Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Pia Ferraz | -- | 3662 | 2022-06-22 10:49:31 | | | |
| 2 | Catherine Yang | Meta information modification | 3662 | 2022-06-22 11:02:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ferraz, M.P. Biomaterials for Ophthalmic Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/24329 (accessed on 08 February 2026).
Ferraz MP. Biomaterials for Ophthalmic Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/24329. Accessed February 08, 2026.
Ferraz, Maria Pia. "Biomaterials for Ophthalmic Applications" Encyclopedia, https://encyclopedia.pub/entry/24329 (accessed February 08, 2026).
Ferraz, M.P. (2022, June 22). Biomaterials for Ophthalmic Applications. In Encyclopedia. https://encyclopedia.pub/entry/24329
Ferraz, Maria Pia. "Biomaterials for Ophthalmic Applications." Encyclopedia. Web. 22 June, 2022.
Copy Citation
Ophthalmology is the branch of medicine that deals with diseases of the eye, the organ responsible for vision, and its attachments. Biomaterials can be made with different types of materials and can replace or improve a function or an organ, specifically the eye in the case of ophthalmic biomaterials. Biomaterials are substances that interact with biological systems for a medical purpose, either as a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic agent, and have continued to improve over the years, leading to the creation of new biomaterials.
ophthalmologic biomaterials
contact lenses
intraocular lenses
1. Introduction
The eye is an organ of great complexity and, compared to our other organs, is easier to observe and easier to access for surgery. It is also the first organ in which a foreign material was implanted to fulfil the function of what is now called a biomaterial. With the introduction of synthetic hydrogels, i.e., polymers capable of addressing and retaining water without dissolving in an aqueous medium, the range of ophthalmic biomaterials has grown considerably [1]. The use of biomaterials in general and ophthalmic biomaterials in particular is growing and integrates knowledge and ideas from multiple disciplines, such as medicine, biology, chemistry, physics, materials science and engineering [2].
The eye is the organ responsible for vision situated in a bony cavity, called the orbit, is connected to the brain via optic pathways and is surrounded by a nutrient membrane, the choroid, as well as a protective membrane, the sclera. The eye is a hollow, spherical structure, surrounded by attachments with a motor paper or protective paper. The anatomy and physiology of the eye are very complex and extensively documented [3][4][5].
The consequences of an increasingly ageing society on the health system have become a topic of great importance and concern. Concerning the maintenance of vision, adequate care is vitally important as it helps to maintain the independence of the elderly. All tissues and all aspects of the eye are affected by ageing to some degree. The most common vision disorders associated with increasing age are presbyopia, dry eyes, cataract or glaucoma [1].
In relation to ophthalmic biomaterials, contact lenses are the oldest and most well-known of these, and are presently composed of hydrogel and silicone hydrogel. Intraocular lenses can replace a clouded eye lens that is removed during cataract operation. They are usually composed of derivative acrylics or silicone copolymers [6][7]. Other biomaterials widely marketed are artificial tears, used mainly for dry eye treatment. They reduce irritation symptoms, reduce friction, increase lubrication, stabilize the tear film and protect against dehydration. They are dispensed in the form of eye drops, gel or ointment [8]. Inlays are used to correct presbyopia and work according to different approaches [9].
Locations for the possible ophthalmic application of biomaterials is provided by several authors [10][11]. Briefly, in the anterior segment, soft contact lenses and artificial tears may be used. Intraocular lenses and inlays can be used in the lens. On the posterior segment, biomaterial application mainly involves vitreous substitutes.
2. Biomaterials in Ophthalmology
The history of ophthalmic biomaterials is relatively brief. Onofrio Abbate, in 1862, implanted a foreign material for a purpose similar to that of a biomaterial. It was a glass disc enclosed in a two-ring skirt forming an artificial cornea. This device was tested on the cornea of animals, but it was unable to stay in place for more than a week. Later, Dimmer attempted to construct an artificial cornea composed of celluloid (a mixture of nitrocellulose and camphor with stabilizers), implanting it in four human patients; however, it was rejected in the first few months. It was not until half a century later that a fully synthetic polymer was used as an implantable ophthalmic biomaterial composed of polyvinyl alcohol (PVA) gel. It was followed by the first artificial cornea made from polymethyl methacrylate (PMMA) [12]. A few years later, a synthetic polymer, poly(1-vinyl-2-pyrrolidone), was implanted in the vitreous cavity as a vitreous substitute. It was mainly thanks to the introduction of synthetic hydrogels that the range of biomaterials to treat ophthalmic lesions developed [1].
