Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marc-Antoine Lauzon | -- | 6947 | 2022-06-29 14:47:51 | | | |
| 2 | Dean Liu | -34 word(s) | 6913 | 2022-06-30 04:03:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kheir, W.E.; Marcos, B.; Virgilio, N.; Paquette, B.; Faucheux, N.; Lauzon, M. Innovative Treatments for Glioblastoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/24637 (accessed on 07 February 2026).
Kheir WE, Marcos B, Virgilio N, Paquette B, Faucheux N, Lauzon M. Innovative Treatments for Glioblastoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/24637. Accessed February 07, 2026.
Kheir, Wiam El, Bernard Marcos, Nick Virgilio, Benoit Paquette, Nathalie Faucheux, Marc-Antoine Lauzon. "Innovative Treatments for Glioblastoma" Encyclopedia, https://encyclopedia.pub/entry/24637 (accessed February 07, 2026).
Kheir, W.E., Marcos, B., Virgilio, N., Paquette, B., Faucheux, N., & Lauzon, M. (2022, June 29). Innovative Treatments for Glioblastoma. In Encyclopedia. https://encyclopedia.pub/entry/24637
Kheir, Wiam El, et al. "Innovative Treatments for Glioblastoma." Encyclopedia. Web. 29 June, 2022.
Copy Citation
Glioblastoma multiforme (GBM) is a grade IV glioma considered the most fatal cancer of the central nervous system (CNS), with less than a 5% survival rate after five years. The tumor heterogeneity, the high infiltrative behavior of its cells, the low resistance of the brain to ionizing radiations and the blood–brain barrier (BBB) that limits the access of therapeutic drugs to the brain are the main reasons hampering the current standard treatment efficiency.
brain cancer
chemoattractant
cancer cell trap
drug delivery systems
nanocarriers
glioblastoma
immunotherapy
gene therapy
CXCL12
1. Some Innovative Treatments in Glioblastoma
1.1. Gene Therapies
Some of the antiangiogenic gene therapies have been successful to suppress tumor angiogenesis and growth [1]. Angiogenic gene therapy strategies consist mostly in disrupting the normal function of VEGF [2]. For example, ofranergene obadenovec (VB-111) is a genetically modified non-replicating adenovirus type 5 containing a specific promoter and a transgene encoding for a chimeric death receptor (proapoptotic Fas-TNFR1 chimeric protein) [3][4]. Przystal et al. first verified that TMZ increases the expression of the endogenous Grp78 protein in human GBM cells (LN229, U87, and SNB19 cells) in a dose-dependent manner. They also showed that RGD4C/AAVP-Grp78 gene expression is strongly increased by TMZ. Interestingly, the combination of the TMZ with the RGD4C/AAVP-Grp78-HSVtk mutant SR39 encoding the Herpes simplex virus type I thymidine kinase in the presence of ganciclovir (GCV) induced strong tumor cell killing both in vitro and in vivo (mice with established intracranial U87 tumors) [5].
-
mRNA and siRNA in GBM Gene Therapy:
mRNA-based gene therapy is an efficient gene transfection tool that emerged to adapt with the high heterogeneity and diffusing invasiveness nature of the GBM. Xiangjun et al. explored a new therapeutic strategy using an in vitro synthesized mRNA encoding for (a) a phosphatase and tensin homolog on chromosome ten (PTEN) that can induce apoptosis or (b) a tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL)- in tumor cells derived from -PTEN-deletion patients [6]. They confirmed that a low survival rate is observed in GBM patients who have a high frequency mutation of PTEN. Using patient-derived primary GBM stem cells with PTEN alteration and a Denver Brain Tumor Research Group (DBTRG)-cell-derived xenograft to detect the cytotoxicity of mRNA in vitro and tumor suppression in vivo, they showed that the combined treatment of PTEN-mRNA and TRAIL-mRNA significantly reduced the growth of both the GBM cells and tumor. The tumor growth is suppressed after two months compared with the control PBS (96.4%) and single mRNA group (PTEN-mRNA (89.7%) or TRAIL-mRNA (84.5%)) [6]. mRNAs can also be encapsulated into nanocarriers such as liposomes and nanoparticles (NP) to overcome the natural barriers and protect them from degradation [7]. The delivery routes and the carrier forms of mRNA depend on the patients glioma grade, stage, surgery, and chemotherapy history [7]. No clinical trial of mRNA-based GBM therapy has been completed, and has not been widely adopted in treating GBM yet.
On another side, small interfering RNA (siRNA) are known by their ability to silence the genes responsible for cancer progression by targeting tumor-promoting factors, such as VEGF and EGFR [8][9]. Since GBM cells are resistant to anti-tumor drugs, the use of siRNA in combination with chemotherapy could be beneficial to enhancing the treatment efficiency [10]. For example, the combination of resveratrol (RES) and heat shock protein 27-knockdown using siRNA (Hsp27) was tested to treat the disease [11]. Hsp27 is a tumor-promoting factor in GBM implicated in ECM remodeling and cell survival. RES at 15 µM decreases the Hsp27 protein level in a similar way than quercetin, a well-known Hsp27 inhibitor (47% and 41%, respectively). However, combining RES at 15 µM with Hsp27 siRNA induces a decrease in the level of Hsp27 by 93.4% in transfected human U87 MG cells [11]. This combined treatment increases the caspase-3 activity by 101% and induces GBM cell apoptosis. [11]. The study proves that the use of Hsp27-siRNA combined with an anti-tumor agent could be beneficial to induce apoptosis in GBM cells. However, the BBB, the degradation by RNAse enzymes, and reaching the tumor site are the main challenges preventing the efficiency of siRNA therapy [12]. Under these conditions, nanocarriers can promote a targeted delivery of siRNA and protect them against degradation at the same time. A wide range of siRNA-loaded nanocarriers have been tested in GBM therapy [13]. For instance, loaded MGMT-siRNA liposomes have been tested in GBM treatment resulting in MGMT downregulation, DNA repair induction, and decreased drug efflux capacities responsible for increasing GBM cell sensitivity to TMZ [13]. In another study, RGD-functionalized pH-responsive polyamidoamine (PAMAM) dendrimers were investigated for delivery of both c-myc siRNA anddoxorubicin (DOX)-loaded Se NP in GBM therapy [14]. The RGD functionalization of PAMAM enhances the uptake of siRNA dendrimers by cancer cells [14]. The nanocarriers were able to penetrate a BBB model in vitro, developed to deliver the drug and enhance the antitumor activity [14]. Moreover, chitosan lipidic nanocapsules were used for galectin-1 and EGFR-siRNAs’ delivery in nude mice-bearing orthotopic U87 MG GBM cells [15]. The mean survival time increased in the mice treated 14 days after tumor implantation with both anti-EGFR and anti-Galectin-1 siRNAs plus TMZ (39 days), in comparison to untreated mice (32 days), or EGFR siRNA plus TMZ or anti-Galectin-1 siRNA plus TMZ (34 days), representing a promising strategy to induce anti-tumor effects in GBM [15]. Furthermore, CXCR4-targeted peptide carriers for VEGF-siRNA delivery were tested in GBM therapy [16]. The peptide carriers were able to condense and protect siRNA from RNAse degradation and induced a 2–6-fold decrease in VEGF expression in the cells, indicating that the surface modification of the nanocarriers can improve their specificity towards GBM cells [16]. However, more studies to develop safe and well-tolerated nanocarriers for siRNA delivery are needed. Another therapy that appeared to be very powerful and hopeful is immunotherapy, in which drug delivery systems are extensively used.
1.2. Immunotherapies
Immune checkpoint inhibitors release the inhibitory brakes of T cells, activating the immune system to induce anti-tumor responses [17]. When the binding to their ligand is inhibited, the checkpoint receptors can promote an effective cell response against GBM [18]. Targeted checkpoint molecules such as Nivolumab (Opdivo®), pembrolizumab (Keytruda®), durvalumab (Imfinzi®), and atezolizumab (Tecentriq®) (an anti-programmed death-ligand 1) have been approved to treat several types of cancer and are currently trialed in GBM treatment [19][20]. For example, Gardell et al. used human monocyte-derived macrophages genetically modified for bispecific T cell engager (BiTE) and proinflammatory cytokine IL-12 secretion [21]. BiTE is specific for the mutation of epidermal growth factor receptor variant 3 (EGFRV3), expressed by the GBM cell. The secreted BiTE, by binding to the tumor antigen, was able to activate T cells as well as their proliferation and degranulation, leading to the elimination of the antigen-specific tumor cells in in vitro and in vivo models. BiTE secretion promotes a reduction in the tumor burden [21]. However, the kinetics release of BiTE protein from the cells still needs to be improved which reveal the importance of the platform optimization in the development of therapeutic approaches. Further, chimeric antigen receptors (CAR), that consist of the use of genetically modified T cells to express CAR genes, have been approved by the FDA for the treatment of hematologic malignancies [22]. O’Rourke et al. were the first to use autologous T cells redirected to the EGFRV3 mutation by CARs on human GBM patients [23]. They demonstrated that the use of CART-EGFRV3 (10 patients) is feasible and safe, since no toxicity was observed [23]. Seven patients had surgery after CART-EGFRV3 treatment, which allowed researchers to gather more information: (a) The trafficking of CART-EGFRV3 cells was observed in the direction of the tumor within the first 2 weeks of treatment. (b) The in-situ studies of the tumor environment also showed an up-regulation of immunosuppressive molecules, such as indoleamine 2,3-dioxygenase 1 (IDO1), programmed cell death ligand 1 (PD-L1) and IL-10, as well as the recruitment of immunosuppressive regulatory T cells expressing FoxP3. Thus, CART therapy for GBM still needs more investigation because of the immunosuppressive tumor microenvironment, cell trafficking, and risks of CNS toxicity [23].
Some approaches based on vaccines are also investigated as a potential adaptive immunotherapy for GBM. An autologous tumor lysate-pulsed dendritic cell vaccine called DCVax®-L, produced by Northwest Biotherapeutics, Inc., Bethesda, MD, USA [24], has been used against glioblastoma and appears to be safe. DCVax-L has been approved for GBM treatment in Switzerland, but is still under clinical trials in the United States [25]. Additionally, a phase 3 trial is ongoing to evaluate the long-term effects of the DCVax®-L vaccine in patients after surgery and chemoradiotherapy [26].
