bacteria, such as Haemophilus influenza, Enterobacter spp., and Escherichia coli, tend to colonize lung cancer. Regarding the gut microbiota of lung cancer patients, when compared to healthy individuals, it displays a lower concentration of Firmicutes and Proteobacteria, combined with relatively higher levels of Bacteroidetes and Fusobacteria [8]. These phyla were found constantly, regardless of microbial changes in cancer.
Furthermore, when the intestinal microbiota and its products are translocated through the epithelial barrier and then into the blood flow, they stimulate a toll-like receptor (TLR) response and the subsequent expansion of T lymphocytes into distant tissues. The translocation of bacteria from the gastrointestinal tract can enhance tumor-specific responses through TLRs or the induction of memory responses, as observed for the relationships between Enterococcus hirae and small-cell lung cancer [9][10].
Increasing evidence indicates that the conversion of the gut microbiota from a mutualistic configuration into a pro-carcinogenic configuration may be favored by triggering factors that comprise inflammation and bacterial infections. Several bacterial pathogens can produce enzymatically active protein toxins that can directly attack and damage DNA or become involved in essential host cell signaling pathways that direct cell proliferation, apoptosis, and inflammation [11]. The E. coli colibactin and cytolethal distending toxin (CDT) are significant examples of bacterial toxins able to induce mutations and genome instability, whereas the Bacteroides fragilis toxin, the E. coli Cif and cytotoxic necrotizing factor 1 (CNF1), the Fusobacterium nucleatum FadA, and the Salmonella AvrA are prototypes of toxins that engage signaling pathways, ultimately leading to transformation [11][12]. Chronic inflammation can contribute to colorectal cancer via several mechanisms, including the induction of the epithelial–mesenchymal transition (EMT), a process involved in metastasis, invasion, and cancer progression. CNF1 and FadA have been reported to trigger the EMT, and also the protein toxins CagA and CagE, from the pro-carcinogenic bacteria Helicobacter pylori, which raises the patients’ risk for gastric cancer [13]. Interestingly, H. pylori infection has also been associated with lung cancer since its inhalation may lead to lung tissue colonization that can cause direct damage and chronic inflammation. One of the H. pylori protein toxins, VacA, exerts a cytotoxic effect in airway epithelial cells and triggers the production and secretion of the pro-inflammatory cytokines Il-6 and IL-8 [14]. Hence, in concomitance with environmental risk factors and genetic predispositions of the host, H. pylori and its toxins could be involved in lung cancer onset [15].
Knowing the lung microbiota composition could represent a valuable tool for prognostic investigation. In fact, in early-stage lung cancer patients, the gut microbiota undergoes characteristic changes that permit the identification of these possible bacterial contributors to lung cancer development [16]. In particular, controls show a higher abundance of the genus Bifidobacterium and Faecalibacterium, whereas Bacillus spp. appears more elevated in lung cancer patients. Bacillus spp. detected in lung sputum have been connected to an increased lung cancer risk [17]. The dysbiosis of gut and sputum microbiota is associated with disease progression and distant metastasis (DM), but the sputum microbiota is best for discriminating stage I to III patients from DM patients [18]. Lu and coworkers observed a progressive worsening in the alpha diversity of sputum microbiota when comparing stage I to stage III patients and to DM patients. The Coriobacteriaceae family and the genera Actinomyces, Streptococcus, and Pseudomonas are significantly increased while Capnocytophaga was decreased in the sputum and the gut microbiota of DM patients. A correlation was found between brain metastasis (BM) and Pseudomonas while trying to identify potential microbial biomarkers. There was no detectable pseudomonas in the other stages of lung cancer or healthy controls, while it was present in the fecal microbiota of BM patients and highly abundant in the sputum; thus, there is a significant association between pseudomonas and BM in non-small-cell lung cancer (NSCLC).