The main goal of developing successive generations of biomaterials is to bridge the gaps or defects of previous generations and to improve safety, efficiency and comfort. Innovations were made to improve quality standards, production or production efficiency to reduce costs. Market pressure to reduce costs exists in order to promote competitiveness and provide better accessibility. Ophthalmic biomaterials are now highly sophisticated devices and their usefulness has increased dramatically in recent years [1]. Many important requirements must be met by ophthalmic biomaterials, including the ability to deliver oxygen to tissues, refractive changes, tissue protection during surgery, tissue integration and healing modulation [13][14].
Contact lenses are especially important as an ophthalmic biomaterial, as they are in contact with ocular surface components, primarily the corneal epithelium. They also, economically speaking, represent the most important category and have undergone the most important and complex evolution [15].
Hydrogels have already been approved for several ophthalmic applications, but currently, several are under investigation. Hydrogels are successfully marketed as soft contact lenses [16], foldable intraocular lenses [17], or in situ gelling vehicles for ophthalmic drug delivery [18]. Efforts are being mounted to improve the sustained release of antibiotics, anti-inflammatory drugs, therapeutic proteins and nucleic acids. Furthermore, hydrogels are also being investigated as potential vitreous substitutes [19]. A wide range of natural, semisynthetic and synthetic polymers can be used as starting materials for hydrogels. Examples of natural origin polymers include alginate, collagen, and hyaluronic acid (HA). Poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), polymers based on acrylate monomers, and siloxanes are examples of synthetic gel-forming materials [10].
Tissue engineering helps to regenerate lost tissue, specifically in severe cases such as burns, ulcers, diabetes, bone defects and liver failure among others. It is based on stem cells, growth factors and biomaterials [20][21]. The general process of tissue engineering is based on the implantation of living cells in a scaffold specially designed to promote tissue replacement and regeneration [22][23]. It is therefore considered as the next step in the development of biomaterials. Regenerative medicine relies on interdisciplinary research and applications based on repair, replacement or the regeneration of cells, tissues or organs to restore impaired function. However, adult humans only have a limited capacity for spontaneous regeneration. Induced regeneration has been reported in conjunctiva and cornea. Tissue engineering in the eye has mainly been reported in the anterior segment (cornea and conjunctiva) and significant progress has been made in cell therapies to treat degenerative diseases of the retina. Developments in biomaterials are being made using new manufacturing techniques that allow for the production of personalized tissues through advances in stem cell programming, generation tissue imaging and computer-aided design [1][24].
2.1. Contact Lenses
Obtaining good visual acuity may require the use of an optical correction, which can be achieved by converging or diverging contact lenses, whose power is expressed in diopters. There are four common refractive disorders, namely myopia; hypermetropia; astigmatism and presbyopia. The first glass contact lenses appeared in the 19th century. In the 1880sm the first scleral glass lenses appeared with Adolf Fick. The process of making contact lenses was described in 1888 [2][25]. Rohm and Haas introduced PMMA, a material considered biologically inert, light, easy to manufacture and resistant to breakage, in 1936. Hard lenses apparently appeared due to an error during the process of manufacturing PMMA scleral lenses and led to the popularization of contact lenses [2][25].
Silicone elastomer lenses were introduced in the 1960s as a silicone soft contact lens that does not retain water, is very permeable to gases, and has a hydrophobic surface [25].
Hydrophilic lenses date back to 1972 with the introduction of the soft lens on the market, which was an immediate success thanks to its comfort and its superior biocompatibility; however, an improvement in their gas permeability was required, after which they were made thinner and had a higher aqueous content [26]. In 1974, the first rigid gas permeable lenses were invented. One of the first materials was cellulose acetate butyrate (CAB), which provided a gas permeability higher than that of PMMA, but was prone to deformation. Norman Gaylord successfully incorporated silicone into the basic structure of PMMA to introduce a new family of polymer contact lenses, silicone acrylate. In 1994, a technique drastically reduced the production costs and helped to conceive of the first daily contact lenses. More than one decade of intensive research and development was needed to develop silicone hydrogel contact lenses which improved hypoxia problems related to contact lenses [25][27][28].
Soft contact lenses are the most popular type of contact lens, accounting for 88% of worn lenses [26][29][30]. Hydrogel contact lenses are made with a component of stable polymer, which can absorb or bind water. Polymer pores allow the liquid to penetrate the material, making it hydrated. Due to the particular environment of the eye, the lens must be safe, inert, non-toxic, biocompatible, easy to produce, maintain a stable and continuous tear film, be permeable to oxygen, maintain normal corneal metabolism, be ion permeable to maintain the movement of eyes, be comfortable and provide a stable and clear view [25][26][29][31][32].
