Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rebeca García Varela | -- | 2301 | 2022-07-01 16:57:52 | | | |
| 2 | Dean Liu | -7 word(s) | 2294 | 2022-07-04 07:52:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Phenolic Compound Family Extracted from Raspberries. Encyclopedia. Available online: https://encyclopedia.pub/entry/24751 (accessed on 07 February 2026).
Lopez-Corona AV, Valencia-Espinosa I, González-Sánchez FA, Sánchez-López AL, Garcia-Amezquita LE, Garcia-Varela R. Phenolic Compound Family Extracted from Raspberries. Encyclopedia. Available at: https://encyclopedia.pub/entry/24751. Accessed February 07, 2026.
Lopez-Corona, Alejandra Vanessa, Illeen Valencia-Espinosa, Fabio Antonio González-Sánchez, Angélica Lizeth Sánchez-López, Luis Eduardo Garcia-Amezquita, Rebeca Garcia-Varela. "Phenolic Compound Family Extracted from Raspberries" Encyclopedia, https://encyclopedia.pub/entry/24751 (accessed February 07, 2026).
Lopez-Corona, A.V., Valencia-Espinosa, I., González-Sánchez, F.A., Sánchez-López, A.L., Garcia-Amezquita, L.E., & Garcia-Varela, R. (2022, July 01). Phenolic Compound Family Extracted from Raspberries. In Encyclopedia. https://encyclopedia.pub/entry/24751
Lopez-Corona, Alejandra Vanessa, et al. "Phenolic Compound Family Extracted from Raspberries." Encyclopedia. Web. 01 July, 2022.
Copy Citation
In plant physiology, a phenolic compound family can act as an antioxidant and antimicrobial agent, and their biosynthesis follows the shikimate and phenylpropanoid pathways.
raspberry
phenolic compounds
antioxidant activity
anthocyanins
cancer
1. Introduction
Red raspberries (Rubus idaeus) are native fruits from Europe. The genera Rubus contains approximately 740 species, which are classified into 15 subgenera. Raspberries belong to the subgenus Idaeobatus [1]. Raspberries have been studied by the pharmaceutical, cosmetical, agricultural and food industries [2][3][4]. They have become popular and highly distinguished for their flavor and characteristic color. Their red color is produced by a pigment known as anthocyanins, which, in other types of berries, can give a blue or purple pigment [5]. This fruit is known to provide the vitamins, minerals, fatty acids, proteins, carbohydrates and dietary fiber needed for healthy nutrition in humans and animals [1][6]. Additionally, they contain a polyphenolic compound profile associated with the prevention and treatment of some pathologies and chronic degenerative diseases [7]. In plant physiology, a phenolic compound family can act as an antioxidant and antimicrobial agent, and their biosynthesis follows the shikimate and phenylpropanoid pathways, described in the following sections [8][9][10]. While raspberries have been reported to have a wide antioxidant capacity, approximately 75% is due to their anthocyanin and ellagitannin contents and 20% to vitamin C; here, a synergistic effect is produced when in contact with polyphenols; the remaining 5% is attributed to other compounds in the matrix [11][12][13][14].
During the development of several common chronic degenerative diseases, the evidence suggests that reactive oxygen species (ROS) and reactive nitrogen species (RNS) play an important role by being responsible for producing oxidative stress in cells. Oxidative stress occurs when ROS and RNS are present at a higher rate than normal; therefore, the antioxidant defenses cannot cope with this imbalance, and the free radicals are unpaired [15]. Different types of ROS, such as superoxide anion radical (O2−), 1O2, hydroxide (HO−) or hydrogen peroxide (H2O2), can be produced in plants when there is an imbalance in the electron transport chain or Calvin reactions [16][17]. Molecules are classified as RNS when they are derivatives from nitric oxide and have a strong oxidizing effect. The most common RNS are nitrosyl cation (NO+), nitrosyl anion (NO−), nitrogen dioxide (NO2), dinitrogen trioxide (N2O3), peroxynitrite (ONOO−), nitrite (NO2−), nitrate (NO3−) and nitroxyl (HNO) [18]. There are endogenous and exogenous factors that can increase the level of ROS and RNS production in the body. The endogenous factors are related to the biochemical reactions in enzymatic and nonenzymatic pathways in the cell. On the other hand, exogenous factors are linked to environmental and lifestyle factors, such as pollution, alcohol, drugs, radiation and diet. The excess of free radicals in cells is related to aging and many chronic diseases, such as cancer and diabetes [19][20]. Antioxidant compounds function as potential aids by decreasing oxidative stress when pairing ROS-free electrons [21]. Additionally, chronic diseases are also accompanied by an inflammatory response in most cases. Inflammation is a self-defense response of cells that can be triggered by a pathogen or an injury/damage. The inflammatory response is produced by the immune system and is usually acute. In some diseases, this can become chronic and have a negative effect on the cells. Some of the consequences of chronic inflammation include: damage of macromolecules, DNA, tissues, ROS and RNS accumulation and an increase of oxidative stress [22][23][24]. Most of the anti-inflammatory assays are based on measuring the proinflammatory proteins and associated compounds, such as interleukins, tumor necrosis factor alpha and cytokines, among others [25].