Distant modulation by the gut microbiota of the immune system, cancer progression, and metastasis growth are well explained in a mouse model of lung metastatic melanoma [19]. The supplementation of specific strains of Lactobacillus and Bifidobacterium, increases the gut microbiota relative abundances of Lachnospiraceae, Streptococcus, and Lachnoclostridium, which are all involved in SCFAs production. In the presence of a sufficient intake of fiber, the concentration of SCFAs in the gut increases and thus in the blood, as well as in the sputum, indicating that they reach the lungs and the airways. The distant effect of SCFAs promotes the expression of CCL20 (also known as LARC or MIP3A) in lung endothelial cells, which in turn recruits Th17 cells that attenuate melanoma cell metastasis in the lung. Moreover, it has been observed that microorganisms are closely related to tumor angiogenesis and metastatic processes by regulating the expression of the vascular endothelial growth factor (VEGF) and inflammation. Wang and collaborators highlighted the ability of H. influenzae, Human papillomavirus, and H. pylori to increase the expression of angiogenic mediators, chemokines, and cytokines that promote angiogenesis and inflammation in lung cancer [20]. In contrast, the fungi zj-14, zj-17, and zj-36, Akkermansia muciniphila, and E. hirae, have been reported to suppress lung cancer angiogenesis. Further studies are needed for a thorough investigation into the potential role of bacteria influencing the metastatic process in lung cancer.
Collectively, these findings indicate that the microbiota plays a crucial role in the development of lung cancer. Modulating the immune response and treating the local and distal microbiota represents a potential new avenue for lung cancer prevention and treatment.
2. Local Microbiota Role in Lung Cancer
The lung is a critical site of immune–microbiota interaction, and homeostasis is maintained by the immune cells resident in the lung. Growing evidence from human and mouse studies has linked bacterial dysbiosis to lung cancer [21]. Many studies have observed reduced alpha diversity, enrichment in specific bacterial taxa, and higher bacterial density associated with lung cancer [22]. Chronic infection of the lungs can be the initial cause of cancer when microbiota dysbiosis results in a more hypoxic, tumor-promoting environment. In addition, anaerobic respiration is observed to increase in lung cancer due to the elective anaerobic qualities of the bacteria that preferentially colonize tumors. They grow in number along with cancer progression, further promoting and stabilizing the hypoxic and proinflammatory tumor environment [9]. Jin and collaborators showed that lung tumor burden was highly correlated with local bacterial abundance in the airways, but not in the intestinal tract, and deduced a relatively more significant contribution of the local microbiota in lung cancer development. Furthermore, they showed that intratracheal inoculation of a pool of bacteria isolated from advanced lung tumors significantly accelerated tumor growth [23] (Figure 2).