2.2. Intraocular Lenses
Cataract is the clouding of the lens and is the leading cause of blindness in the world. It is due to changes in lens proteins, a natural phenomenon that occurs during aging, but can be accentuated by various factors, such as exposure to UV rays or smoking. It is treated by replacing the biological lens with a polymer-based substitute [1]. The most common procedure is phacoemulsification (FACO), creating a small incision in the cornea through which a probe is used to break the cloudy lens which is removed by suction. An intraocular lens artificial device (IOL) is then placed in the intact capsular bag [1][33].
The first IOL was in PMMA [34][35] a rigid, non-collapsible and hydrophobic polymer requiring a large incision. Thomas Mazzocco invented the first foldable silicone lens that can be implanted through a 3 mm incision [36].
An ideal IOL should provide the patient with good vision for a long period of time and the surgeon should be able to implant it easily, without causing complications [34]. The material and its design must allow for a low degree of postoperative inflammation and the production process must be relatively simple for the IOL to be accessible [37]. Currently, all IOLs include a chromophore in their composition to block UV light. Blue light is harmful and can cause damage to the retina due to increased oxidative stress [37].
IOLs can be divided into the following two groups: an acrylic/methacrylate polymer comprising hydrophobic acrylic, hydrophilic acrylic or hydrogel (rigid or flexible) and hydrophobic (flexible) silicone/silicone elastomers. Non-folding PMMA lenses from the group of acrylic polymers are almost non-existent in Europe and the USA due to the size of the incision needed for implantation [38][39][40][41].
2.3. Artificial Tears
Dry eye is defined as a multifactorial disease of the ocular surface characterized by a loss of tear film homeostasis and accompanied by ocular symptoms, including instability, tear film hyperosmolarity, inflammation, an ocular surface and sensorineural abnormalities [42]. It is a disorder of the ocular surface, affecting millions of people worldwide, with varying degrees in severity ranging from simple discomfort to pain or fluctuating vision [43]. Dry eye is associated with several names, including keratoconjunctivitis sicca (KCS); dry eye syndrome (DES); dry eye disease (DED) or dysfunctional tear syndrome (STD). KCS is the traditional name that involves the drying and inflammation of the ocular surface. DES or DED is the most widely recognized term [44][45].
Aetiologias of DED are often classified as environmental, aqueous tear deficient or evaporative [43][46]. A problem in lipid secretion, mucins or water or an increase in tear film evaporation can cause a DED, which often has a multifactorial origin [43][47]. DED is usually related to other pathologies and may be triggered by the environment, be a side effect of a drug and its prevalence increases with age. Dry eye is classified according to risk factors and pathophysiology characteristics to improve diagnosis and treatment. Dry eye diagnosis is complex, due to the lack of consistent results from current clinical trials, as well as individual variability and the subjective nature of symptoms [43]. DED can be episodic or chronic. Patients often complain of eye irritation and occasional blurred vision, but if patients with chronic DED are not treated, symptoms may persist and cause eye damage without impairing vision. Cornea or conjunctiva erosion are rare complications. The treatment of DED reduces symptoms and prevents eye damage; furthermore, artificial tears are the most used treatment for this condition, regardless of disease severity [46].
2.4. Inlays
The history of ophthalmic inlays begins in the 1940s, when Barraquer introduced the idea of correcting corneal refraction through an implant to increase cornea curvature [48]. Barraquer proved that no existing material could be used due to their lack of biocompatibility (corneal inflammation, nutritional deprivation, lack of precision in refractive correction). The use of living corneal tissue did not prove to be a viable alternative due to difficult surgical procedures [48][49].
The discovery of hydrogels deepened the concept of the intracorneal inlay [9]. It was Dohlman, in the late 1960s, who first described the use of a hydrophilic hydrogel polymer to permit nutritional flow. Inlay migration, epithelial growth at the interface and crystal formation represent some of the most common complications. The shapes were limited by the materials and available equipment used to modify surfaces.
Inlays continued to be improved with the emergence of new biocompatible materials and were more tolerated. Femtosecond lasers developed at the end of 1990s made obtaining more accurate stromal pouches possible; therefore, a better centralization of the intracorneal inlay and a better estimation of the implementation depth were achieved [9].
Presbyopia is a progressive decrease in the amplitude of accommodation, related with age, which is responsible for a reduction in near visual acuity. Presbyopia is a growing problem in view of an aging global population and increasingly, patients desire spectacle-free solutions to address this condition. This loss in accommodation amplitude is due to the aging of the zonula, capsule, lens and ciliary bodies. The lens becomes thicker and stiffer, the capsule increases in thickness and decreases in elasticity, and the zonula becomes more fragile and rigid; therefore, the ciliary body remains in a contracted position because of elasticity loss in the lens. Non-invasive methods to correct presbyopia include corrective lenses and contact lenses, however, these do not restore the accommodation process. Surgical methods can reduce visual acuity and quality of vision. Deploying inlays is technically easy and incurs less risk than an intraocular procedure [9].