On another scale of immunotherapies targeting GBM, viral-based therapy involves genes delivery via viral vectors. For example, Desjardins et al. focused on the use of the convection-enhanced intratumoral delivery of the recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO) which recognizes the neurotropic poliovirus receptor CD155 widely expressed by GBM cells and improves the survival rate of the patients [27]. In summary, immunotherapies demonstrated promising results in terms of feasibility, safety, and even signs of efficacy. The challenges ahead are still numerous, including the optimization of the dosing, the modulation of immunosuppressive tumor microenvironment, the molecular marked heterogeneity of GBM and the understanding of the chemokines network. Thus, drug delivery systems have emerged to overcome some of those limitations.
2. Drugs Delivery Systems for Glioblastoma Treatments
2.1. Systemic Delivery
The BBB is considered as the major hurdle in drug delivery-based therapies because of its low permeability that hampers the passage of anti-cancer agents (ACA). The transport of ACA is achieved via two mechanisms: (a) passive transport (diffusion of water-soluble compounds and lipophilic molecules with a molecular weight less than 500 Da), and (b) active transport (mediated by membrane protein carriers of small molecules) [28]. Therefore, in order to promote the passage of ACA, different strategies have been explored, including small molecules capable of crossing the BBB, chemical modification of ACA, drug-loaded nanocarriers, and cell delivery systems [29].
-
Cell-Mediated Delivery:
Cell-mediated delivery utilizes cells such as leukocytes and stem cells to carry drug carriers themselves [30]. The strategy had several advantages such as long circulation times, flexible morphology, and cellular signaling [31]. The drugs can be loaded into the cells through biological pathways (endocytosis, ligand-receptor interactions), physical approaches (hypotonic hemolysis, electroporation), or chemical modifications (covalent conjugation onto surface markers, biotinylation, click chemistry) [32].
Macrophages are commonly used because of their particularity to migrate to the tumor side in response to the secretion of cytokines and chemokines [32]. Cell-mediated delivery has been widely explored in GBM. For example, neural stem cells (NSC) have been used to secrete and deliver the proapoptotic protein TRAIL to human intracranial glioma xenografts [33]. The use of high doses of TRAIL in patients can induce issues of toxicity and a danger of excessive antiviral host immune responses; for this reason, the molecule was delivered by NSCs. NSCs were able to migrate to the tumor site and secrete TRAIL, resulting in the apoptosis of the cancer cells without toxicity for the normal brain parenchyma. They also induced a significant reduction in the tumor size [33]. Wang et al. found that monocyte-mediated DOX (also known as Adriamycin®) delivery through NPs, with the surface coated with polyglycerol and RGD peptides for GBM treatment, caused tumor cell damage both in vitro and in vivo in mice orthotopic GBM xenografts. [30]. In the same context, Pang et al. loaded NPs into macrophages such as ”Trojan horses” to deliver DOX for GBM treatment [34]. The viability of the cells encapsulating the NPs was not affected and an improvement of the macrophages’ penetration into the core of the spheroids model was observed, which mimics the behavior of the cells in an in vivo model [34]. To conclude, the tumor targeting was enhanced after loading the NPs into the cells, which indicates that macrophages can improve glioma therapy and underline the importance of using NPs [34].
-
Nanocarriers:
Nanotechnology and nanocarrier-based drug delivery have recently gained remarkable attention due to their characteristics of biosafety, sustained drug release, and enhanced drug bioactivity and BBB penetrability [35]. Based on preparation methods, nanocarriers can be classified into nanocapsules, nanospheres, and NPs that are the mostly used in GBM treatment.
Nanocapsules are small vesicles of 100–200 nm in which hydrophobic drugs are encapsulated in the empty space by a polymer membrane. Polymers such as poly(lactic acid) (PLA) and Poly Lactic-co-Glycolic Acid (PLGA) can be used to prepare these nanomaterials [36]. NPs can be loaded by different therapeutic agents and are characterized by particular properties that allow them to pass the BBB [37] and achieve the tumor site. The particle size, surface charge, hydrophobicity, and coating material are the NPs’ physiochemical properties that play an important role in the targeting process [38]. The size of the NPs is a critical factor for the NPs’ delivery; small size NPs < 200 nm are preferred and suitable for systemic administration and can smoothly reach the leaky blood vessels of the tumor microenvironment [39][40][41]. The shape, stability, and charge of the NPs are also important due to their implication in fluid dynamics and their interaction with cell charge membranes and proteins [42][43]. Based on their composition and characteristics, NPs can be classified into lipidic NPs, organic NPs, and inorganic NPs [44][45][46].
Lipid-based nanocarriers regroup liposomes, nanostructured lipid carriers, solid lipid NPs, and lipid micelles (Figure 1). Liposomes are small vesicles composed of a phospholipidic bilayer that surrounds a water-soluble core similar to the cell membrane [47]. Liposomes are characterized by an easy encapsulation of ACA, an easy preparation, biodegradability, and a favorable biocompatibility [48]. Nanostructured lipid carriers (NLCs) are composed of a matrix with solid and liquid lipids forming an unordered matrix, providing a large space for drug incorporation [49]. Solid lipids NPs (SLNs) are formed from a solid hydrophobic lipid core and have demonstrated a higher stability compared with liposomes [50]. Further, SLNs have the ability to cross the BBB and deliver a wide spectrum of GBM-targeted ACAs, such as large molecules, genes, oligonucleotides, and enzymes [51]. Finally, lipidic micelles are spherical amphiphilic aggregates with a hydrophobic core and a hydrophilic shell in which drugs are loaded in the central core or can be linked to the lipids [49].
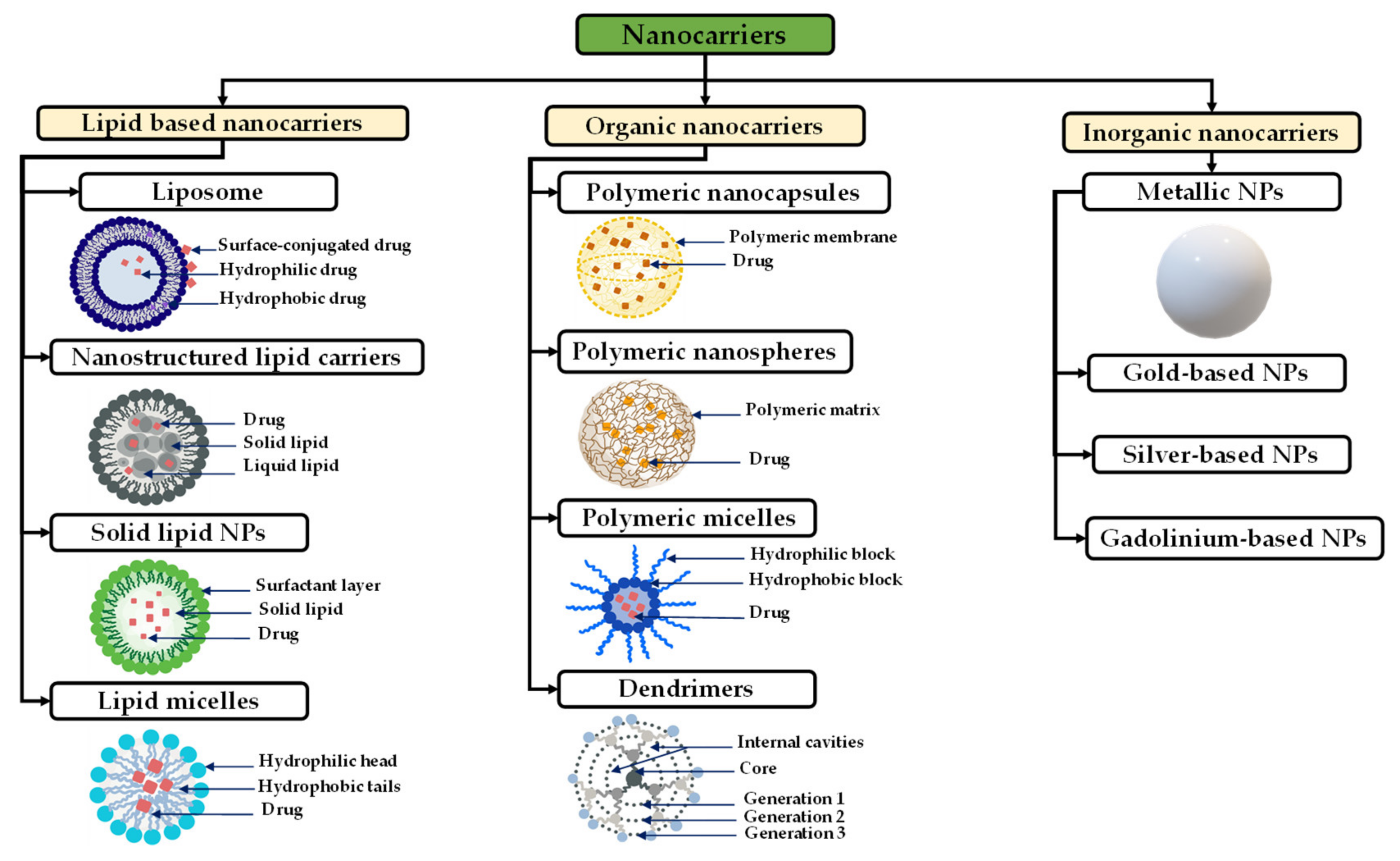
Figure 1. Schematic representation of different types of nanocarriers and their structures used in GBM treatment.
Inorganic NPs are generally composed of mineral compounds, such as metals. Among various inorganic NPs: iron oxide NPs are commonly used as contrast agents for MRI, and gold NPs are used to improve photothermal therapy [52][53][54].