Various phenolic family compounds have been proven to have an antioxidant and anti-inflammatory capacity when administered to humans and animals for epidemiological studies. Therefore, members of the phenolic family are considered nutraceuticals, and this is based on the health benefits they confer. Great interest and resources have been focused in developing sustainable protocols to extract these polyphenols from multiple berries with the purpose of producing supplements with a higher concentration compared to its original source. Nevertheless, side effects have been reported when high doses are consumed, mostly through supplements that have been produced by isolating these compounds from their food matrix [26]. The adverse side effects are liver injuries or stroke. Although some supplements contain multiple bioactive compounds and polyphenols, it could be assumed that the side effects linked to supplements are caused by the phenolic family compounds or, if there is a synergetic effect when combining them, with other vitamins or isolated compounds. These risks may be related to the difficulty in mimicking the in vivo conditions when performing the in vitro models of study [27]. Hence, several authors keep performing in vitro and in vivo assays with high doses of polyphenols to determine their cytotoxicity.
There is a vast quantity of antioxidant, anti-inflammatory and cytotoxicity assays that are the key to performing preclinical and clinical trials before selling these supplements. Multiple studies have shown that phenolic family compounds have the ability to scavenge free radicals [28][29][30][31][32]. These findings are based on the data obtained by applying methodologies to determine their antioxidant activity; such as: the oxygen radical absorbance capacity (ORAC), 2,2-diphenyl-1picrylhydrazyl (DPPH), ferric-reducing antioxidant power (FRAP) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assays [33]. The nitric oxygen (NO) assay and protein denaturation inhibition assay are commonly used to evaluate the anti-inflammatory effects on cells. Researchers discusses the antioxidant and anti-inflammatory activity reported in chemical, in vitro and in vivo assays using phenolic family compounds obtained from raspberries; additionally, it explores the antioxidant and anti-inflammatory activity potential attributed to raspberries due to their phenolic compound contents that act as effective nutraceuticals. Cytotoxicity assays are examined and compared with the reported doses used in cancer cell lines from several published studies. An overview of the metabolism, mechanism of action and the beneficial effects that have been observed in patients with chronic degenerative diseases, such as cancer, when provided with raspberry extracts will be further discussed. Further studies are needed to determine if consuming polyphenols in their original food matrix have increased benefits compared to the isolated forms in the human body.
2. Phenolic Compound Family Found in Raspberries
2.1. Role of the Phenolic Compound Family in Plant Physiology
Phenolic compounds are the most abundant secondary metabolites in plants, and they play an important physiological role; however, that is not the case in plant development [34]. Bioactive compound profiles may vary depending on conditions such as the growth stage and raspberry variety. Particularly, polyphenols serve a self-protection role in plants, and their concentration can be increased as a response to abiotic and biotic stresses, including pests, bacteria, diseases, ultraviolet light, low temperatures, soil nutrients and drought [35]. Since they are sessile to soil, it is difficult for them to attack pathogens and react against adverse environmental conditions. Plants have developed molecular, physiological and morphological mechanisms to overcome stress, included the production of secondary metabolites [36][37][38]. For example, the synthesis of polyphenols contributes to the stability and robustness of raspberries by adapting certain environmental conditions and as a defense against mechanical damage [10]. Anthocyanins and ellagitannins have been reported as the main contributors of the antioxidant effect in raspberries and other plants from the genera Rubus [11][13]. A transcriptomic analysis by RNA-Seq conducted by Gutiérrez-Albanchez et al. showed a plant protection of up to 88% in blackberries (Rubus ulmifolius) when the regulating and core genes of the flavonol–anthocyanin pathway were expressed in the plant [39]. Ellagitannins from raspberries were proven to have an in vitro and in situ antifungal capacity against Geotrichum candidum [40].