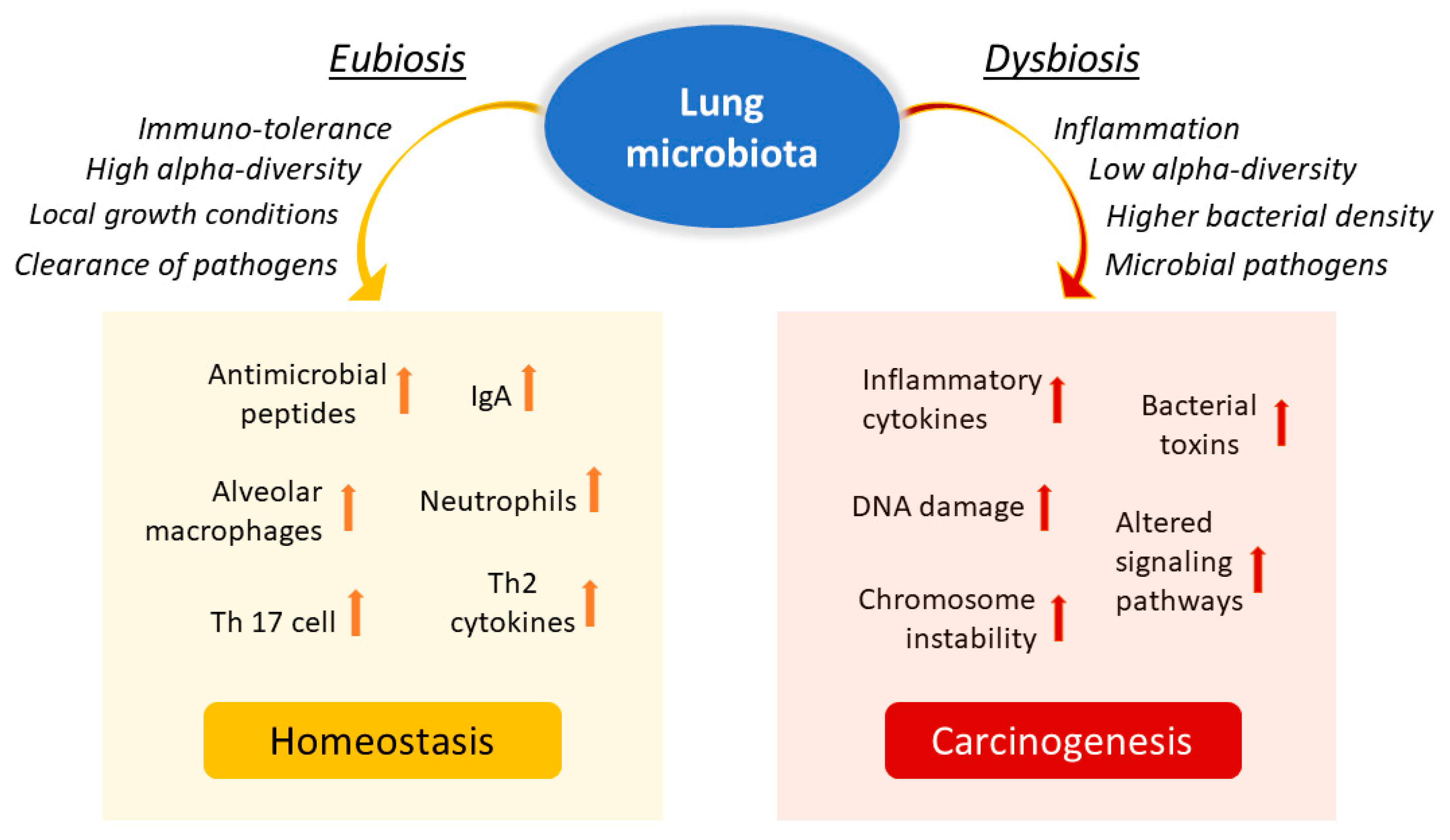
Figure 2. Influence of lung microbiota on homeostasis maintenance or carcinogenesis induction. The lung microbiota is crucial in driving local inflammation and tumor promotion. While the condition of eubiosis promotes immune tolerance and the formation of a homeostatic environment, dysbiosis and chronic infection of the lungs can cause alterations in the inflammatory response and result in a more hypoxic, tumor-promoting environment. Reduced alpha diversity and increased bacterial density are associated with lung cancer by stimulating the production of specific cytokines that promote inflammation (e.g., IL-17, IL-22) and the infiltration of neutrophils and other effector molecules that enhance the proliferation of cancer cells. Furthermore, enrichment of potential pathogenic bacterial taxa may facilitate changes in oncogenic pathways, possibly through some microbial toxins.
While recent literature has focused primarily on the gut microbiota, it is unclear how the distal gut microbiota and the local microbiota work together to regulate the balance between tumor-promoting inflammation and antitumor immunity.
A decrease in the alpha diversity of the bacterial community in tumor tissues compared to non-malignant lung tissues has been reported in patients with lung cancer, while the beta diversity does not vary between healthy and malignant lung tissue [20]. Although there are no shared definitions for healthy or harmful lung microbiota, interesting correlations between specific taxa or genera and lung cancer have been identified by several authors. Jin and coworkers demonstrated the importance of microbiota-immunity cross-talk in promoting inflammation and lung cancer development in an indigenous mouse model [23]. Specifically, they found that some bacterial families, such as Herbaspirillum and Sphingomonadaceae were enriched in tumor-bearing lung tissues compared to healthy lungs. In contrast, other taxa, including Aggregatibacter and Lactobacillus, were enriched in healthy lungs. The increase in local bacterial load and the altered composition of the lung microbiota stimulated the production of IL-17, promoting inflammation and infiltration of neutrophils and IL-22 and other effector molecules that promote the proliferation of cancer cells. Germ-free mice or antibiotic-treated mice had significantly reduced lung tumor growth, which agrees with pulmonary physiology, providing an objective low microbial load. Yu and coworkers [24] observed an increase in the genera Thermus and Legionella pneumophila in patients with advanced lung cancer (IIIB, IV) and with metastases, respectively.