For example, Liu et al. prepared magnetic iron-based NPs (MNP) modified by PEG-transferrin (Tf-PEG) and polylysine (PLL) to condense small interference RNA against polo-like kinase I (PLK1) (Tf-PEG-PLL/MNP@siPLK1). The Tf was used to target GBM cells since they express a high amount of transferrin receptors. PLK1 is involved in G2/M transition in the cell cycle and is related to tumor progression and recurrence [55]. They found that Tf-PEG-PLL/MNP@siPLK1 at the dose of 1.6 mg/kg prolonged the survival time of GBM mice, 80% of them being alive at three months. In contrast, a median survival time of about one month was observed in Tf-PEG-PLL/MNP@Scrambled PLK1-treated GBM-mice [55]. On the other hand, Zhu et al. developed ruthenium Tf and aptamer AS1411 co-grafted NPs loaded with [Ru(bpy)2(tip)]2+ (RBT), an antitumor drug for cell apoptosis. Using photodynamic therapy for GBM, they proved that RBT@MRN-SS-Tf/Apt killed glioma cells in vivo and in vitro under laser irradiation, prolonging the median survival rate [56].
Polymeric nanoparticles can be prepared using a different range of materials and are used as carriers for different drugs, such as chemotherapeutic drugs [36][57]. Polymeric nanoparticles are more advantageous over other types of NPs because of their biocompatibility, biodegradability, and the improvement they achieved in the kinetics release as reported in many reviews on the subject [58][59]. Polymeric NPs preparation can be very flexible in terms of composition, structure, and properties [60]. Polymeric NPs can be prepared using: (a) Synthetic polymer NPs such as PLA, poly(ε-caprolactone) (PCL), poly(glycolic acid) (PGA), PLGA, and poly (amino acids) [57][61][62], these polymers are biocompatible and degrade hydrolytically [63]. (b) Natural polymers which are composed of polymers such alginate [64][65], chitosan [64][65], dextran [66], and HA [67].
Polymeric micelles are highly biocompatible amphiphilic nanoparticles capable of delivering different therapeutic agents and characterized by their flexibility in terms of design modification [68]. Polymeric micelles have core–shell-type NPs formed through the self-assembly of block copolymers, and which have a controllable size range of 10–100 nm [69]. Biodegradable polyesters such as PCL, poly (D,L-lactide) (PDLA), and poly (D,L-lactide-co-glycolide) (P(DLA-co-G) are commonly used to form the core which helps to prolong the half-life of the loaded drugs for more than 10 h [44][70]. Dendrimers are small particles with sizes less than 12 nm, composed of repeating monomeric or oligomeric units with an internal cavity surrounded by reactive terminal groups [71]. Dendrimers are used to encapsulate different drug agents, such as siRNA, and are highly efficient for BBB crossing, but are toxic to normal tissue because of their interactions with the cell membrane and their less controllable release behavior [72].
The use of nanocarriers has been well established over the past decade both in pharmaceutical research and clinical settings to enhance the in vivo treatment efficiency [73]. For GBM, such treatments are in development as well.
-
Lipid-Based Nanocarriers for Glioblastoma Treatment
Lipid based NPs have been extensively used to target GBM. They can efficiently encapsulate multiple drugs that act synergistically to kill GBM cells or drugs with poor physicochemical properties (e.g., poor water-soluble drugs). For example, in a study conducted by Zhang et al., glucose-functionalized liposomes (gLTP) that co-load TMZ and pro-apoptotic peptide (PAP) can cross the BBB through the glucose–GLUT1 pathway to deliver these drugs to the tumor site. PAP affects the mitochondria reducing ATP generation, while enhancing the sensitivity of GBM cells to TMZ [74]. In the same way, Papachristdoulou et al. delivered, via liposomes, O6-(4-bromothenyl) guanine derivates (O6BTG) targeting MGMT to enhance TMZ efficacy in vitro [75]. The magnetic resonance image-guided microbubble was also used to enhance the low-intensity pulsed focused ultrasound that permits the opening of the BBB and better deliver the liposomes [75]. The O6BTG-liposomes combined with TMZ reduced the tumor growth and increased mice survival [75].
Among many types of lipid-based nanoparticles, NLCs and SLNs gained an extreme focus for developing GBM treatments, especially in the delivery of GBM-targeted ACA [44][76][77]. Song et al. prepared dual-ligand-commodified NLCs using both lactoferrin, a member of the Tf family, and RGD peptides recognized by integrins overexpressed in GBM cells. They demonstrated that the use of these dual-ligand-comodified NLCs (139 nm) for TMZ and vincristine delivery induce a higher cytotoxic effect in vitro on human GBM cells (U87MG cells) compared to single-drug-loaded NLCs or free drugs. The same results were gained in vivo on the inhibition of tumor growth in U87 MG cell-bearing nude mice [78]. Furthermore, the size of the tumor treated by dual lactoferrin/RGD-NLCs was reduced compared to the tumor treated by single-ligand NLCs [78]. The use of dual ligands improves the GBM cell targeting in the brain. However, the lipid composition of NLCs can also greatly influence the ability of the liposome to cross the BBB and target GBM cells. Zwain et al. prepared NLCs using four liquid lipids alone or in combination (propylene glycol monolaurate, propylene glycol monocaprylate, caprylocaproylmacrogol-8-glycerides, and/or polyox-yl-15-hydroxystearate) to encapsulate the poorly water-soluble docetaxel (DTX), also known as Taxotere®. They observed that NLCs composed of the four lipids had not only the highest drug loading (almost 89%), but they also crossed the BBB in the in vitro model without the loss of the barrier integrity [79]. These NLCs, with an average particle size of 123.3 nm, were internalized more efficiently by U87MG cells compared with non-cancerous cells. They were also more efficient to reduce the size of U87MG spheroids than the free drug, by inhibiting both the cell cycle via the G2/M phase and mitochondria activity [79]. Another strategy to target GBM cells is the use of antibodies against specific growth factor receptors such as the epidermal growth factor receptor (EGFR). For example, carmustine-loaded cationic SLNs grafted with an anti-EGFR permit and affective delivery of the drug resulting in an antiproliferative efficacy against the tumor growth [80].
SLNs and NLCs are effective as drug carriers for GBM. However, despite all the promising results in the literature, none of these carriers have been successfully developed by a pharmaceutical company. Therefore, more efforts should be focused on the development of reproducible nanocarriers.
-
Polymeric-Based Nanocarriers for Glioblastoma Treatment
Due to their biocompatibility, low toxicity, and biodegradability, polymers offer many advantages [81]. Han et al., used paclitaxel (Taxol®)-loaded into dextran NPs coated with RVG29 peptide for targeted chemotherapy in glioma [82]. RVG29 is a peptide with a high affinity to the nicotinic acetylcholine receptor (nAchR) on neuronal cells and is highly implicated in drug resistance [82]. The use of NPs either in vitro or in vivo exhibited a higher cell growth inhibition rate against C6 cells compared with the non-grafted NPs [82]. The knowledge, these polymers are not used much in the context of GBM treatment compared to other cancers such as breast cancer [83], cervical cancer [84], and lung cancer [85].
On the other side, PLGA, PLA, and PGA-based nanocarriers are the most extremely used polymers in brain delivery [86]. In the context of GBM, these nanocarriers are mainly used for the encapsulation of chemotherapeutic molecules to control their release. For example, DOX-loaded PLGA-PEG NPs with a size around 200 nm were prepared for the in vitro study of the drug kinetic release, no burst release was observed, and a sustained release was maintained for up to 96 h [87]. Ramalho et al. used PLGA-NPs functionalized with OX26, a monoclonal antibody for a transferrin receptor, and loaded with TMZ for targeting U215 and U87 cell lines. The NPs showed an encapsulation efficiency of 48% and a size of 194 nm, both free and encapsulated TMZ induced a decrease of cell growth in the studied lines, but the use of NPs exhibits a longer and stronger action on the cells [88]. Caban-Toktas et al. studied paclitaxel co-loaded in PLGA NPs with R-Flurbiprofen, a nonsteroidal anti-inflammatory drug known for its strong anticancer activity [89]. In the same study, chitosan-modified PLGA NPs were also co-loaded with paclitaxel and R-Flurbiprofen for an efficient delivery to the tumor site [89]. Sixty percent of the paclitaxel was released from the NPs for five days until reaching the pseudo-plateau. On the other hand, R-flurbiprofen was released quickly in the firsts 6 h. Additionally, the NPs showed efficient cytotoxic activity and were well integrated by the tumor cells, resulting in anti-tumoral activity against glioma [89]. The in vivo studies confirmed that the use of paclitaxel-loaded NPs with R-flurbiprofen-loaded NPs induces a significantly higher reduction of the tumor compared to when the drug-NPs are used individually [89].
Due to the protection they provide, NPs can also be used to encapsulate and deliver peptides and small proteins, such as CXCL12, in the aim of controlling GBM cell migration as a therapeutic approach. Researcher's team strongly believe that the control of GBM cells migration via the CXCL12–CXCR4 axis can be a promising approach to rule the spread of those cells and facilitate their elimination. For this aim, researchers have developed composite alginate–chitosan NPs with an average size of 250 nm for CXCL12 encapsulation which, upon its release, increases GBM cell migration [90]. Three initial mass loadings were tested (0.372 µg/mg NPs, 0.744 µg/mg NPs, and 1.490 µg/mg NPs). The results showed that the alginate–chitosan NPs entrapped CXCL12 with a percentage of ~98% without loss of the molecule [90]. For all the conditions tested, a burst released in the first 2 h was observed, followed by a sustained release that reach a pseudo-plateau after 72 h without a complete release of the chemokine [90]. The releasing profile observed was coherent with a diffusion-based system which led researchers to evaluate the driving mass transport phenomenon [90]. Using the experimental data, researchers performed mathematical modeling using Fick’s second law of diffusion for a spherical geometry, which considered the size distribution of NPs (class method) and boundary conditions that allowed researchers to model the interactions between the CXCL12 molecules and the NPs. The cumulative mass release vs. time and position equation was solved using a finite difference approach and mechanistic parameters (effective diffusion coefficient, Deff; overall mass transfer coefficient at the surface, k) which were estimated using an evolutionary algorithm, leading to coefficients of a determination > 0.97 [90]. Small values of Deff (~2 × 10−19 m2/s) were obtained, which can be associated with the presence of electrostatic interactions between the positive charge of CXCL12 and the negative one of the alginates composing the NPs [90]. However, since the experiments were conducted in static conditions, it is impossible to deny that in vivo, other types of mass transport phenomena may occur, such as convective interstitial brain fluid flow, that may be a reason for an increased releasing rate. Furthermore, the migration assays proved that CXCL12 significantly controlled the invasion of the F98 cell line, which highlights the importance of using CXCL12 in delivery systems for GBM targeting [90].