2.5. Vitreous Substitutes
The vitreous body, also called the vitreous or vitreous humor, fills the posterior space of the eye, between the lens and the retina. It occupies more than two thirds of the eye volume. It is a clear gel, highly transparent, inhomogeneous and consists of several parts with different densities and biochemical compositions [19][50][51]. The vitreous is composed of water, proteins (mainly collagen), GAG (hyaluronic acid, chondroitin sulfate and heparan), metabolites (glucose and lactic acid), ascorbic acid, amino acid, fatty acid, prostaglandin, cells (hyalocytes, fibrocytes/fibroblasts and macrophages) and enzymes [19][51][52][53].
The viscoelastic properties of the vitreous are due to collagen fibers, while the hyaluronic acid provides it with shock-absorbing properties. This protects the structures and tissue of the eye, maintaining the shape of the eyeball and keeping the lens and retina in place [19][50]. The vitreous has four main functions, which are as follows: a structural function, it supports the eye growth, volume and elasticity; an optical function while maintaining transparency and improving accommodation; a barrier function, forming a barrier to biochemical substances; and a nutritional role in providing nutrients and for metabolism [52].
At birth, the vitreous body is completely gelatinous, with age, the vitreous body gradually liquefies. It is this liquefaction of the vitreous that plays a role in posterior vitreous detachment (PVD), corresponding to vitreous cortex detachment of the retina [19][51].
An ideal vitreous substitute should be (i) non-toxic and biocompatible with eye tissues, (ii) clear and transparent with a refractive index and density similar to natural glass, (iii) must remain transparent without opacifying after the operation, (iv) an effective buffering agent, (v) allow for the transfer of metabolites, proteins and solutes, (vi) if possible non-absorbable and not biodegradable, (vii) hydrophilic and not soluble in water, (viii) injectable in a small needle, (ix) retain its properties after injection, (x) can be preserved and sterilized without the loss of properties mentioned above. The ideal vitreous substitute does not yet exist and remains a goal to be achieved [19][51].
The various vitreous substitutes available today make it possible to replace the mechanical role of the vitreous, but they are toxic in the long run. They can be classified into the following broad categories: gas (air, sulfur hexafluoride (SF6)), perfluoropropane (C3F8); liquid (physiological solution, perfluorocarbon fluids (PFCL), semi-fluorinated alkanes (SFA), natural and semi-synthetic polymers namely hyaluronic acid and chitosan, silicone oil; and experimental substitutes.
Currently, silicone oil (OS) is the only substance used for long-term vitreous replacement despite some clinical complications [51].
Regarding experimental substitutes, these correspond to the search for a substance with the same molecular structure than the vitreous, as well as the same chemical and physiological properties. These are mainly synthetic polymers, especially hydrogels that can be divided into hydrogels and smart hydrogels. They seem to be promising materials because they have excellent transparency, biocompatibility and can absorb viscoelastic shocks, thus mimicking the behavior of natural vitreous [19].
Hydrogels and smart hydrogels seem to be good candidates as long-term vitreous substitutes once they show excellent transparency and good biocompatibility. They can act as viscoelastic shock-absorbing materials, thereby closely mimicking the behaviour of the natural vitreous body. Hydrogels are networks of polymer chains that contain 99.9% water, they are hydrophilic and not flowable, they swell in aqueous solutions without being solved, can be injected in an aqueous form forming a gel in situ [54]. However, many issues, such as retinal toxicity, increased intraocular pressure, and the formation of opacities still need to be addressed. Fragmentation and changes in viscoelastic properties and resiliency after injection through a small-gauge needle have also been found in some types of hydrogels.
Smart hydrogels are a relatively new class of stimuli-sensitive hydrogels. They possess the common properties of conventional hydrogels, and they can respond to a variety of signals, including PH, temperature, light, pressure, electric fields, and chemicals [50][51][52][53]. These interactions lead to better gelation, drug diffusion, and gel expansion. However, there is still insufficient information about its toxicity or inflammatory action. These materials are still in the experimental phase, as certain complications are still not well understood. Generally, smart hydrogels appear promising, but research on their use is still at an early experimental stage, and their effects on long-term toxicity are unknown [5].