Using diagnostic bronchoscopy samples, Tsay and coworkers [25] found that lung cancer patients had increased oral taxa, particularly Streptococcus and Veillonella, compared to controls. The increased prevalence of oral taxa has been associated with PI3K and ERK (MAPkinase pathway) upregulation. In vitro experiments that exposed airway epithelial cells to Veillonella, Prevotella, and Streptococcus also led to the upregulation of the ERK and PI3K pathway, this last being implicated as an early event in lung carcinogenesis [26], and thus upregulation of this pathway by commensal microbiota dysbiosis facilitates carcinogenesis. Still, the results of Greathouse and coworkers [22] have suggested an association between TP53 and lung microbiota dysbiosis. The genus Acidovorax was enriched in squamous cell carcinoma lung biopsy specimens, and it was found that this same taxon is further enriched in lung biopsies of patients with TP53-mutated squamous cell carcinoma. In the lung cancer-associated microbiota, a significant enrichment of Firmicutes Granulicatella, Abiotrophia, and Streptococcus and a decrease in bacterial community diversity have been evidenced [27]. Moreover, the genus Thermus is more abundant in the tissues of advanced-stage cancer patients, while the presence of Legionella is more consistent in people who develop metastases [21]. Liu and coworkers provided evidence that the lung cancer-associated microbiota is enriched in Streptococcus while it is deficient in Staphylococcus [28], suggesting a deleterious role for the former and a protective role for the latter in lung cancer development [1]. In contrast, other researchers showed that Staphylococcus might induce DNA damage while Streptococcus may play a role in cancer prevention [29]. Such contradictory results, however, could be explained by the difficulties in identifying actual species or strains involved in carcinogenesis, or by the fact that different taxa could play distinct roles in different body niches or that even the same taxa may have protective or harmful functions in the same place, depending on the presence of other stimuli. It should be considered that the composition of the lung microbiota is associated with lifestyle, pollution, and tobacco smoke. Differences are also observable between patients with chronic bronchitis or tumors. It is worth noting that smokers’ intratumoral and intracellular bacterial taxa show enrichment in the chemical breakdown pathways of cigarette smoke, indicating an association between intratumoral microbiota and cancer etiology [30]. Hence, it appears crucial to consider the microbiota within the microenvironment in which it develops to favor its balance.
Also, dysregulation of lung microbial communities probably facilitates changes in oncogenic pathways, potentially through specific microbial components such as toxins [21][31]. For instance, Apopa and collaborators [32] found an abundance of Cyanobacteria in NSCLC biopsies and related the microcystin toxin produced by the Cyanobacteria themselves with the local inflammatory state and the onset of lung cancer. Conversely, Yaghoobi and collaborators [33] have shown the anticancer properties of the CDT secreted by some pathogenic gram-negative bacteria. CDT induced apoptosis in the A549 human lung adenocarcinoma cell line, and potential antitumor use as a drug against lung cancer was speculated for the toxin. Intriguingly, CDT has also been indicated, among others, as a pro-carcinogenic toxin in the colon [11]. This apparent discrepancy, with the same microbial factor playing opposite roles in different environments, is not a new finding and certainly deserves a deeper investigation.
These findings strongly suggest the importance of the local microbiota in driving local inflammation and tumor promotion.
3. Probiotic Therapies and Next-Generation Probiotics (NGPs)
A probiotic is currently indicated as a specific bacterial strain that can effectively promote human health and that should not carry or transfer antibiotic resistance to other strains in the microbiota. Although probiotics do not need to colonize the target organ, such as the intestine, at least a certain number of bacteria must reach the colon, where they can experience the local intestinal ecology, physiology, and metabolism [34]. By definition, probiotics should be safe in animals, resistant to acidity and bile acids, and able to adhere and colonize in the intestine.
Available data indicate that probiotic bacteria can modulate the immune system by promoting the host’s endogenous defense systems and can modify various immune parameters, including humoral, cellular, and non-specific immunity. Emerging data also indicate that probiotics potentiate NK cell activity in the elderly and modulate non-specific host defenses. A reversal of the age-related decrease in cytokine production was demonstrated in elderly mice fed probiotic supplements. Immunomodulatory mechanisms that have been demonstrated with probiotics include induction of mucus production, activation of macrophages by Lactobacilli signaling, stimulation of secretory IgA and neutrophils, inhibition of the release of inflammatory cytokines, and stimulation of elevated peripheral immunoglobulins. In addition, probiotics can modulate DC surface phenotype and cytokine release. Other bacteria seem to be involved in the control of inflammation, side effects and chemotherapy-related toxicity in cancer patients. A combination of selected strains of Lactobacilli and Bifidobacteria has proven significant efficacy in hematological toxicity, affecting red blood cell and platelet count, and an improvement in the control of AST, GGT, urea, and creatinine levels in mice affected by pancreas cancer treated with gemcitabine plus probiotics compared to mice receiving chemotherapy alone [35]. Clearly, further investigation is needed to characterize candidate probiotic bacteria immunomodulatory properties and tailor their application to specific target populations.