NPs provide a series of advantages for delivery applications to enhance the therapeutic efficiency of the drugs, but the major challenge remaining is the transport to the tumor site without degradation [91]. Despite all the advantages of systemic drug delivery across the BBB, systemic delivery needs to address different challenges for further improvement. The long distance between the delivery route and the target site and the drug digestion remain the biggest limitations of systemic delivery.
2.2. Local Delivery
Local delivery consists of delivering chemotherapeutic agents in the surgical cavity after the tumor resection for GBM therapy improvement [92]. A series of factors make local delivery advantageous in GBM treatment [93]. Metastasis occurs within approximately ̴2 cm of the tumor’s original site, which means it is close to where the drugs were loaded locally; the administration of the drugs will no longer face the BBB limitations [93]. Different strategies for local delivery to improve the GBM survival rate have been developed, including intranasal drugs delivery, convection enhanced delivery, and direct injection of the drugs including rigid implants and hydrogels [94].
-
Intranasal Drugs Delivery:
The intranasal route can bypass the BBB and achieve reaching the brain for the delivery of drugs. It is important to understand the anatomy of the nasal cavity and the mechanisms of compound transport through the intranasal route. Bruinsmann et al., described this aspect in detail [95]. In this paragraph, researchers focus on discussing the different GBM treatments via the intranasal approach to deliver drugs to the tumor.
Blacher et al. used “anthranoid 4,5-dihydroxyanthraquinone-2-carboxylic acid”, also known as rhein, to inhibit CD38 by intranasal injection in mice. CD38 deficiency is known to regulate microglial activation and attenuates glioma progression [96][97][98]. The tumor in the mice treated decreased, concluding that the intranasal drugs’ administration is effective and rhein can be a therapeutic target in GBM [96]. Li et al. used intranasal drug delivery to administer TMZ in a rat model bearing orthotopic C6 glioma xenografts showing a significantly reduction in the tumor growth compared with intravenous injection or gavage [99]. The results suggest that the intranasal route should be further considered as an option for TMZ delivery into the brain [99]. However, different therapeutic agents are under investigation for GBM treatment, but the use of a delivery system has been proved to be more beneficial in term of maintaining the drug release.
NPs are also good vehicles to control the drug release and overcome some limitations of the intranasal drug delivery, such as the poor capacity of crossing the nasal mucosa and enzymatic degradation [95]. Polymeric NPs (PLGA-based [95][100], PCL-based [101][102]) and lipid-based NPs are the most commonly used for nose-to-brain delivery [44][103]. For instance, PLGA and oligomeric chitosan composite NPs were designed to co-deliver alpha-cyano-4-hydroxycinnamic acid (CHC) and the monoclonal antibody cetuximab (CTX) into the brain by nasal administration to ensure the therapeutic efficacy for GBM treatment [100]. CHC and CTX are known to have a therapeutic effect against angiogenesis, cancer cell invasion, and metastasis [100]. In vitro assays using a chicken chorioallantoic membrane assay showed no reduction of cell viability for U251 and SW1088 glioma cell lines, but the designed NPs showed a stability that reached three months and a high encapsulation of the drugs was reached [100]. More recently, they designed a new platform using PLGA and chitosan composite NPs to carry CHC. CHC-NPs were covalently coated with CTX. An ex vivo study using a porcine mucosa demonstrated the capacity of the NPs to promote CHC and CTX permeation, whereas the chicken chorioallantoic membrane assay demonstrated its capacity to reduce the tumor size [104]. Sousa et al. also used PLGA-based NPs to administer the monoclonal antibody bevacizumab, an anti-VEGF used as an anti-cancer drug, intranasally in mice [105]. The use of bevacizumab-loaded NPs when administered intranasally into CD-1 mice showed higher brain bioavailability compared to the free drug. Furthermore, used in a GBM nude mouse model, the NPs-based delivery system also induced a reduction in the tumor growth after 14 days, with a high anti-angiogenic effect of bevacizumab compared to free drug administration [105]. PCL-based NPs have also been used to route drugs addressing the brain tissue intranasally. De Oliveira et al. used PCL based NPs loaded with melatonin to target the U87 MG GBM cell line [102]. The NPs revealed to be non-cytotoxic on healthy cells (MRC-5) and increased the water solubility of the drug in addition to promoting strong activity against U87 MG cells. In vivo assays in rats through intranasal injection increased the drug uptake in the brain compared to when administrated directly without nanocarriers [102]. Conversely, Alex et al. develop PCL-based NPs to encapsulate the anticancer drug carboplatin to target GBM via the nasal route [101]. The optimized formulation was a 311.6 nm particle size, and they observed a burst release of the drug followed by a slow continued release. However, ex vivo permeation studies through sheep nasal mucosa showed a lower drug permeation, which was attributed to the nasal mucosa complexity. While improving the delivery and accumulation of drugs to the brain, those results highlight the complexity of the nose-to-brain route. Despite all the advantages given by intranasal delivery, the low volumes of the drugs delivered remain the main problem that limits its use.
-
Convection Enhanced Delivery:
Convection enhanced delivery (CED) is a local therapeutic method that aims to enhance intracerebral drugs diffusion to the CNS by bypassing the BBB, allowing the introduction of high doses of therapeutic agents with different ranges of molecular weight through the interstitial spaces [106]. CED is based on the principles of “bulk flow” which refer to the extracellular flow of fluid delivered via a pressure gradient rather than the normal passive diffusion transport [106]. CED fundamental procedures consist of the stereotactic placement of a microcatheter directly into the tumor and generating an external pressure using a motor-driven pump to induce fluid convection in the brain [107]. CED permits a deeper penetration and distribution of the drugs, eliminating the problem of depletion frequently seen using the direct injection [108]. In addition, CED is used even with agents with a high molecular weight, including proteins, nucleic acids, and antibodies [109][110].
Several studies have proved the safety and feasibility of CED [107][111][112]. Additionally, drug encapsulation into nano-sized carriers proved to be more beneficial to increase the efficiency of delivery. For instance, Séhédic et al. developed lipid nanocapsules (LNCs) to incorporate radionuclides and implant them in the brain using stereotactic injections for locoregional therapy [113]. Using the CED, they demonstrated that lipophilic thiobenzoate complexes of rhenium-188 loaded in LNCs (LNC188Re) with a function-blocking antibody (12G5) directed at the CXCR4 on its surface enhance the median survival and show major clinical improvement in Scid mice [113]. The retention of rhenium in the brain and the outcomes achieved (distribution, efficacy, gradient) were principally ensured by LNCs, which accentuate the interest of using nanocarriers. Zhang et al. used cisplatin-loaded NPs of 70 nm in diameter functionalized with PEG for administration by CED to control the release of cisplatin and kill the tumor cells that they reach without causing toxicity [114]. Their small size and dense PEG corona prevented them from being trapped as they moved within the brain tissue while controlling the delivery of the drug, making them efficient brain-penetrating drug delivery vehicles. Their results also showed a significant increase in the survival rate of a GBM rat brain tumor model, thus highlighting the advantages of using NPs. CED, combined with the delivery of the favorable physicochemical properties ensured by NPs, has demonstrated a great potential to improve clinical outcomes. For instance, Stephen et al. used magnetic NPs coated with a chitosan-PEG copolymer to deliver MGMT inhibitor O6-benzylguanine via CED for GBM targeting as a treatment for GBM patients showing resistance to TMZ [115]. They showed that the distribution of the NPs in the mice’s brains was excellent, whereas the activity of MGMT decreased significantly, which, in the presence of TMZ, increased the median survival rate [115]. In another study, Chen et al. proved that the nanoliposomal formulation of irinotecan with CED technology enhanced the survival time of the treated mice when combined with radiation, as compared with the systemic injection of irinotecan plus radiotherapy [116]. CED for GBM treatment has been also reported in clinical trials. Cruickshank et al. injected irinotecan drug-loaded beads suspended in an alginate solution into patients after surgical resection. Studies are still under investigation, however, only one patient has died, due to causes which were not associated with the treatment, thus suggesting that the use of irinotecan drug-loaded beads may be a promising, stable, and safe platform to assess the local delivery of new agents [117]. All these studies focus on combining CED with nanocarriers for a better control of the drug release in the aim of increasing survival. Despite the promising results achieved, different physical and technical limitations and challenges are still to be overcome. The main obstacle occurring is the backflow, sometimes referred as reflux, that takes place when the perfusate is not well penetrated in the tissues [106]. Hopefully, backflow resistant catheters have been developed, which may solve this issue.
-
Direct Drug Injection:
The direct drug injection for the delivery of ACA within the tumor resection cavity emerged to resolve the bypassing BBB limitations and increase the drugs’ concertation in the tumor site. This method has lot of advantages, including side effects reduction, safe administration of different molecules, and the depletion of toxicity actions [118].
-
Rigid Implants:
The direct drug injection opens the doors for local implant-based GBM treatments. The only local delivery system approved by the FDA and currently used for GBM treatment is the polifeprosan 20 with the carmustine-loaded wafer Gliadel® [119]. Gliadel® wafers are 14.5 mm in diameter and 1 mm in thickness, the wafer is made from a biodegradable hydrophobic co-polymer 1,3-bis-(p-carboxyphenoxy)propane (pCPP) and sebacic acid [119]. When in contact with aqueous fluids, the wafers start releasing the carmustine into the surrounding tissue [120]. After approving Gliadel® wafers, several chemotherapeutic agents have been tested in preclinical models such as paclitaxel [121], acriflavine [122], and DOX [123]. Due to the rigidity of the device compared with the soft nature of the brain tissue, different adverse reactions occur such as necrosis, infection, and convulsions [122]. The main problem remaining using these wafers is the drug release profile. For this, many studies have explored the possibility of reducing the burst release profile and sustaining the release for a long period [94][124][125]. For example, Shapira-Furman et al. used Gliadel wafers co-loaded with 50% w/w of TMZ and BCNU in PLGA for sustaining the release of the drugs for four weeks [126]. The drugs were first coated with the polymer to form core–shell particles, in which the coating shell served as a membrane for the drug particles [126]. The median survival was 15 days in the group treated with BCNU wafers alone, whereas the group with TMZ wafers alone had a median survival of 19 days [126]. The group treated with combined BCNU and TMZ wafers had a median survival of 28 days, suggesting that the combination of drugs can achieve a big improvement for local drug delivery [126]. However, rapid drug release, cell migration, drug resistance, and side effects are different problems that prevent Gliadel wafers from being an effective option to treat GBM.