Anthocyanins are mainly found in the vacuoles of plant cells. In raspberries, the ones identified at higher concentrations are: cyanidin-3-O-sophoroside, cyanidin-3-O-glucosylrutinoside and cyanidin-3-O-glucoside [41][42]. Regarding ellagitannins, sanguiin H-6 and lambertianin C have also been found at significant amounts in raspberries [43][44]. In a chemical composition study of raspberry seeds conducted by Kosmala et al., the content of ellagitannins was analyzed. Their results showed that sanguiin H-6 was almost 50% of the total ellagitannins content, whereas lambertianin C represented 34% of the total phenolic content [45]. The reported contents of the phenolic compounds by each author may vary; this is due to the variety in origin, location and the chosen extraction method.
The physicochemical characteristics are linked to biotic and abiotic stresses. Some factors that may contribute to these variations in the content values are harvesting, transportation, storage conditions and applied treatments [46]. Mazur et al. [47] evaluated the raspberry genotype variations in three different harvesting seasons, proving that the phenolic content, color and general characteristics were significantly affected. In a recent study, twelve different cultivars of raspberries from Spain and Morocco were examined to prove that the chemical composition results varied even when applying the same conditions [29]. On the other hand, some authors have focused their studies on a specific phenolic family. Chen et al. evaluated the anthocyanin degradation kinetics on raspberry juices at different times of storage. Their results confirmed that the anthocyanins concentration was reduced when longer storage times were applied [48]. In another study, the ellagitannin and anthocyanin contents were quantified from frozen and fresh raspberries; for both compounds, higher concentrations were found in the frozen samples [49]. Ponder & Hallmann performed a phenolic analysis between conventional and organic cultivars of raspberries; the variables they considered were the type of soil, location, dosage and time of the given fertilizers. The antioxidant activity results showed higher values for raspberries that were cultivated under organic conditions than the conventional method [35]. In brief, the harvesting season, region and storage conditions have a strong effect on the physicochemical properties of raspberries.
2.2. Classification
The phenolic family compounds are divided and classified into two major groups: flavonoids and non-flavonoids, also called simple phenols. Flavonoids have two phenyl rings linked by a pyran ring and may or may not be attached to a sugar molecule. Glycosides are bound to any sugar apart from glucose; on the other hand, glucosides are only bound to glucose, and aglycones are not bound to a sugar molecule. Aglycones have a flavylium ion (2-phenylbenzopyrilium) with a cation function and are composed of two aromatic groups: a benzopyrilium and a phenolic ring [50][51]. As shown in Figure 1, flavonoids are subdivided into six subclasses: anthocyanidins, flavonols, flavanones, flavanols, flavones and isoflavones. Non-flavonoids are subdivided in four subclasses: stilbenes, tannins, coumarins and phenolic acids [51][52]. Phenolic acids are ordered into three categories: hydroxybenzoic, hydroxyphenylacetic and hydroxycinnamic acids [53].