In general, widely used traditional probiotics, such as Bifidobacterium spp., Lactobacillus spp., and many others, have been selected by chance or through the collection of life experiences. Although most of them show biological safety and some may show ameliorative efficacy, the overall effects and functions on disease improvement are statistically marginal. On the other hand, the administration of traditional probiotics does not target specific diseases.
Through analyses using the latest generation sequencing and bioinformatics platforms, many potential NGPs are currently under intensive development. Emerging NGPs include a class of organisms developed exclusively for pharmaceutical applications and as novel preventive tools. These comprise, for example, Prevotella copri and Christensenella minuta, which control insulin resistance, Parabacteroides goldsteinii, A. muciniphila, and Bacteroides thetaiotaomicron, which reverse obesity and insulin resistance, Faecalibacterium prausnitzii, which protects mice from intestinal disease, and B. fragilis, which reduces inflammation and antitumor effect. Some other bacterial species have also shown a promising efficacy in promoting anticancer immunotherapies. These bacteria include Eubacterium limosum, E. hirae, Enterococcus faecium, Collinsella aerofaciens, and Burkholderia cepacia.
Many of the aforementioned bacteria (A. muciniphila, F. prausnitzii, Bifidobacterium spp., and B. fragilis) are associated with good clinical responses to immunotherapy. In contrast, they have also been found in various disease groups and associated with pathology. For example, according to some studies, A. muciniphila is positively correlated with Parkinson’s disease and constipation-predominant irritable bowel syndrome [36][37]. Other authors evaluate the pathogenic potential of A. muciniphila for its ability to adhere to and degrade the intestinal mucus layer as a starter of early pathogenic behaviors. Unlike pathogens, however, A. muciniphila as a mucin-degrading agent remains mainly in the outer mucous layer and does not reach the inner mucous layer, and it has been shown that bacteria reaching the inner layer are necessary for pathogenicity. Although mucin degradation itself is pathogen-like behavior, it is considered a normal process in the intestinal self-renewal balance. Furthermore, it has been reported that A. muciniphila can maintain host–gut microbial balance by converting mucin into beneficial products [38]. Up to now, A. muciniphila alone has not been proved to cause pathogenicity, although it is not known whether it can cause diseases in synergy with other bacteria.
The underlying reason for the ambivalent correlations of such microorganisms may be that the abundance of these health-associated bacteria may reflect the presence of a well-balanced intestinal microflora, leading to a homeostatic host–microbiota ecosystem associated with good health. The other possibility is that a small number of these NGP candidates may be directly responsible for the aberrant “immune set point”. However, it still remains unclear whether a single bacterial strain is sufficient to obtain such ameliorative effects or whether cocktails are required to obtain the effects of live bacterial biotherapy [39].
4. Treatment of the Gut Microbiota in Lung Cancer
The gut microbiota characteristics in lung cancer patients vary widely compared to healthy subjects, suggesting its possible involvement in lung cancer prognosis and therapy. The gut microbiota may significantly influence immunotherapy by altering the differentiation of regulatory T cells, thus resulting in changes in immunomodulation mechanisms [40]. The same authors found that supplementation with A. muciniphila increases response to immunotherapy, while an abnormal gut microbiota composition is associated with resistance to the treatment [40]. The gut microbiota in lung cancer patients who respond to immunotherapy differs significantly from that of unresponsive patients. Furthermore, a significantly higher response to anti-PD-1 therapy in lung cancer patients was found to be positively correlated with A. muciniphila species abundance [1].