Recent research in vitreous substitutes mostly include cross-linked hydrogels; these materials show an enhanced retention time in the eye and are capable of acting as a tamponade agent. New developments in existing hydrogel-based vitreous substitutes have been reported. A study conducted by Leone et al. reported PVA hydrogels synthesis through cross-linking with non-toxic trisodium trimetaphosphate (STMP), resulting in rheological properties similar to the natural vitreous body. These properties were preserved after injection and in vitro cytotoxicity presented good results. In vivo studies to prove the long-term compatibility of this promising material have yet to be conducted [55]. Swindle-Reilly et al. copolymerized acrylamide with bisacryloylcystamine, obtaining good mechanical properties similar to the natural vitreous humor with in vivo animal testing showing good biocompatibility. However, long-term testing is still needed [56]. Tao et al. proposed a different cross-linking approach using two reactive PEG derivatives and tested it on rabbits. The obtained hydrogel was stable during the time of the in vivo rabbit study (9 months), the mechanical and optical properties were very similar to the natural vitreous body and no adverse reactions were found [57].
Natural polymers for vitreous replacement have also been investigated with hyaluronic acid (HA) the most promising material. Schramm et al. compared two different HA hydrogels regarding their suitability as a vitreous substitute with different cross-linking methods (adipic dihydrazide (ADH) as well as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and the photopolymerization of glycidyl methacrylate groups. During in vitro cell culture experiments, ADH/EDC cross-linked hydrogels induced mild cytotoxicity, whereas photopolymerized HA gels presented no toxicity. During 6 weeks of in vivo testing, the photopolymerized gels showed appropriate biocompatibility [58]. However, another study found no toxic effects with similar ADH cross-linked HA hydrogels not only in vitro but also in vivo [59].
2.6. Tissue Engineering
Tissue engineering evolved from biomaterials development and is based on the combination of scaffolds, cells, and biologically active molecules to promote the development of functional tissues. The goal of tissue engineering is to assemble functional constructs that restore, maintain, or improve damaged tissues or whole organs. The approach was conceived to address the critical gap between the growing number of patients on the waiting list for organ transplantation and the limited number of donated organs available [23].
The general process of tissue engineering is based on the implantation of living cells in a scaffold specially designed to promote tissue replacement and regeneration [22][23] as is schematized in Figure 1. It is therefore considered as the next step in the development of biomaterials. Regenerative medicine relies on interdisciplinary research and applications based on repair, replacement or the regeneration of cells, tissues or organs to restore impaired functions. However, adult humans have only a limited capacity for spontaneous regeneration. Induced regeneration has been reported in conjunctiva and cornea.