-
New Innovative Drug-Delivery Approaches in Glioblastoma Treatments:
-
Hydrogels:
Hydrogels can be defined as 3D polymeric hydrophilic networks within an aqueous medium [127]. Due to their ability to encapsulate different agents and control their release, hydrogels are used for different biomedical applications and medicine such as artificial skin, membranes for biosensors, 3D platforms, and drug delivery devices [128][129][130]. Further, the use of hydrogels can be more beneficial than the Gliadel wafers because of their ability to mimic the mechanical properties and the softness of the brain tissue. For instance, Wang et al. have shown, using PEG-based hydrogels bearing GRGDS adhesion peptides and U87 human GBM cells, that matrix stiffness induces differential GBM cell proliferation, morphology, and migration [131]. Increasing the matrix stiffness (associated with tumor-like mechanical properties) led to delayed U87 cell proliferation, but the authors observed that cells formed denser spheroids with extended cell protrusions.
Various studies explored the use of hydrogels for GBM treatments in this context [131][132][133][134]. Bastiancich et al. reviewed the different types of hydrogels as drug delivery systems for GBM local treatment recently used in preclinical and clinical studies, suggesting that loaded hydrogels with one or many chemotherapeutic agents are advantageous for GBM treatment [135]. Hydrogels fill the gap between the tumor resection and the administration of chemotherapy and radiotherapy, allowing a sustained release of the drugs, which may lead to better results than a conventional CED approach [135]. Akbar et al. developed a biodegradable hydrogel from PLGA:plasticizers with a ratio of 40:60 for TMZ delivery in C6 glioma rats [136]. A significant reduction of the tumor was observed, and no mortality was associated with the gel matrix treatment, concluding that the gels can be safe and effective when used in vivo [136]. In another recent study, hydrogel loaded with the quisinostat drug and radiopaque gold NPs (AuNP) has been explored in GBM. Radiopaque NPs were used as the contrast agent that would release the drugs when irradiated. The release of quisinostat in vitro was high, which inhibited the tumor growth in the in vivo mice model bearing xenografted human GBM tumors [137]. The platform developed can also be used simultaneously for radiation therapy. OncoGel™ is a PLGA-PEG-based thermo-sensitive hydrogel used for paclitaxel delivery in GBM treatment, which has been shown to prolong the survival in a rodent glioma model [138]. OncoGel™ provides a sustained release of paclitaxel for 50 days, maintaining a high local concentration and biodegrades after four to six weeks [139]. In 2007, the first clinical trial (NCT00479765) using OncoGel™ for recurrent glioma in order to evaluate the safety and tolerability of the system in the patients started, but could not be ended for sponsor businesses [135]. Déry et al. used a biodegradable hydrogel (GlioGel) loaded with three chemoattractants (CXCL10, CCL2, and CCL11) to attract murine F98 and U87 GBM cells toward a therapeutic trap using an agarose drop assay [140]. The zones with high concentrations of CXCL10 display the highest number of the cells attracted compared to the control due to the chemoattractant gradient. CCL2 showed a very similar response to CXCL10 for the F98 cells, but the U87MG cells were less responsive, and both cells did not show any significant effect on chemotaxis to CCL11 [140]. The team performed in vivo assays using an orthotopic syngeneic F98-Fischer rat model. Three days after the implantation of the F98 tumor cells, the GlioGels containing the chemoattractant were inoculated. Many peritumoral clusters were observed when CXCL10, CCL2, and CCL11 were implanted in the controlateral hemisphere compared to those implanted in ipsilateral hemisphere [140]. These results support the hypothesis that the use of the GlioGel with chemokines can modify the migration behavior of the GBM cells.
Nonetheless, for a sustained controlled release of chemotherapeutic drugs, nanocarriers can be confined in a hydrogel. Several reviews have highlighted that a better controlled release can be obtained when NPs are loaded in hydrogels [141][142]. For instance, Brachi et al. have shown that a multi-component system composed of polymeric NPs BODIPY-loaded and embedded within a thermosensitive hydrogel, revealed to be more efficient in terms of drug retention within the tumor in an orthotopic GBM mice model compared to NPs alone [143]. Zhao et al. used PLGA NPs for paclitaxel encapsulation, then loaded them into photopolymerizable hydrogels that had been implanted in the resection cavity [144]. They found that the system enhanced long-term survival (<150 days) in the U87 cells in vivo mice model compared with the mice where the hydrogels were implanted empty (they tolerated the hydrogels and had a long healthy life for up to four months) [144]. Furthermore, for biocompatibility, biodegradability, a better control of the gelation time, and a sustained release of hydrophobic and hydrophilic drugs, some prepolymer hydrogels have shown remarkable results, but still need to be investigated in GBM [145][146][147]. However, because of the many advantages of combining NPs to hydrogels, this approach can also be transposed to the development of a chemoattractant releasing device as part of cancer cell traps.
-
Cancer Cells Trap:
Since the main cause of GBM recurrence is the infiltrated GBM cells that migrate from the tumor, as discussed earlier, researchers propose to inverse the direction of GBM cell migration towards a well confined area in which they will be trapped and can be eliminated with localized radiotherapy using a chemoattractant gradient of CXCL12 (Figure 2).
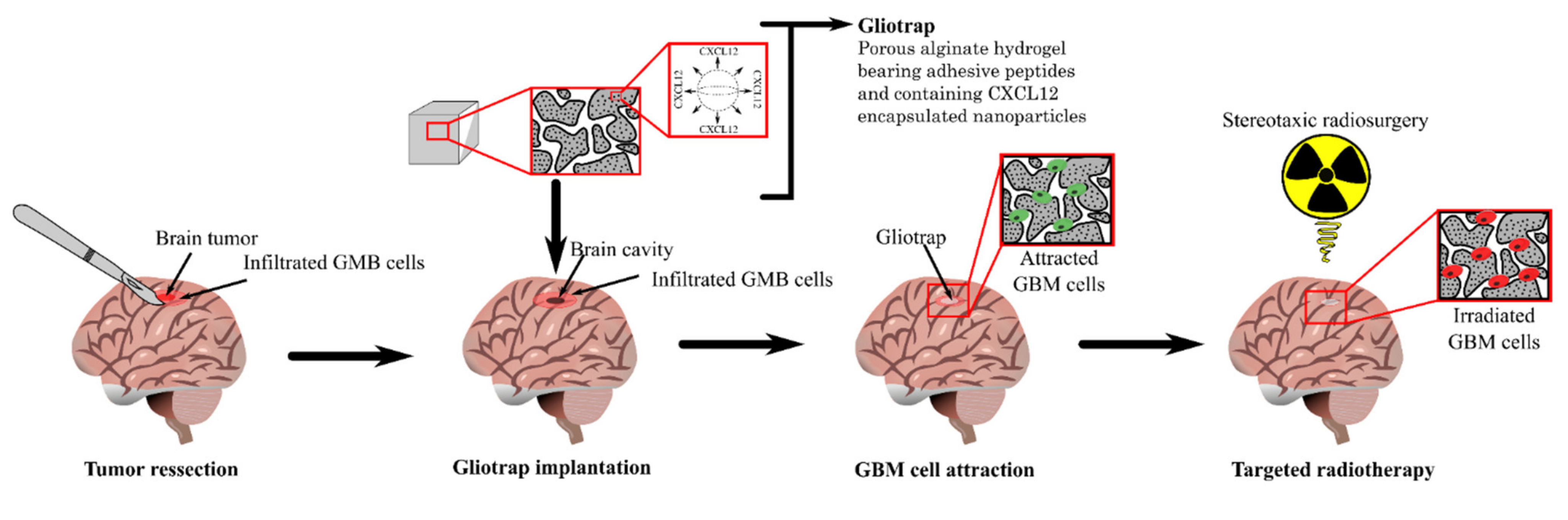
Figure 2. The strategy proposed by researcher's team for GBM treatment.
The device proposed takes the name of gliotrap (GBM-trapping), and it combines an alginate macroporous hydrogel functionalized with RGD peptides (for cells catching) and alginate–chitosan composite NPs encapsulating CXCL12 (for cells attraction). The functionalization of the hydrogel with RGD peptides aims to promote the GBM cell adhesion inside the matrices via the interactions between the peptides and the αvβ3 and αvβ5 integrins widely expressed by those cells, as discussed above [148]. Hydrogels will be loaded with the NPs’ delivery system and implanted into the surgical cavity of the tumor after its resection to ensure a CXCL12 gradient maintained for a very long period. The NPs are designed to promote a controlled release of CXCL12 and so create a cancer cell attracting gradient. This will help to maintain the CXCL12 gradient under a fast release that could happen because of the fluid flow in the brain.
References
- Yamamoto, M.; Curiel, D.T.T.; Ph, D.; South, S.; South, S. Cancer gene therapy. Technol. Cancer Res. Treat. 2005, 4, 315–330.
- Oka, N.; Soeda, A.; Inagaki, A.; Onodera, M.; Maruyama, H.; Hara, A.; Kunisada, T.; Mori, H.; Iwama, T. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2007, 360, 553–559.
- Triozzi, P.L.; Borden, E.C. VB-111 for cancer. Expert Opin. Biol. Ther. 2011, 11, 1669–1676.
- Arend, R.C.; Beer, H.M.; Cohen, Y.C.; Berlin, S.; Birrer, M.J.; Campos, S.M.; Rachmilewitz Minei, T.; Harats, D.; Wall, J.A.; Foxall, M.E.; et al. Ofranergene obadenovec (VB-111) in platinum-resistant ovarian cancer; favorable response rates in a phase I/II study are associated with an immunotherapeutic effect. Gynecol. Oncol. 2020, 157, 578–584.
- Przystal, J.M.; Waramit, S.; Pranjol, M.Z.I.; Yan, W.; Chu, G.; Chongchai, A.; Samarth, G.; Olaciregui, N.G.; Tabatabai, G.; Carcaboso, A.M. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 2019, 11, e8492.