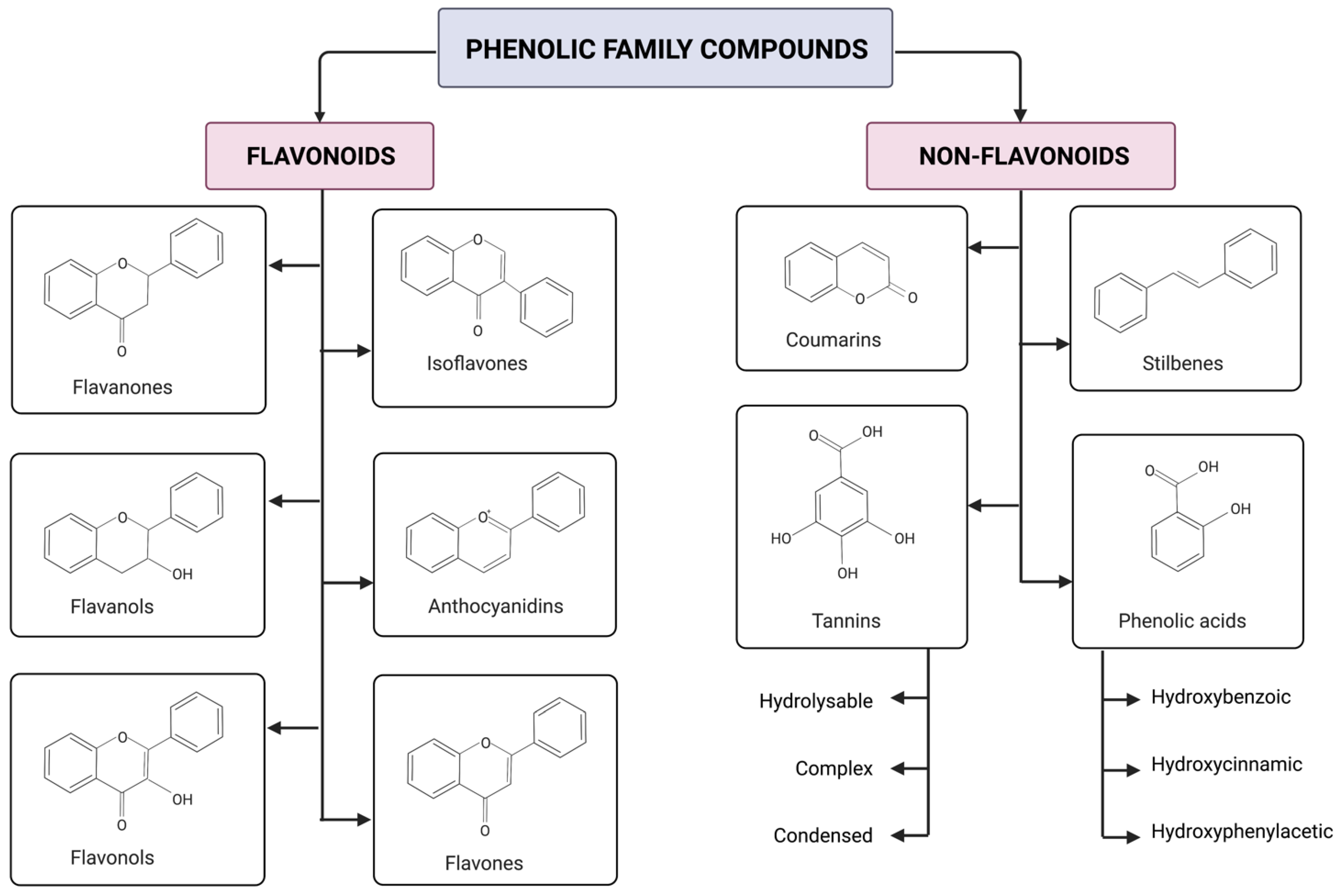
Figure 1. Phenolic family compound basic skeletal structure and their classification in subclasses as stated by [50]. There are two major groups: flavonoids and non-flavonoids. Flavonoids have six subclasses: anthocyanidins, flavonols, flavanones, flavanols, flavones and isoflavones. Anthocyanidins become anthocyanins when sugars are linked in their chemical structures. Non-flavonoids are subdivided into four subclasses: stilbenes, tannins, coumarins and phenolic acids. Tannins are categorized into condensed, hydrolysable and complex. For phenolic acids, a more specified classification is to divide them into three groups: hydroxybenzoic, hydroxyphenylacetic and hydroxycinnamic acids. Created using licensed BioRender (2022).
Tannins are categorized in three groups: hydrolysable tannins and condensed tannins; this last one is also known as proanthocyanidins [51]. The characterization is according to the chemical bonds that link their monomers. Hydrolysable tannins have ester bonds, whereas condensed tannins have carbon–carbon and carbon–oxygen–carbon bonds. The conjugations between the condensed and hydrolysable are known as complex tannins [54]. Ellagitannins are categorized as hydrolysable tannins since they release gallic and ellagic acid when hydrolyzed. Gallic acid and multiple hexahydroxydiphenoyl (HHDP) moieties are the core subunits in the ellagitannins chemical structure [55][56]. Anthocyanins are anthocyanidins with sugar molecules coupled to their chemical structure. Anthocyanins and ellagitannins are two of the most representative and studied phenolics found in raspberries [7][47][57].