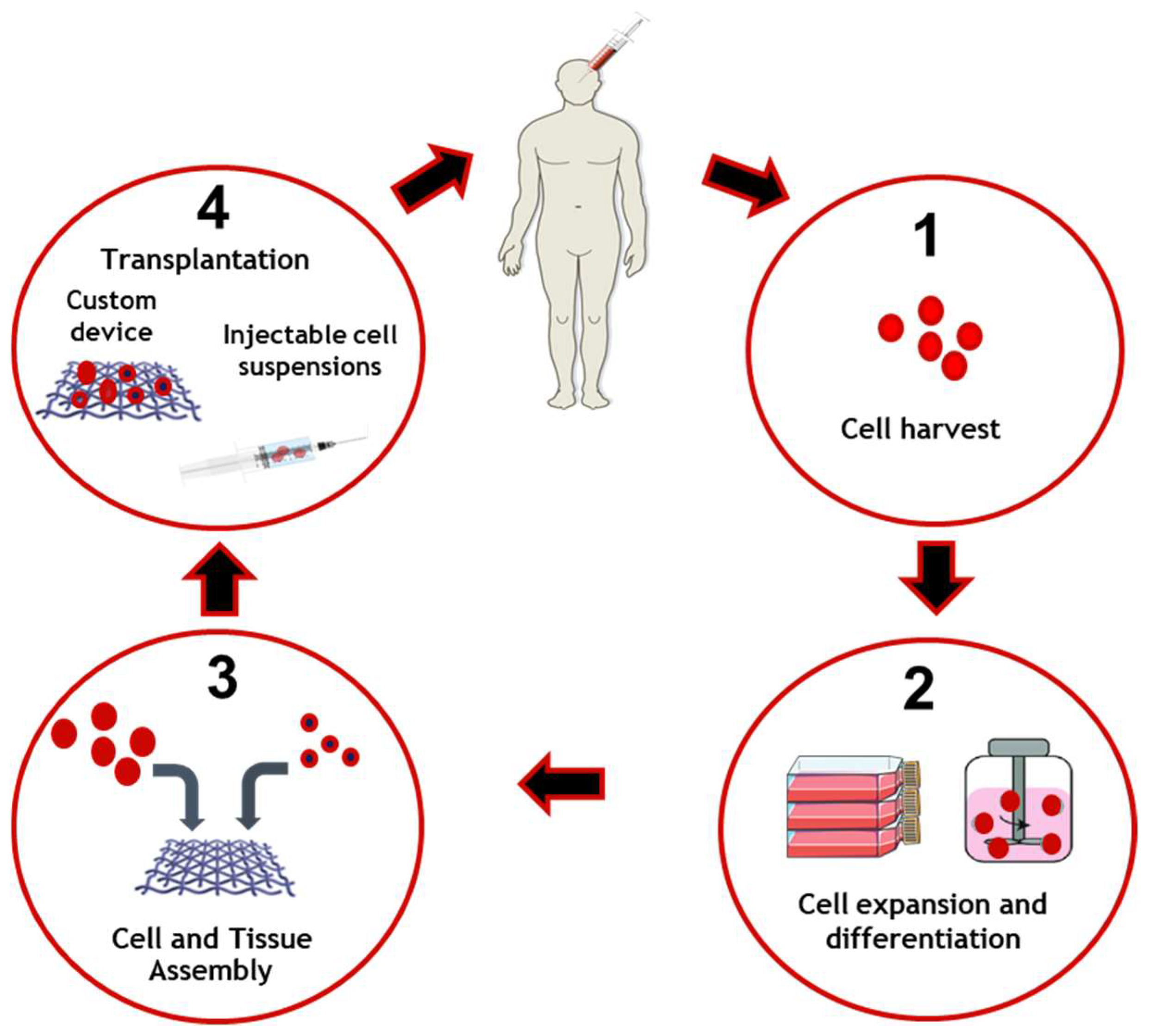
Figure 1. Tissue engineering applied to cell and tissue manufacture and delivery. Cells are collected and harvested in order to perform expansion and differentiation. Cells and bioengineered scaffolds are then assembled to be able to be transplanted on the patient.
Tissue engineering in the eye has been reported mainly in the anterior segment (cornea and conjunctiva) and significant progress has been made in cell therapies to treat degenerative diseases of the retina. Ocular regenerative therapies are a new tool to treat several blinding disorders, namely corneal disease, cataract, glaucoma, retinitis pigmentosa, and age-related macular degeneration. Several transplantable products, delivered as cell suspensions or as preformed 3D scaffolds combining cells and natural or artificial substrates, are being studied. Bioengineering approaches with advance cell product manufacturing are being developed, thereby enhancing stem cell-based medicine [60].
References
- Chirila, T.; Harkin, D.; ScienceDirect. Biomaterials and Regenerative Medicine in Ophthalmology; Woodhead Publishing: Duxford, UK, 2016.
- Amato, S.; Amato, S.F.; Ezzell, R.M.; Amato, S.F. ProQuest. In Regulatory Affairs for Biomaterials and Medical Devices, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015.
- Friedman, N.J.; Kaiser, P.K. Essentials of Ophthalmology, 1st ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007; 294p.
- Basak, S.K. Essentials of Ophthalmology, 7th ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2019; 606p.
- Decker, S. Essentials of Ophthalmology; Foster Academics: New York, NY, USA, 2021; 432p.
- Winterton, L.C.; Lally, J.M.; Sentell, K.B.; Chapoy, L.L. The elution of poly (vinyl alcohol) from a contact lens: The realization of a time release moisturizing agent/artificial tear. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 424–432.
- Powell, C.H.; Lally, J.M.; Hoong, L.D.; Huth, S.W. Lipophilic versus hydrodynamic modes of uptake and release by contact lenses of active entities used in multipurpose solutions. Contact Lens Anterior Eye 2010, 33, 9–18.
- Hopkins, G.A.; Hopkins, G.; Pearson, R.M.; ScienceDirect. Ophthalmic Drugs: Diagnostic and Therapeutic Uses, 5th ed.; Butterworth-Heinemann Elsevier: Edinburgh, UK, 2007.
- Moarefi, M.A.; Bafna, S.; Wiley, W. A Review of Presbyopia Treatment with Corneal Inlays. Ophthalmol. Ther. 2017, 6, 55–65.
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238.
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228.
- Santos, L.; Ferraz, M.P.; Shirosaki, Y.; Lopes, M.A.; Fernandes, M.H.; Osaka, A.; Santos, J.D. Degradation studies and biological behavior on an artificial cornea material. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4274–4281.
- Zhang, X.; Williams, D.F. Definitions of Biomaterials for the Twenty-First Century: Proceedings of a Consensus Conference Held in Chengdu, People’s Republic of China, June 11th and 12th 2018, Organized under the Auspices of the International Union of Societies for Biomaterials Science & Engineering; Hosted and Supported by Sichuan University, Chengdu and the Chinese Society for Biomaterials, China; Elsevier: Amsterdam, The Netherlands, 2019.