- Tang, X.; Peng, H.; Xu, P.; Zhang, L.; Fu, R.; Tu, H.; Guo, X.; Huang, K.; Lu, J.; Chen, H. Synthetic mRNA-based gene therapy for glioblastoma: TRAIL-mRNA synergistically enhances PTEN-mRNA-based therapy. Mol. Ther. 2022, 24, 707–718.
- Tang, X.; Zhang, S.; Fu, R.; Zhang, L.; Huang, K.; Peng, H.; Dai, L.; Chen, Q. Therapeutic prospects of mRNA-based gene therapy for glioblastoma. Front. Oncol. 2019, 9, 1208.
- Long, Y.; Tao, H.; Karachi, A.; Grippin, A.J.; Jin, L.; Chang, Y.E.; Zhang, W.; Dyson, K.A.; Hou, A.Y.; Na, M. Dysregulation of glutamate transport enhances treg function that promotes VEGF blockade resistance in glioblastoma. Cancer Res. 2020, 80, 499–509.
- Tian, R.-F.; Li, X.-F.; Xu, C.; Wu, H.; Liu, L.; Wang, L.-H.; He, D.; Cao, K.; Cao, P.-G.; Ma, J.K. SiRNA targeting PFK1 inhibits proliferation and migration and enhances radiosensitivity by suppressing glycolysis in colorectal cancer. Am. J. Transl. Res. 2020, 12, 4923.
- Yang, B.; Hao, A.; Chen, L. Mirror siRNAs loading for dual delivery of doxorubicin and autophagy regulation siRNA for multidrug reversing chemotherapy. Biomed. Pharmacother. 2020, 130, 110490.
- Önay Uçar, E.; Şengelen, A. Resveratrol and siRNA in combination reduces Hsp27 expression and induces caspase-3 activity in human glioblastoma cells. Cell Stress Chaperones 2019, 24, 763–775.
- Zhang, C.; Yuan, W.; Wu, Y.; Wan, X.; Gong, Y. Co-delivery of EGFR and BRD4 siRNA by cell-penetrating peptides-modified redox-responsive complex in triple negative breast cancer cells. Life Sci. 2021, 266, 118886.
- Mirzaei, S.; Mahabady, M.K.; Zabolian, A.; Abbaspour, A.; Fallahzadeh, P.; Noori, M.; Hashemi, F.; Hushmandi, K.; Daneshi, S.; Kumar, A.P.; et al. Small interfering RNA (siRNA) to target genes and molecular pathways in glioblastoma therapy: Current status with an emphasis on delivery systems. Life Sci. 2021, 275, 119368.
- Huang, W.; Liang, Y.; Sang, C.; Mei, C.; Li, X.; Chen, T. Therapeutic nanosystems co-deliver anticancer drugs and oncogene SiRNA to achieve synergetic precise cancer chemo-gene therapy. J. Mater. Chem. B 2018, 6, 3013–3022.
- Danhier, F.; Messaoudi, K.; Lemaire, L.; Benoit, J.-P.; Lagarce, F. Combined anti-Galectin-1 and anti-EGFR siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int. J. Pharm. 2015, 481, 154–161.
- Egorova, A.; Shubina, A.; Sokolov, D.; Selkov, S.; Baranov, V.; Kiselev, A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int. J. Pharm. 2016, 515, 431–440.
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249.
- Maxwell, R.; Jackson, C.M.; Lim, M. Clinical Trials Investigating Immune Checkpoint Blockade in Glioblastoma. Curr. Treat. Options Oncol. 2017, 18, 51.
- Romani, M.; Pistillo, M.P.; Carosio, R.; Morabito, A.; Banelli, B. Immune Checkpoints and Innovative Therapies in Glioblastoma. Front. Oncol. 2018, 8, 464.
- Lukas, R.V.; Rodon, J.; Becker, K.; Wong, E.T.; Shih, K.; Touat, M.; Fassò, M.; Osborne, S.; Molinero, L.; O’Hear, C. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J. Neuro-Oncol. 2018, 140, 317–328.
- Gardell, J.L.; Matsumoto, L.R.; Chinn, H.; DeGolier, K.R.; Kreuser, S.A.; Prieskorn, B.; Balcaitis, S.; Davis, A.; Ellenbogen, R.G.; Crane, C.A. Human macrophages engineered to secrete a bispecific T cell engager support antigen-dependent T cell responses to glioblastoma. J. Immunother. Cancer 2020, 8, e001202.
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 2017, 68, 139–152.
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984.
- Polyzoidis, S.; Ashkan, K. DCVax®-L—Developed by northwest biotherapeutics. Hum. Vaccin. Immunother. 2014, 10, 3139–3145.
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142.
- Jain, K.K. A critical overview of targeted therapies for glioblastoma. Front. Oncol. 2018, 8, 419.
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161.
- Gopalan, D.; Pandey, A.; Udupa, N.; Mutalik, S. Receptor specific, stimuli responsive and subcellular targeted approaches for effective therapy of Alzheimer: Role of surface engineered nanocarriers. J. Control Release 2020, 319, 183–200.
- Angeli, E.; Nguyen, T.T.; Janin, A.; Bousquet, G. How to make anticancer drugs cross the blood–brain barrier to treat brain metastases. Int. J. Mol. Sci. 2019, 21, 22.
- Wang, C.; Li, K.; Li, T.; Chen, Z.; Wen, Y.; Liu, X.; Jia, X.; Zhang, Y.; Xu, Y.; Han, M.; et al. Monocyte-mediated chemotherapy drug delivery in glioblastoma. Nanomedicine 2017, 13, 157–178.
- Anselmo, A.C.; Mitragotri, S. Cell-mediated delivery of nanoparticles: Taking advantage of circulatory cells to target nanoparticles. J. Control. Release 2014, 190, 531–541.
- Su, Y.; Xie, Z.; Kim, G.B.; Dong, C.; Yang, J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS Biomater. Sci. Eng. 2015, 1, 201–217.
- Ehtesham, M.; Kabos, P.; Gutierrez, M.A.R.; Chung, N.H.C.; Griffith, T.S.; Black, K.L.; Yu, J.S. Induction of Glioblastoma Apoptosis Using Neural Stem Cell-mediated Delivery of Tumor Necrosis Factor-related Apoptosis-inducing Ligand1. Cancer Res. 2002, 62, 7170–7174.
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091.
- Liao, W.; Fan, S.; Zheng, Y.; Liao, S.; Xiong, Y.; Li, Y.; Liu, J. Recent advances on glioblastoma multiforme and nano-drug carriers: A review. Curr. Med. Chem. 2019, 26, 5862–5874.
- Reis, C.P.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21.
- Frellsen, A.F.; Hansen, A.E.; Jølck, R.I.; Kempen, P.J.; Severin, G.W.; Rasmussen, P.H.; Kjær, A.; Jensen, A.T.I.; Andresen, T.L. Mouse Positron Emission Tomography Study of the Biodistribution of Gold Nanoparticles with Different Surface Coatings Using Embedded Copper-64. ACS Nano 2016, 10, 9887–9898.
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893.
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103.
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169.
- Shu, X.Z.; Zhu, K.J. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. Int. J. Pharm. 2000, 201, 51–58.
- Jo, D.H.; Kim, J.H.J.H.; Lee, T.G.; Kim, J.H.J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611.
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142.
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: An update. Cancers 2021, 13, 195.
- Paroha, S.; Chandel, A.K.S.; Dubey, R.D. Nanosystems for drug delivery of coenzyme Q10. Environ. Chem. Lett. 2018, 16, 71–77.
- Paroha, S.; Chandel, A.K.S.; Dubey, R.D. Nanotechnology delivery systems of coenzyme Q10: Pharmacokinetic and clinical implications. In Nanoscience in Food and Agriculture 4; Springer: Cham, Switzerland, 2017; pp. 213–228.
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted nanotechnology in glioblastoma multiforme. Front. Pharmacol. 2017, 8, 166.
- Hosseini, M.; Haji-Fatahaliha, M.; Jadidi-Niaragh, F.; Majidi, J.; Yousefi, M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1051–1061.
- Gonçalves, C.; Ramalho, M.J.; Silva, R.; Silva, V.; Marques-Oliveira, R.; Silva, A.C.; Pereira, M.C.; Loureiro, J.A. Lipid Nanoparticles Containing Mixtures of Antioxidants to Improve Skin Care and Cancer Prevention. Pharmaceutics 2021, 13, 2042.
- Patidar, A.; Thakur, D.S.; Kumar, P.; Verma, J. A review on novel lipid based nanocarriers. Int. J. Pharm. Pharm. Sci. 2010, 2, 30–35.
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Smart Drug Delivery Systems in the Treatment of Glioblastoma Multiforme. Pharmaceutics 2020, 12, 860.
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Marchenko, Y.Y.; Dobrodumov, A.V.; Mikhrina, A.L.; Martynova, M.G.; Bystrova, O.A.; Yakovenko, I.V.; Ischenko, A.M. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int. J. Nanomedicine 2014, 9, 273–287.
- Shi, Y. Self-Assembled Gold Nanoplexes for Cancer-Targeted SiRNA Delivery. Master’s Thesis, University of Southern Mississippi, Hattiesburg, MS, USA, 2014. Available online: https://aquila.usm.edu/cgi/viewcontent.cgi?article=1052&context=masters_theses (accessed on 30 April 2022).
- Guglielmelli, A.; Rosa, P.; Contardi, M.; Prato, M.; Mangino, G.; Miglietta, S.; Petrozza, V.; Pani, R.; Calogero, A.; Athanassiou, A. Biomimetic keratin gold nanoparticle-mediated in vitro photothermal therapy on glioblastoma multiforme. Nanomedicine 2020, 16, 121–138.
- Liu, D.; Cheng, Y.; Cai, R.; Wang, W.; Cui, H.; Liu, M.; Mei, Q.; Zhou, S. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 991–1003.
- Zhu, X.; Zhou, H.; Liu, Y.; Wen, Y.; Wei, C.; Yu, Q.; Liu, J. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018, 82, 143–157.
- Taghipour-Sabzevar, V.; Sharifi, T.; Moghaddam, M.M. Polymeric nanoparticles as carrier for targeted and controlled delivery of anticancer agents. Ther. Deliv. 2019, 10, 527–550.