2.3. Phenolic Compound Family Biosynthesis
Phenolic compound family biosynthesis is known to follow the phenylpropanoid and flavonoid pathways in the rough endoplasmic reticulum of the plant cell. This plant metabolic pathway is based on secondary metabolites generated from the shikimate pathway, which takes place in the plastids [10]. The shikimate pathway synthesizes secondary metabolites such as ellagitannins, gallotannins and phenylalanine, as depicted in Figure 2. The production of secondary metabolites through the shikimate pathway takes place in a wide variety of bacteria, fungi and plants [58]. It starts with the production of dihydroshikimate when erythrose 4-phosphate and phosphoenolpyruvate (PEP) have an aldol condensation reaction [58]. The enzyme shikimate dehydrogenase (SDH) catalyzes the production of gallic and shikimic acid. The final products of this pathway are ellagitannins, gallotannins and phenylalanine [59][60].
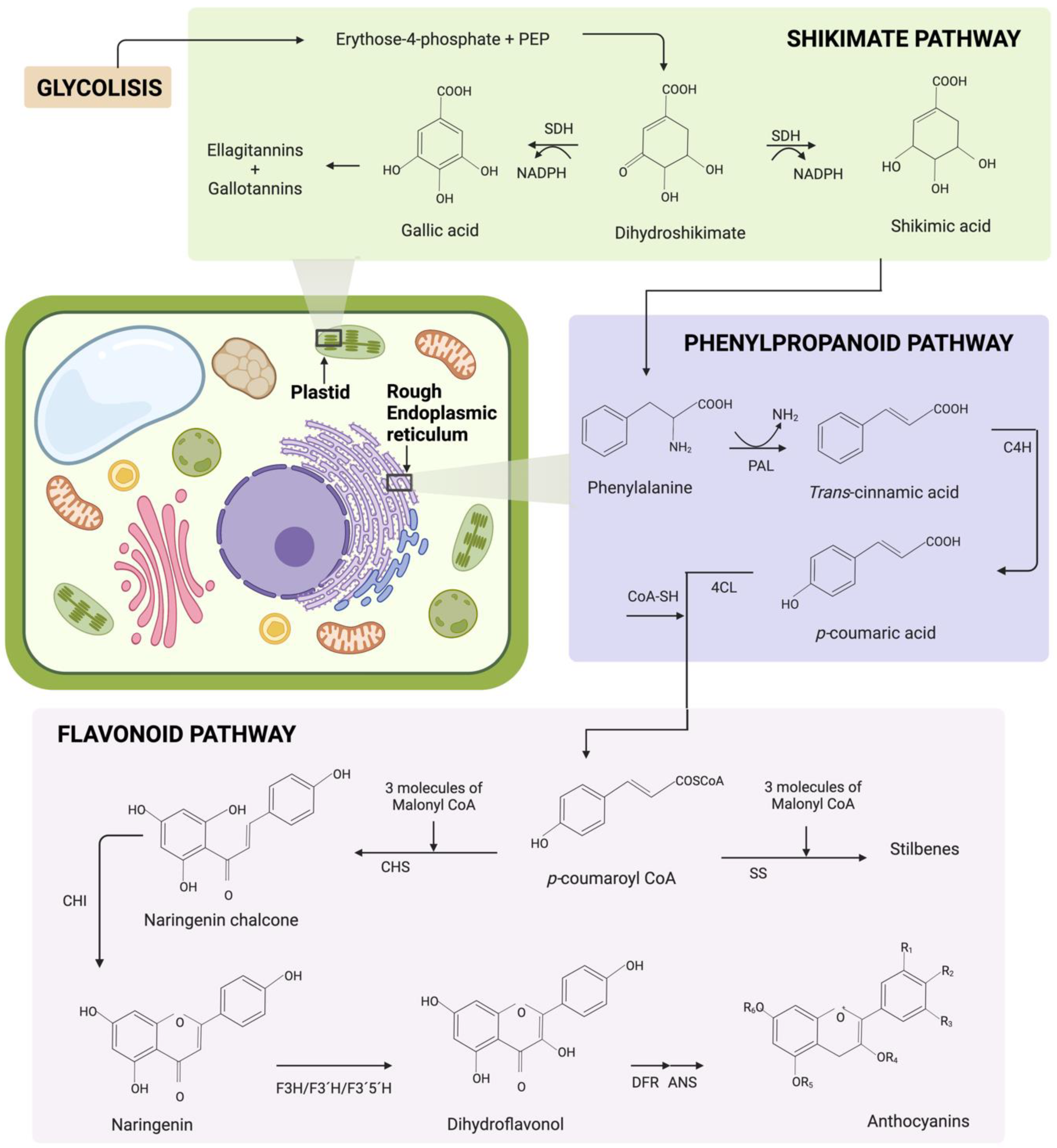
Figure 2. Biosynthesis of the phenolic compound family in the plant cell through the shikimate, phenylpropanoid and flavonoid pathways. The shikimate pathway begins with the reaction of erythrose 4-phosphate and phosphoenolpyruvate (PEP) to produce dehydroshikimate. With this precursor, the enzyme shikimate dehydrogenase (SDH) can catalyze the production of ellagitannins, gallotannins and phenylalanine. The phenylpropanoid pathway begins with the condensation of phenylalanine and acetate by the action of the phenylalanine ammonia lyase enzyme (PAL). Consequently, p-coumaryl CoA is produced by the action of the cinnamate 4-hydoxylase enzyme (C4H) and 4-coumarate CoA ligase (4CL). The precursor of the flavonoid pathway is p-coumaryl CoA, which reacts with three molecules of Malonyl CoA to later produce multiple phenolic family compounds, such as anthocyanins and stilbenes. The enzymes involved in the flavonoid pathway are the following: chalcone synthase (CHS), stilbene synthase (SS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavanone 3′-hydroxylase (F3′H), flavanone 3′5′-hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR) and anthocyanidin synthase (ANS). Created using licensed BioRender (2022).