- Williams, D.F. Essential Biomaterials Science; Cambridge University Press: Cambridge, UK, 2014.
- Morgan, P.B.; Efron, N. Global contact lens prescribing 2000–2020. Clin. Exp. Optom. 2022, 105, 298–312.
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283.
- Findl, O.; Leydolt, C. Meta-analysis of accommodating intraocular lenses. J. Cataract Refract. Surg. 2007, 33, 522–527.
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402.
- Baino, F. Towards an ideal biomaterial for vitreous replacement: Historical overview and future trends. Acta Biomater. 2011, 7, 921–935.
- Teixeira, S.; Fernandes, M.H.; Ferraz, M.P.; Monteiro, F.J. Proliferation and mineralization of bone marrow cells cultured on macroporous hydroxyapatite scaffolds functionalized with collagen type I for bone tissue regeneration. J. Biomed. Mater. Res. A 2010, 95, 1–8.
- Teixeira, S.; Yang, L.; Dijkstra, P.J.; Ferraz, M.P.; Monteiro, F.J. Heparinized hydroxyapatite/collagen three-dimensional scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2385–2392.
- Mozafari, M.; Mozafari, M.; Sefat, F.; Atala, A.; ScienceDirect. Handbook of Tissue Engineering Scaffolds; Woodhead Publishing: Duxford, UK, 2019.
- Lanza, R.P.; Langer, R.; Vacanti, J.; Atala, A.; Science, D. Principles of Tissue Engineering, 5th ed.; Academic Press: London, UK, 2020.
- Sun, M.T.; O’Connor, A.J.; Wood, J.; Casson, R.; Selva, D. Tissue Engineering in Ophthalmology: Implications for Eyelid Reconstruction. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 157–162.
- Efron, N. Contact Lens Practice; Elsevier: Edinburgh, UK, 2018.
- Hom, M.M.; Bruce, A.S. Manual of Contact Lens Prescribing and Fitting with CD-ROM; Butterworth Heinemann: Oxford, UK, 2006.
- Guillon, M.; Dumbleton, K.; Patel, T.; Patel, K.; Gupta, R.; Maissa, C.A. Corrigendum to “Quantification of contact lens wettability after prolonged visual device use under low humidity conditions” . Contact Lens Anterior Eye 2020, 43, 91.
- Morgan, P.B.; McCullough, S.J.; Saunders, K.J. Corrigendum to “Estimation of ocular axial length from conventional optometric measures” . Contact Lens Anterior Eye 2020, 43, 413.
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261.
- Pillay, R.; Hansraj, R.; Rampersad, N. Historical Development, Applications and Advances in Materials Used in Spectacle Lenses and Contact Lenses. Clin. Optom. 2020, 12, 201–202.
- Millis, E.A.W. Medical Contact Lens Practice; Elsevier Butterwoth Heinemann: Edinburgh, UK; New York, NY, USA, 2005.
- Bennett, E.S.; Bennett, E.S.; Henry, V.A. Clinical Manual of Contact Lenses, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020.
- Khandelwal, S.S.; Jun, J.J.; Mak, S.; Booth, M.S.; Shekelle, P.G. Effectiveness of multifocal and monofocal intraocular lenses for cataract surgery and lens replacement: A systematic review and meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 863–875.
- Güell, J.L. Cataract; Karger: Basel, Switzerland, 2013; 153p.
- Shekelle, P.G.; Khandelwal, S.; Jun, J. United StatesDepartment of Veterans AffairsHealth Services, RDevelopment, S.Quality Enhancement Research, I.West Los Angeles, V.A.M.C.E.-B.S.P.CComparative Effectiveness of Multifocal, Accommodative, and Monofocal Intraocular Lenses for Cataract Surgery and Lens Replacement; Department of Veterans Affairs, Veterans Health Administration, Quality Enhancement Research Initiative, Health Services Research & Development Service: Washington, DC, USA, 2018.
- Buratto, L.; Brint, S.F.; Boccuzzi, D.; Ebook Central Academic Complete. Cataract Surgery and Intraocular Lenses; Slack Incorporated: Thorofare, NJ, USA, 2014.
- Traxler, L.; Bayer, N.; Reutterer, B.; Drauschke, A. Improvement of Optics, Mechanics and the Usability of a Mechanical Eye Model for Vision Quality Evaluation of IOLs. IFAC-PapersOnLine 2015, 48, 1–18.