Recent work has shown that gut microbiota diversity in fecal bacteria (Proteobacteria, Firmicutes, Bacteroidetes, and Actinobacteria) increases the response to anti-PD-1 immunotherapy [41]. Additionally, a previous study stated that in patients with NSCLCs that responded to nivolumab, the gut microbiota composition was relatively stable, and greater diversity was noted. Extended progression-free survival has also been demonstrated in patients with high microbiome diversity versus those with low diversity. Analysis of systemic immune responses by multicolor flow cytometry revealed that patients with high gut microbiome diversity have a higher frequency of CD8 + T cells with unique memory and subsets of NK cells in the periphery in response to anti-PD-1 [23]. A retrospective evaluation study of 118 patients with advanced NSCLC and treated with immunotherapy showed that the addition of supplemental therapy with Clostridium butyricum before and/or after immunotherapy resulted in significantly prolonged progression-free survival and overall survival of patients [42].
In addition to the observed improvements in response to immunotherapy, the gut microbiota also affects the efficacy of chemotherapy treatment in lung cancer. For example, the oral administration of Lactobacillus acidophilus during cisplatin treatment in mouse models of lung cancer has improved the anticancer efficacy of cisplatin, reduced tumor size, and improved survival rate. These results suggest that co-administration of probiotics enhances the antigrowth and pro-apoptotic effects of cisplatin [43]. In addition, patients with end-stage lung cancer and undergoing chemo-immunotherapy, who additionally received E. hirae and Barnesiella intestinihominis, had longer progression-free survival [44]. Therefore, the increased survival of these patients can be attributed to the improvement of the immunomodulatory effect.
A study by Liu et al. [45], in which the gut microbiota of 30 lung cancer patients and 16 healthy subjects was analyzed, found that each lung cancer group had a loss of bacterial diversity and a shortage of the probiotic genera Blautia, Coprococcus, Bifidobacterium, and Lachnospiraceae, compared to healthy controls. The genus Blautia, belonging to the Firmicutes phylum, has the role of helping to digest complex carbohydrates. Decreases in the Blautia genus have also been observed in irritable bowel syndrome, non-alcoholic fatty liver disease, Crohn’s disease, and diabetes. Coprococcus, observed only in the control group, is a beneficial butyrate-producing genus. Other studies have shown that high consumption of yogurt is beneficial as it has been shown to cause a significant reduction in lung cancer risk by 30%, implying that prebiotics and probiotics may have a protective effect on lung carcinogenesis [46]. This evidence indicates that the composition and development of bacterial communities vary in lung cancer with different biomarkers. Therefore, it is possible that some special microbiomes may serve for diagnosis, prognosis, therapeutic target or for fecal microbiota transplantation in lung cancer therapy.
However, the role of the gut microbiota in the development and progression of lung cancer needs further investigation, and the potential actions of the microbiome in the effective modulation of anticancer treatment should be further explored and evaluated.
References
- Zhao, Y.; Liu, Y.; Li, S.; Peng, Z.; Liu, X.; Chen, J.; Zheng, X. Role of lung and gut microbiota on lung cancer pathogenesis. J. Cancer Res. Clin. Oncol. 2021, 147, 2177–2186.
- GLOBOCAN Lung 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf (accessed on 5 May 2022).
- Dickson, R.P.; Huffnagle, G.B. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLOS Pathog. 2015, 11, e1004923.
- Dickson, R.P.; Martinez, F.J.; Huffnagle, G.B. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014, 384, 691–702.
- Dickson, R.P.; Erb-Downward, J.R.; Huffnagle, G.B. The role of the bacterial microbiome in lung disease. Expert Rev. Respir. Med. 2013, 7, 245–257.
- Dickson, R.P.; Huang, Y.J.; Martinez, F.J.; Huffnagle, G.B. The lung microbiome and viral-induced exacerbations of chronic obstructive pulmonary disease: New observations, novel approaches. Am. J. Respir. Crit. Care Med. 2013, 188, 1185–1186.
- Otoshi, T.; Nagano, T.; Park, J.; Hosomi, K.; Yamashita, T.; Tachihara, M.; Tabata, T.; Sekiya, R.; Tanaka, Y.; Kobayashi, K.; et al. The Gut Microbiome as a Biomarker of Cancer Progression among Female Never-smokers with Lung Adenocarcinoma. Anticancer Res. 2022, 42, 1589–1598.
- Zhang, W.Q.; Zhao, S.K.; Luo, J.W.; Dong, X.P.; Hao, Y.T.; Li, H.; Shan, L.; Zhou, Y.; Shi, H.B.; Zhang, Z.Y.; et al. Alterations of fecal bacterial communities in patients with lung cancer. Am. J. Transl. Res. 2018, 10, 3171–3185.