The general phenylpropanoid pathway begins with the condensation of phenylalanine and acetate by the action of the phenylalanine ammonia lyase enzyme (PAL) to produce trans-cinnamic acid. The cinnamate 4-hydoxylase enzyme (C4H) catalyzes trans-cinnamic acid into p-coumaric acid. Afterwards, p-coumaric acid reacts with 4-coumarate CoA ligase (4CL) to produce p-coumaroyl CoA [61]. Overall, in the case of phenolic acids, p-coumaric acid functions as a precursor, while flavonoids use p-coumaroyl CoA [62]. The flavonoid pathway starts p-coumaroyl CoA. Multiple phenolic compound family biosynthesis follows the general phenylpropanoid pathway, as shown in Figure 2, including anthocyanins and stilbenes. For this purpose, enzymes such as chalcone synthase (CHS), stilbene synthase (SS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavanone 3′-hydroxylase (F3′H), flavanone 3′5′-hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR) and anthocyanidin synthase (ANS) are also required [8][63][64].
References
- Hummer, K.E. Rubus pharmacology: Antiquity to the present. HortScience 2010, 45, 1587–1591.
- Gomes, M.D.S.; Cardoso, M.D.G.; Guimarães, A.C.G.; Guerreiro, A.C.; Gago, C.M.L.; Vilas Boas, E.V.D.B.; Dias, C.M.B.; Manhita, A.C.C.; Faleiro, M.L.; Miguel, M.G.C.; et al. Effect of edible coatings with essential oils on the quality of red raspberries over shelf-life. J. Sci. Food Agric. 2017, 97, 929–938.
- Brodowska, A. Raspberry pomace—Composition, properties and application. Eur. J. Biol. Res. 2017, 7, 86–96.
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J. Cosmet. Dermatol. 2019, 18, 539–544.
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588.
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2016, 68, 18–28.
- Del Bo, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917.
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90.
- Zha, J.; Koffas, M.A.G. Production of anthocyanins in metabolically engineered microorganisms: Current status and perspectives. Synth. Syst. Biotechnol. 2017, 2, 259–266.
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20.
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165.
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246.
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883.