- Cardona, G.; Alonso, S.; Yela, S. Compliance versus Risk Awareness with Contact Lens Storage Case Hygiene and Replacement. Optom. Vis. Sci. 2022, 99, 449–454.
- Gasson, A.; Morris, J.; Askews. The Contact Lens Manual: A Practical Guide to Fitting, 4th ed.; Butterworth/Heinemann: Edinburgh, UK, 2010.
- Leung, T.G.; Lindsley, K.; Kuo, I.C. Types of intraocular lenses for cataract surgery in eyes with uveitis. Cochrane Database Syst. Rev. 2014, 3, CD007284.
- Wang, X. Intraocular Lens; IntechOpen: London, UK, 2020.
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283.
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223.
- Yao, W.; Davidson, R.S.; Durairaj, V.D.; Gelston, C.D. Dry eye syndrome: An update in office management. Am. J. Med. 2011, 124, 1016–1018.
- Ocular surface, d.; Holland, E.J.; Mannis, M.J.; Lee, W.B.; ScienceDirect. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film; Elsevier/Saunders: London, UK; New York, NY, USA, 2013.
- Marshall, L.L.; Roach, J.M. Treatment of Dry Eye Disease. Consult. Pharm. 2016, 31, 96–106.
- Bron, A.J.; Tomlinson, A.; Foulks, G.N.; Pepose, J.S.; Baudouin, C.; Geerling, G.; Nichols, K.K.; Lemp, M.A. Rethinking dry eye disease: A perspective on clinical implications. Ocul. Surf. 2014, 12, S1–S31.
- Binder, P.S. Intracorneal Inlays for the Correction of Presbyopia. Eye Contact Lens 2017, 43, 267–275.
- Fenner, B.J.; Moriyama, A.S.; Mehta, J.S. Inlays and the cornea. Exp. Eye Res. 2021, 205, 108474.
- Donati, S.; Caprani, S.M.; Airaghi, G.; Vinciguerra, R.; Bartalena, L.; Testa, F.; Mariotti, C.; Porta, G.; Simonelli, F.; Azzolini, C. Vitreous substitutes: The present and the future. Biomed. Res. Int. 2014, 2014, 351804.
- Kleinberg, T.T.; Tzekov, R.T.; Stein, L.; Ravi, N.; Kaushal, S. Vitreous substitutes: A comprehensive review. Surv. Ophthalmol. 2011, 56, 300–323.
- Alovisi, C.; Panico, C.; de Sanctis, U.; Eandi, C.M. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery. J. Ophthalmol. 2017, 2017, 3172138.
- Gao, Q.Y.; Fu, Y.; Hui, Y.N. Vitreous substitutes: Challenges and directions. Int. J. Ophthalmol. 2015, 8, 437–440.
- Barros, J.A.R.; Melo, L.D.R.; Silva, R.; Ferraz, M.P.; Azeredo, J.; Pinheiro, V.M.C.; Colaco, B.J.A.; Fernandes, M.H.R.; Gomes, P.S.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine 2020, 24, 102145.
- Leone, G.; Consumi, M.; Aggravi, M.; Donati, A.; Lamponi, S.; Magnani, A. PVA/STMP based hydrogels as potential substitutes of human vitreous. J. Mater. Sci. Mater. Med. 2010, 21, 2491–2500.
- Swindle-Reilly, K.E.; Shah, M.; Hamilton, P.D.; Eskin, T.A.; Kaushal, S.; Ravi, N. Rabbit study of an in situ forming hydrogel vitreous substitute. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4840–4846.
- Tao, Y.; Tong, X.; Zhang, Y.; Lai, J.; Huang, Y.; Jiang, Y.R.; Guo, B.H. Evaluation of an in situ chemically crosslinked hydrogel as a long-term vitreous substitute material. Acta Biomater. 2013, 9, 5022–5030.
- Schramm, C.; Spitzer, M.S.; Henke-Fahle, S.; Steinmetz, G.; Januschowski, K.; Heiduschka, P.; Geis-Gerstorfer, J.; Biedermann, T.; Bartz-Schmidt, K.U.; Szurman, P. The cross-linked biopolymer hyaluronic acid as an artificial vitreous substitute. Investig. Ophthalmol. Vis. Sci. 2012, 53, 613–621.
- Su, W.Y.; Chen, K.H.; Chen, Y.C.; Lee, Y.H.; Tseng, C.L.; Lin, F.H. An injectable oxidated hyaluronic acid/adipic acid dihydrazide hydrogel as a vitreous substitute. J. Biomater. Sci. Polym. Ed. 2011, 22, 1777–1797.
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 23, 453.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Revisions:
2 times
(View History)
Update Date:
22 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