- Georgiou, K.; Marinov, B.; Farooqi, A.A.; Gazouli, M. Gut Microbiota in Lung Cancer: Where Do We Stand? Int. J. Mol. Sci. 2021, 22, 10429.
- Goubet, A.G.; Daillère, R.; Routy, B.; Derosa, L.; Roberti, P.M.; Zitvogel, L. The impact of the intestinal microbiota in therapeutic responses against cancer. Comptes Rendus Biol. 2018, 341, 284–289.
- Fiorentini, C.; Carlini, F.; Germinario, E.A.P.; Maroccia, Z.; Travaglione, S.; Fabbri, A. Gut microbiota and colon cancer: A role for bacterial protein toxins? Int. J. Mol. Sci. 2020, 21, 6201.
- Piciocchi, A.; Germinario, E.A.P.; Garcia Etxebarria, K.; Rossi, S.; Sanchez-Mete, L.; Porowska, B.; Stigliano, V.; Trentino, P.; Oddi, A.; Accarpio, F.; et al. Association of polygenic risk score and bacterial toxins at screening colonoscopy with colorectal cancer progression: A multicenter case-control study. Toxins 2021, 13, 569.
- Pappas-Gogos, G.; Tepelenis, K.; Fousekis, F.; Katsanos, K.; Pitiakoudis, M.; Vlachos, K. The Implication of Gastric Microbiome in the Treatment of Gastric Cancer. Cancers 2022, 14, 2039.
- Nakashima, S.; Kakugawa, T.; Yura, H.; Tomonaga, M.; Harada, T.; Hara, A.; Hara, S.; Nakano, M.; Yamasaki, E.; Sakamoto, N.; et al. Identification of Helicobacter pylori VacA in human lung and its effects on lung cells. Biochem. Biophys. Res. Commun. 2015, 460, 721–726.
- Samareh-Fekri, M.; Hashemi Bajgani, S.M.; Shafahi, A.; Asadi-Zarandi, M.; Mollaie, H.; Paghalhe, A.J. Detection of Helicobacter pylori in the Bronchoalveolar Lavage of Patients with Lung Cancer Using Real-Time PCR. Jundishapur J. Microbiol. 2016, 9, e32144.
- Zheng, Y.; Fang, Z.; Xue, Y.; Zhang, J.; Zhu, J.; Gao, R.; Yao, S.; Ye, Y.; Wang, S.; Lin, C.; et al. Specific gut microbiome signature predicts the early-stage lung cancer. Gut Microbes 2020, 11, 1030.
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64.
- Lu, H.; Gao, N.L.; Tong, F.; Wang, J.; Li, H.; Zhang, R.; Ma, H.; Yang, N.; Zhang, Y.; Wang, Y.; et al. Alterations of the Human Lung and Gut Microbiomes in Non-Small Cell Lung Carcinomas and Distant Metastasis. Microbiol. Spectr. 2021, 9, e00802-21.
- Chen, L.; Zhou, X.; Wang, Y.; Wang, D.; Ke, Y.; Zeng, X. Propionate and Butyrate Produced by Gut Microbiota after Probiotic Supplementation Attenuate Lung Metastasis of Melanoma Cells in Mice. Mol. Nutr. Food Res. 2021, 65, 2100096.
- Wang, D.; Cheng, J.; Zhang, J.; Zhou, F.; He, X.; Shi, Y.; Tao, Y. The role of respiratory microbiota in lung cancer. Int. J. Biol. Sci. 2021, 17, 3646–3658.
- Ramírez-Labrada, A.G.; Isla, D.; Artal, A.; Arias, M.; Rezusta, A.; Pardo, J.; Gálvez, E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer 2020, 6, 86–97.
- Greathouse, K.L.; White, J.R.; Vargas, A.J.; Bliskovsky, V.V.; Beck, J.A.; von Muhlinen, N.; Polley, E.C.; Bowman, E.D.; Khan, M.A.; Robles, A.I.; et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol. 2018, 19, 123.
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013.
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016, 17, 163.