- Kshatriya, D.; Li, X.; Giunta, G.M.; Yuan, B.; Zhao, D.; Simon, J.E.; Wu, Q.; Bello, N.T. Phenolic-enriched raspberry fruit extract (Rubus idaeus) resulted in lower weight gain, increased ambulatory activity, and elevated hepatic lipoprotein lipase and heme oxygenase-1 expression in male mice fed a high-fat diet. Nutr. Res. 2019, 68, 19–33.
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020.
- Turkan, I. ROS and RNS: Key signalling molecules in plants. J. Exp. Bot. 2018, 69, 3313–3315.
- Moldogazieva, N.T.; Mokhosoev, I.M.; Feldman, N.B.; Lutsenko, S.V. ROS and RNS signalling: Adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018, 52, 507–543.
- Król, M.; Kepinska, M. Human nitric oxide synthase—Its functions, polymorphisms, and inhibitors in the context of inflammation, diabetes and cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 56.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Davies, M.J. Quantification and Mechanisms of Oxidative Stress in Chronic Disease. Proceedings 2019, 11, 18.
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96.
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635.
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41.
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50.
- Hariram Nile, S.; Won Park, S. Optimized Methods for In Vitro and In Vivo Anti-Inflammatory Assays and Its Applications in Herbal and Synthetic Drug Analysis. Mini-Rev. Med. Chem. 2013, 13, 95–100.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant supplements and mortality. Curr. Opin. Clin. Nutr. Metab. Care 2013, 17, 1.
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem. 2010, 119, 592–599.
- García, A.V.; Pérez, S.E.M.; Butsko, M.; Moya, M.S.P.; Sanahuja, A.B. Authentication of “adelita” raspberry cultivar based on physical properties, antioxidant activity and volatile profile. Antioxidants 2020, 9, 593.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Hidalgo, G.-I.; Almajano, M. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7.
- Plaza, M.; Domínguez-Rodríguez, G.; Castro-Puyana, M.; Marina, M.L. Polyphenols analysis and related challenges. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 177–232. ISBN 9780128135723.
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red raspberries and their bioactive polyphenols: Cardiometabolic and neuronal health links. Adv. Nutr. 2016, 7, 44–65.
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus l.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458.
- Nejat, N.; Mantri, N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 2017, 23, 1–16.
- Méndez Espinoza, C.; Vallejo Reyna, M.Á. Mecanismos de respuesta al estrés abiótico: Hacia una perspectiva de las especies forestales. Rev. Mex. Cienc. For. 2019, 10, 33–64.
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86.
- Gutiérrez-Albanchez, E.; Gradillas, A.; García, A.; García-Villaraco, A.; Gutierrez-Mañero, F.J.; Ramos-Solano, B. Elicitation with Bacillus QV15 reveals a pivotal role of F3H on flavonoid metabolism improving adaptation to biotic stress in blackberry. PLoS ONE 2020, 15, e0232626.
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from Raspberry (Rubus idaeus L.) Fruit as Natural Inhibitors of Geotrichum candidum. Molecules 2016, 21, 908.
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from Polana Raspberry (Rubus idaeus L.) fruit and juice—In Vitro Study. Molecules 2018, 23, 1812.
- Mihailović, N.R.; Mihailović, V.B.; Ćirić, A.R.; Srećković, N.Z.; Cvijović, M.R.; Joksović, L.G. Analysis of Wild Raspberries (Rubus idaeus L.): Optimization of the Ultrasonic-Assisted Extraction of Phenolics and a New Insight in Phenolics Bioaccessibility. Plant Foods Hum. Nutr. 2019, 74, 399–404.
- García-Villalba, R.; Giménez-Bastida, J.A.; Ávila-Gálvez, M.A.; Tomás-Barberán, F.A.; Espín, J.C.; González-Sarrías, A. Ellagitannins and Their Gut Microbiota-Derived Metabolites: Urolithins. In Dietary Polyphenols; Wiley: Hoboken, NJ, USA, 2020; pp. 319–364.
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196.
- Kosmala, M.; Zduńczyk, Z.; Juśkiewicz, J.; Jurgoński, A.; Karlińska, E.; Macierzyński, J.; Jańczak, R.; Rój, E. Chemical composition of defatted strawberry and raspberry seeds and the effect of these dietary ingredients on polyphenol metabolites, intestinal function, and selected serum parameters in rats. J. Agric. Food Chem. 2015, 63, 2989–2996.