- Shi, J.; Xiao, Z.; Kamaly, N.; Farokhzad, O.C. Self-assembled targeted nanoparticles: Evolution of technologies and bench to bedside translation. Acc. Chem. Res. 2011, 44, 1123–1134.
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 1–29.
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102.
- Haji Mansor, M.; Najberg, M.; Contini, A.; Alvarez-Lorenzo, C.; Garcion, E.; Jérôme, C.; Boury, F. Development of a non-toxic and non-denaturing formulation process for encapsulation of SDF-1α into PLGA/PEG-PLGA nanoparticles to achieve sustained release. Eur. J. Pharm. Biopharm. 2018, 125, 38–50.
- Pourgholi, F.; Hajivalili, M.; Farhad, J.-N.; Kafil, H.S.; Yousefi, M. Nanoparticles: Novel vehicles in treatment of Glioblastoma. Biomed. Pharmacother. 2016, 77, 98–107.
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450.
- Rescignano, N.; Fortunati, E.; Armentano, I.; Hernandez, R.; Mijangos, C.; Pasquino, R.; Kenny, J.M. Use of alginate, chitosan and cellulose nanocrystals as emulsion stabilizers in the synthesis of biodegradable polymeric nanoparticles. J. Colloid Interface Sci. 2015, 445, 31–39.
- Allouss, D.; Makhado, E.; Zahouily, M. Recent Progress in Polysaccharide-Based Hydrogel Beads as Adsorbent for Water Pollution Remediation. Funct. Polym. Nanocomposites Wastewater Treat. 2022, 323, 55–88.
- He, W.; Hosseinkhani, H.; Mohammadinejad, R.; Roveimiab, Z.; Hueng, D.; Ou, K.; Domb, A.J. Polymeric nanoparticles for therapy and imaging. Polym. Adv. Technol. 2014, 25, 1216–1225.
- Elzoghby, A.O.; Abd-Elwakil, M.M.; Abd-Elsalam, K.; Elsayed, M.T.; Hashem, Y.; Mohamed, O. Natural polymeric nanoparticles for brain-targeting: Implications on drug and gene delivery. Curr. Pharm. Des. 2016, 22, 3305–3323.
- Morshed, R.; Cheng, Y.; Auffinger, B.; Wegscheid, M.; Lesniak, M.S. The potential of polymeric micelles in the context of glioblastoma therapy. Front. Pharmacol. 2013, 4, 157.
- Nishiyama, N.; Matsumura, Y.; Kataoka, K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci. 2016, 107, 867–874.
- Saxena, V.; Hussain, M.D. Formulation and in vitro evaluation of 17-allyamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric mixed micelles for glioblastoma multiforme. Colloids Surf. B Biointerfaces 2013, 112, 350–355.
- Fu, Z.; Xiang, J. Aptamer-functionalized nanoparticles in targeted delivery and cancer therapy. Int. J. Mol. Sci. 2020, 21, 9123.
- Stenström, P.; Manzanares, D.; Zhang, Y.; Ceña, V.; Malkoch, M. Evaluation of amino-functional polyester dendrimers based on Bis-MPA as nonviral vectors for siRNA delivery. Molecules 2018, 23, 2028.
- Torchilin, V.P. Nanocarriers. Pharm. Res. 2007, 24, 2333–2334.
- Zhang, Y.; Qu, H.; Xue, X. Blood–brain barrier penetrating liposomes with synergistic chemotherapy for glioblastoma treatment. Biomater. Sci. 2022, 10, 423–434.
- Papachristodoulou, A.; Signorell, R.D.; Werner, B.; Brambilla, D.; Luciani, P.; Cavusoglu, M.; Grandjean, J.; Silginer, M.; Rudin, M.; Martin, E.; et al. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J. Control. Release 2019, 295, 130–139.
- Shirazi, A.S.; Varshochian, R.; Rezaei, M.; Ardakani, Y.H.; Dinarvand, R. SN38 loaded nanostructured lipid carriers (NLCs); preparation and in vitro evaluations against glioblastoma. J. Mater. Sci. Mater. Med. 2021, 32, 78.
- Paraiso, W.K.D.; Garcia-Chica, J.; Ariza, X.; Zagmutt, S.; Fukushima, S.; Garcia, J.; Mochida, Y.; Serra, D.; Herrero, L.; Kinoh, H. Poly-ion complex micelles effectively deliver CoA-conjugated CPT1A inhibitors to modulate lipid metabolism in brain cells. Biomater. Sci. 2021, 9, 7076–7091.
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408.
- Zwain, T.; Alder, J.E.; Sabagh, B.; Shaw, A.; Burrow, A.J.; Singh, K.K. Tailoring functional nanostructured lipid carriers for glioblastoma treatment with enhanced permeability through in-vitro 3D BBB/BBTB models. Mater. Sci. Eng. C 2021, 121, 111774.
- Kuo, Y.-C.; Liang, C.-T. Inhibition of human brain malignant glioblastoma cells using carmustine-loaded catanionic solid lipid nanoparticles with surface anti-epithelial growth factor receptor. Biomaterials 2011, 32, 3340–3350.
- Sun, Y.; Wang, Y.; Liu, Y.; Zou, Y.; Zheng, M.; Shi, B. Recent advances in polymeric nanomedicines for glioblastoma therapy. Sci. Sin. Vitae 2021, 51, 819–835.
- Han, H.; Zhang, Y.; Jin, S.; Chen, P.; Liu, S.; Xie, Z.; Jing, X.; Wang, Z. Paclitaxel-loaded dextran nanoparticles decorated with RVG29 peptide for targeted chemotherapy of glioma: An in vivo study. New J. Chem. 2020, 44, 5692–5701.
- Esfandiarpour-Boroujeni, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H.; Amanpour, S. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr. Polym. 2017, 168, 14–21.
- Sekar, V.; Rajendran, K.; Vallinayagam, S.; Deepak, V.; Mahadevan, S. Synthesis and characterization of chitosan ascorbate nanoparticles for therapeutic inhibition for cervical cancer and their in silico modeling. J. Ind. Eng. Chem. 2018, 62, 239–249.
- Yin, T.; Bader, A.R.; Hou, T.K.; Maron, B.A.; Kao, D.D.; Qian, R.; Kohane, D.S.; Handy, D.E.; Loscalzo, J.; Zhang, Y.-Y. SDF-1α in glycan nanoparticles exhibits full activity and reduces pulmonary hypertension in rats. Biomacromolecules 2013, 14, 4009–4020.
- Saulnier, P.; Benoit, J. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967.
- Geldenhuys, W.; Wehrung, D.; Groshev, A.; Hirani, A.; Sutariya, V. Brain-targeted delivery of doxorubicin using glutathione-coated nanoparticles for brain cancers. Pharm. Dev. Technol. 2015, 20, 497–506.
- Ramalho, M.J.; Sevin, E.; Gosselet, F.; Lima, J.; Coelho, M.A.N.; Loureiro, J.A.; Pereira, M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018, 545, 84–92.
- Caban-Toktas, S.; Sahin, A.; Lule, S.; Esendagli, G.; Vural, I.; Oguz, K.K.; Soylemezoglu, F.; Mut, M.; Dalkara, T.; Khan, M. Combination of Paclitaxel and R-flurbiprofen loaded PLGA nanoparticles suppresses glioblastoma growth on systemic administration. Int. J. Pharm. 2020, 578, 119076.
- Gascon, S.; Solano, A.G.; El Kheir, W.; Therriault, H.; Berthelin, P.; Cattier, B.; Marcos, B.; Virgilio, N.; Paquette, B.; Faucheux, N.; et al. Characterization and mathematical modeling of alginate/chitosan-based nanoparticles releasing the chemokine cxcl12 to attract glioblastoma cells. Pharmaceutics 2020, 12, 356.
- Bastiancich, C.; Bozzato, E.; Henley, I.; Newland, B. Does local drug delivery still hold therapeutic promise for brain cancer? A systematic review. J. Control. Release 2021, 337, 296–305.
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466.
- Bregy, A.; Shah, A.H.; Diaz, M.V.; Pierce, H.E.; Ames, P.L.; Diaz, D.; Komotar, R.J. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 2013, 13, 1453–1461.
- Alghamdi, M.; Gumbleton, M.; Newland, B. Local delivery to malignant brain tumors: Potential biomaterial-based therapeutic/adjuvant strategies. Biomater. Sci. 2021, 9, 6037–6051.
- Bruinsmann, F.A.; Richter Vaz, G.; de Cristo Soares Alves, A.; Aguirre, T.; Raffin Pohlmann, A.; Stanisçuaski Guterres, S.; Sonvico, F. Nasal Drug Delivery of Anticancer Drugs for the Treatment of Glioblastoma: Preclinical and Clinical Trials. Molecules 2019, 24, 4312.
- Blacher, E.; Ben Baruch, B.; Levy, A.; Geva, N.; Green, K.D.; Garneau-Tsodikova, S.; Fridman, M.; Stein, R. Inhibition of glioma progression by a newly discovered CD38 inhibitor. Int. J. Cancer 2015, 136, 1422–1433.
- Levy, A.; Blacher, E.; Vaknine, H.; Lund, F.E.; Stein, R.; Mayo, L. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro-Oncology 2012, 14, 1037–1049.
- Mayo, L.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Moutin, M.-J.; Lund, F.E.; Stein, R. Dual role of CD38 in microglial activation and activation-induced cell death. J. Immunol. 2008, 181, 92–103.
- Li, Y.; Gao, Y.; Liu, G.; Zhou, X.; Wang, Y.; Ma, L. Intranasal administration of temozolomide for brain-targeting delivery: Therapeutic effect on glioma in rats. J. South. Med. Univ. 2014, 34, 631–635.
- Ferreira, N.N.; Granja, S.; Boni, F.I.; Prezotti, F.G.; Ferreira, L.M.B.; Cury, B.S.F.; Reis, R.M.; Baltazar, F.; Gremião, M.P.D. Modulating chitosan-PLGA nanoparticle properties to design a co-delivery platform for glioblastoma therapy intended for nose-to-brain route. Drug Deliv. Transl. Res. 2020, 10, 1729–1747.