- Tsay, J.C.J.; Wu, B.G.; Badri, M.H.; Clemente, J.C.; Shen, N.; Meyn, P.; Li, Y.; Yie, T.A.; Lhakhang, T.; Olsen, E.; et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 1188–1198.
- Gustafson, A.M.; Soldi, R.; Anderlind, C.; Scholand, M.B.; Qian, J.; Zhang, X.; Cooper, K.; Walker, D.; Mcwilliams, A.; Gang, L.; et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci. Transl. Med. 2010, 2, 26ra25.
- Lee, S.H.; Sung, J.Y.; Yong, D.; Chun, J.; Kim, S.Y.; Song, J.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer comparing with benign mass like lesions. Lung Cancer 2016, 102, 89–95.
- Liu, H.X.; Tao, L.L.; Zhang, J.; Zhu, Y.G.; Zheng, Y.; Liu, D.; Zhou, M.; Ke, H.; Shi, M.M.; Qu, J.M. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int. J. Cancer 2018, 142, 769–778.
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reida, G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048.
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980.
- Xu, N.; Wang, L.; Li, C.; Ding, C.; Li, C.; Fan, W.; Cheng, C.; Gu, B. Microbiota dysbiosis in lung cancer: Evidence of association and potential mechanisms. Transl. Lung Cancer Res. 2020, 9, 1554–1568.
- Apopa, P.L.; Alley, L.; Penney, R.B.; Arnaoutakis, K.; Steliga, M.A.; Jeffus, S.; Bircan, E.; Gopalan, B.; Jin, J.; Patumcharoenpol, P.; et al. PARP1 Is Up-Regulated in Non-small Cell Lung Cancer Tissues in the Presence of the Cyanobacterial Toxin Microcystin. Front. Microbiol. 2018, 9, 1757.
- Yaghoobi, H.; Bandehpour, M.; Kazemi, B. Apoptotic Effects of the B Subunit of Bacterial Cytolethal Distending Toxin on the A549 Lung Cancer Cell Line. Asian Pac. J. Cancer Prev. 2016, 17, 299–304.
- Bourlioux, P.; Koletzko, B.; Guarner, F.; Braesco, V. The intestine and its microflora are partners for the protection of the host: Report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am. J. Clin. Nutr. 2003, 78, 675–683.
- Panebianco, C.; Pisati, F.; Ulaszewska, M.; Andolfo, A.; Villani, A.; Federici, F.; Laura, M.; Rizzi, E.; Potenza, A.; Latiano, T.P.; et al. Tuning gut microbiota through a probiotic blend in gemcitabine-treated pancreatic cancer xenografted mice. Clin. Transl. Med. 2021, 11, e580.
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98.
- Gobert, A.P.; Sagrestani, G.; Delmas, E.; Wilson, K.T.; Verriere, T.G.; Dapoigny, M.; Del’Homme, C.; Bernalier-Donadille, A. The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci. Rep. 2016, 6, 39399.
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; De Vos, W.M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648.
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622.
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396.
- Song, P.; Yang, D.; Wang, H.; Cui, X.; Si, X.; Zhang, X.; Zhang, L. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac. Cancer 2020, 11, 1621–1632.
- Tomita, Y.; Ikeda, T.; Sakata, S.; Saruwatari, K.; Sato, R.; Iyama, S.; Jodai, T.; Akaike, K.; Ishizuka, S.; Saeki, S.; et al. Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol. Res. 2020, 8, 1236–1242.
- Gui, Q.F.; Lu, H.F.; Zhang, C.X.; Xu, Z.R.; Yang, Y.M. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651.
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943.
- Liu, F.; Li, J.; Guan, Y.; Lou, Y.; Chen, H.; Xu, M.; Deng, D.; Chen, J.; Ni, B.; Zhao, L.; et al. Dysbiosis of the Gut Microbiome is associated with Tumor Biomarkers in Lung Cancer. Int. J. Biol. Sci. 2019, 15, 2381–2392.
- Yang, J.J.; Yu, D.; Xiang, Y.B.; Blot, W.; White, E.; Robien, K.; Sinha, R.; Park, Y.; Takata, Y.; Lazovich, D.A.; et al. Association of Dietary Fiber and Yogurt Consumption with Lung Cancer Risk: A Pooled Analysis. JAMA Oncol. 2020, 6, e194107.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
3 times
(View History)
Update Date:
12 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