- Piasecka, A.; Kachlicki, P.; Stobiecki, M. Analytical methods for detection of plant metabolomes changes in response to biotic and abiotic stresses. Int. J. Mol. Sci. 2019, 20, 379.
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240.
- Chen, J.Y.; Du, J.; Li, M.L.; Li, C.M. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT 2020, 128, 109448.
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. An exploratory study of red raspberry (: Rubus idaeus L.) (poly)phenols/metabolites in human biological samples. Food Funct. 2018, 9, 806–818.
- Aguilera-Otíz, M.; Reza-Vargas, M.d.C.; Chew-Madinaveita, R.G.; Meza-Velázquez, J.A. Functional Properties of Anthocyanins. Biotecnia 2011, 13, 16–22.
- Wahle, K.W.J.; Brown, I.; Rotondo, D.; Heys, S.D. Plant phenolics in the prevention and treatment of cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51.
- Veeramuthu, D.; Raja, W.R.T.; Al-Dhabi, N.A.; Savarimuthu, I. Flavonoids: Anticancer Properties. In Flavonoids—From Biosynthesis to Human Health; InTech: London, UK, 2017.
- Białecka-Florjańczyk, E.; Fabiszewska, A.; Zieniuk, B. Phenolic Acids Derivatives—Biotechnological Methods of Synthesis and Bioactivity. Curr. Pharm. Biotechnol. 2018, 19, 1098–1113.
- Bule, M.; Khan, F.; Farrukh Nisar, M.; Niaz, K. Tannins (Hydrolysable Tannins, Condensed Tannins, Phlorotannins, Flavono-Ellagitannins); Elsevier: Amsterdam, The Netherlands, 2020.
- Ky, I.; Le Floch, A.; Zeng, L.; Pechamat, L.; Jourdes, M.; Teissedre, P.L. Tannins. In Encyclopedia of Food and Health; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 247–255. ISBN 9780123849533.
- Al-Harbi, S.A.; Abdulrahman, A.O.; Zamzami, M.A.; Khan, M.I. Urolithins: The Gut Based Polyphenol Metabolites of Ellagitannins in Cancer Prevention, a Review. Front. Nutr. 2021, 8, 258.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262.
- Rehan, M. Biosynthesis of Diverse Class Flavonoids via Shikimate and Phenylpropanoid Pathway. In Bioactive Compounds-Biosynthesis, Characterization and Applications; IntechOpen: London, UK, 2021.
- Muir, R.M.; Ibáñez, A.M.; Uratsu, S.L.; Ingham, E.S.; Leslie, C.A.; McGranahan, G.H.; Batra, N.; Goyal, S.; Joseph, J.; Jemmis, E.D.; et al. Mechanism of gallic acid biosynthesis in bacteria (Escherichia coli) and walnut (Juglans regia). Plant Mol. Biol. 2011, 75, 555–565.
- Bontpart, T.; Marlin, T.; Vialet, S.; Guiraud, J.L.; Pinasseau, L.; Meudec, E.; Sommerer, N.; Cheynier, V.; Terrier, N. Two shikimate dehydrogenases, VvSDH3 and VvSDH4, are involved in gallic acid biosynthesis in grapevine. J. Exp. Bot. 2016, 67, 3537–3550.
- Emiliani, G.; Fondi, M.; Fani, R.; Gribaldo, S. A horizontal gene transfer at the origin of phenylpropanoid metabolism: A key adaptation of plants to land. Biol. Direct 2009, 4, 7.
- de Dios–López, A.; Montalvo–González, E.; Andrade–González, I.; Gómez–Leyva, J.F. Inducción de antocianinas y compuestos fenólicos en cultivos celulares de jamaica (Hibiscus sabdariffa L.) in vitro. Rev. Chapingo Ser. Hortic. 2011, 17, 77–87. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1027-152X2011000200002 (accessed on 26 March 2022).
- Peña-Sanhueza, D.; Inostroza-Blancheteau, C.; Ribera-Fonseca, A.; Reyes-Díaz, M. Anthocyanins in Berries and Their Potential Use in Human Health. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; InTech: London, UK, 2017.
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780203508732.
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
04 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