- Alex, A.T.; Joseph, A.; Shavi, G.; Rao, J.V.; Udupa, N. Development and evaluation of carboplatin-loaded PCL nanoparticles for intranasal delivery. Drug Deliv. 2016, 23, 2144–2153.
- de Oliveira Junior, E.R.; Nascimento, T.L.; Salomão, M.A.; da Silva, A.C.G.; Valadares, M.C.; Lima, E.M. Increased nose-to-brain delivery of melatonin mediated by polycaprolactone nanoparticles for the treatment of glioblastoma. Pharm. Res. 2019, 36, 131.
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334.
- Ferreira, N.N.; de Oliveira Junior, E.; Granja, S.; Boni, F.I.; Ferreira, L.M.B.; Cury, B.S.F.; Santos, L.C.R.; Reis, R.M.; Lima, E.M.; Baltazar, F.; et al. Nose-to-brain co-delivery of drugs for glioblastoma treatment using nanostructured system. Int. J. Pharm. 2021, 603, 120714.
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release 2019, 309, 37–47.
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neuro-Oncol. 2021, 151, 415–427.
- Ung, T.H.; Malone, H.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery for glioblastoma: Targeted delivery of antitumor therapeutics. CNS Oncol. 2015, 4, 225–234.
- Chakroun, R.W.; Zhang, P.; Lin, R.; Schiapparelli, P.; Quinones-Hinojosa, A.; Cui, H. Nanotherapeutic systems for local treatment of brain tumors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1479.
- Debinski, W.; Tatter, S.B. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev. Neurother. 2009, 9, 1519–1527.
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080.
- Cornelison, R.C.; Brennan, C.E.; Kingsmore, K.M.; Munson, J.M. Convective forces increase CXCR4-dependent glioblastoma cell invasion in GL261 murine model. Sci. Rep. 2018, 8, 17057.
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A Phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479.
- Séhédic, D.; Chourpa, I.; Tétaud, C.; Griveau, A.; Loussouarn, C.; Avril, S.; Legendre, C.; Lepareur, N.; Wion, D.; Hindré, F.; et al. Locoregional Confinement and Major Clinical Benefit of (188)Re-Loaded CXCR4-Targeted Nanocarriers in an Orthotopic Human to Mouse Model of Glioblastoma. Theranostics 2017, 7, 4517–4536.
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release 2017, 263, 112–119.
- Stephen, Z.R.; Kievit, F.M.; Veiseh, O.; Chiarelli, P.A.; Fang, C.; Wang, K.; Hatzinger, S.J.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O 6-benzylguanine to brain tumors. ACS Nano 2014, 8, 10383–10395.
- Chen, P.-Y.; Ozawa, T.; Drummond, D.C.; Kalra, A.; Fitzgerald, J.B.; Kirpotin, D.B.; Wei, K.-C.; Butowski, N.; Prados, M.D.; Berger, M.S.; et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro-Oncology 2013, 15, 189–197.
- Cruickshank, G.; Fayeye, O.; Ngoga, D.; Connor, J.; Detta, A. ATNT-05: Intraoperative Intraparenchymal Injection of Irinotecan Drug Loaded Beads in Patients with Recurrent Glioblastoma (Gbm): A Safe New Depot Approach for Loco-Regional Therapy (NCT02433392). Neuro-Oncology 2015, 17, v11.
- Kim, D.G.; Kim, K.H.; Seo, Y.J.; Yang, H.; Marcusson, E.G.; Son, E.; Lee, K.; Sa, J.K.; Lee, H.W.; Nam, D.-H. Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy. Oncotarget 2016, 7, 29400.
- Wait, S.D.; Prabhu, R.S.; Burri, S.H.; Atkins, T.G.; Asher, A.L. Polymeric drug delivery for the treatment of glioblastoma. Neuro-Oncology 2015, 17, ii9–ii23.
- Valtonen, S.; Timonen, U.I.; Toivanen, P.; Kalimo, H.; Kivipelto, L.; Heiskanen, O.; Unsgaard, G.; Kuurne, T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: A randomized double-blind study. Neurosurgery 1997, 41, 44–49.
- Walter, K.A.; Cahan, M.A.; Gur, A.; Tyler, B.; Hilton, J.; Colvin, O.M.; Burger, P.C.; Domb, A.; Brem, H. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994, 54, 2207–2212.
- Mangraviti, A.; Raghavan, T.; Volpin, F.; Skuli, N.; Gullotti, D.; Zhou, J.; Asnaghi, L.; Sankey, E.; Liu, A.; Wang, Y. HIF-1α-targeting acriflavine provides long term survival and radiological tumor response in brain cancer therapy. Sci. Rep. 2017, 7, 14978.
- Lesniak, M.S.; Upadhyay, U.; Goodwin, R.; Tyler, B.; Brem, H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005, 25, 3825–3831.
- Ranganath, S.H.; Fu, Y.; Arifin, D.Y.; Kee, I.; Zheng, L.; Lee, H.-S.; Chow, P.K.-H.; Wang, C.-H. The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials 2010, 31, 5199–5207.
- Ranganath, S.H.; Wang, C.-H. Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials 2008, 29, 2996–3003.
- Shapira-Furman, T.; Serra, R.; Gorelick, N.; Doglioli, M.; Tagliaferri, V.; Cecia, A.; Peters, M.; Kumar, A.; Rottenberg, Y.; Langer, R. Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer. J. Control. Release 2019, 295, 93–101.
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627.
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23.
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360.
- Peppas, N.A.; Bures, P.; Leobandung, W.S.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46.
- Wang, C.; Tong, X.; Yang, F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol. Pharm. 2014, 11, 2115–2125.
- Hosseinzadeh, R.; Mirani, B.; Pagan, E.; Mirzaaghaei, S.; Nasimian, A.; Kawalec, P.; da Silva Rosa, S.C.; Hamdi, D.; Fernandez, N.P.; Toyota, B.D. A drug-eluting 3D-printed mesh (GlioMesh) for management of glioblastoma. Adv. Ther. 2019, 2, 1900113.
- Schiapparelli, P.; Zhang, P.; Lara-Velazquez, M.; Guerrero-Cazares, H.; Lin, R.; Su, H.; Chakroun, R.W.; Tusa, M.; Quiñones-Hinojosa, A.; Cui, H. Self-assembling and self-formulating prodrug hydrogelator extends survival in a glioblastoma resection and recurrence model. J. Control. Release 2020, 319, 311–321.
- Turabee, M.H.; Jeong, T.H.; Ramalingam, P.; Kang, J.H.; Ko, Y.T. N, N, N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr. Polym. 2019, 203, 302–309.
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42.
- Akbar, U.; Jones, T.; Winestone, J.; Michael, M.; Shukla, A.; Sun, Y.; Duntsch, C. Delivery of temozolomide to the tumor bed via biodegradable gel matrices in a novel model of intracranial glioma with resection. J. Neuro-Oncol. 2009, 94, 203–212.
- Bouché, M.; Dong, Y.C.; Sheikh, S.; Taing, K.; Saxena, D.; Hsu, J.C.; Chen, M.H.; Salinas, R.D.; Song, H.; Burdick, J.A.; et al. Novel Treatment for Glioblastoma Delivered by a Radiation Responsive and Radiopaque Hydrogel. ACS Biomater. Sci. Eng. 2021, 7, 3209–3220.
- Vellimana, A.K.; Recinos, V.R.; Hwang, L.; Fowers, K.D.; Li, K.W.; Zhang, Y.; Okonma, S.; Eberhart, C.G.; Brem, H.; Tyler, B.M. Combination of paclitaxel thermal gel depot with temozolomide and radiotherapy significantly prolongs survival in an experimental rodent glioma model. J. Neuro-Oncol. 2013, 111, 229–236.
- Zentner, G.M.; Rathi, R.; Shih, C.; McRea, J.C.; Seo, M.-H.; Oh, H.; Rhee, B.G.; Mestecky, J.; Moldoveanu, Z.; Morgan, M. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control. Release 2001, 72, 203–215.
- Déry, L.; Charest, G.; Guérin, B.; Akbari, M.; Fortin, D. Chemoattraction of Neoplastic Glial Cells with CXCL10, CCL2 and CCL11 as a Paradigm for a Promising Therapeutic Approach for Primary Brain Tumors. Int. J. Mol. Sci. 2021, 22, 12150.
- Ye, E.; Loh, X.J. Polymeric hydrogels and nanoparticles: A merging and emerging field. Aust. J. Chem. 2013, 66, 997–1007.
- Najberg, M.; Mansor, M.H.; Boury, F.; Alvarez-Lorenzo, C.; Garcion, E. Reversing the Tumor Target: Establishment of a Tumor Trap. Front. Pharmacol. 2019, 10, 887.
- Brachi, G.; Ruiz-Ramírez, J.; Dogra, P.; Wang, Z.; Cristini, V.; Ciardelli, G.; Rostomily, R.C.; Ferrari, M.; Mikheev, A.M.; Blanco, E. Intratumoral injection of hydrogel-embedded nanoparticles enhances retention in glioblastoma. Nanoscale 2020, 12, 23838–23850.
- Zhao, M.; Danhier, F.; Bastiancich, C.; Joudiou, N.; Ganipineni, L.P.; Tsakiris, N.; Gallez, B.; des Rieux, A.; Jankovski, A.; Bianco, J.; et al. Post-resection treatment of glioblastoma with an injectable nanomedicine-loaded photopolymerizable hydrogel induces long-term survival. Int. J. Pharm. 2018, 548, 522–529.
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid prepolymer-based in situ formation of degradable poly (ethylene glycol)-linked-poly (caprolactone)-linked-poly (2-dimethylaminoethyl) methacrylate amphiphilic conetwork gels showing polarity driven gelation and bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619.
- Chandel, A.K.S.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually crosslinked injectable hydrogels of poly (ethylene glycol) and poly -b-poly (N-isopropyl acrylamide) as a wound healing promoter. J. Mater. Chem. B 2017, 5, 4955–4965.
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran- graft -poly Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153.
- Solano, A.G.; Dupuy, J.; Therriault, H.; Liberelle, B.; Faucheux, N.; Lauzon, M.-A.; Virgilio, N.; Paquette, B. An alginate-based macroporous hydrogel matrix to trap cancer cells. Carbohydr. Polym. 2021, 266, 118115.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
30 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




