
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Osvalda Senneca | -- | 4805 | 2022-06-08 16:46:09 | | | |
| 2 | Peter Tang | Meta information modification | 4805 | 2022-06-09 03:34:18 | | |
Video Upload Options
In the delayed coker the residues of the vacuum distillation units are cracked into lighter hydrocarbons, producing coker gas oil, but also a solid carbonaceous byproduct which is called “pet-coke”. This is a highly viscous, black, sticky material, it remains liquid as long as it is transported hot, but solidifies otherwise. Its insoluble fraction in n-heptane is called asphaltene, which thus represents the heaviest fraction of crude oil. The detailed and complete analysis of heavy petroleum fractions requires the use of many different analytical tools because of their chemical complexity and low volatility. However, the molecular weight (MW), the aromaticity and, in general, the chemical functionalities, are the key properties to determine for guiding their processing technology development. In particular, size exclusion chromatography (SEC) and mass spectrometry (MS) are the most eligible techniques for MW determination, while Raman and Fourier Transform Infrared (FTIR) spectroscopy can provide information on aromaticity and chemical functionalities, respectively.
1. Terminology for Refinery Residues
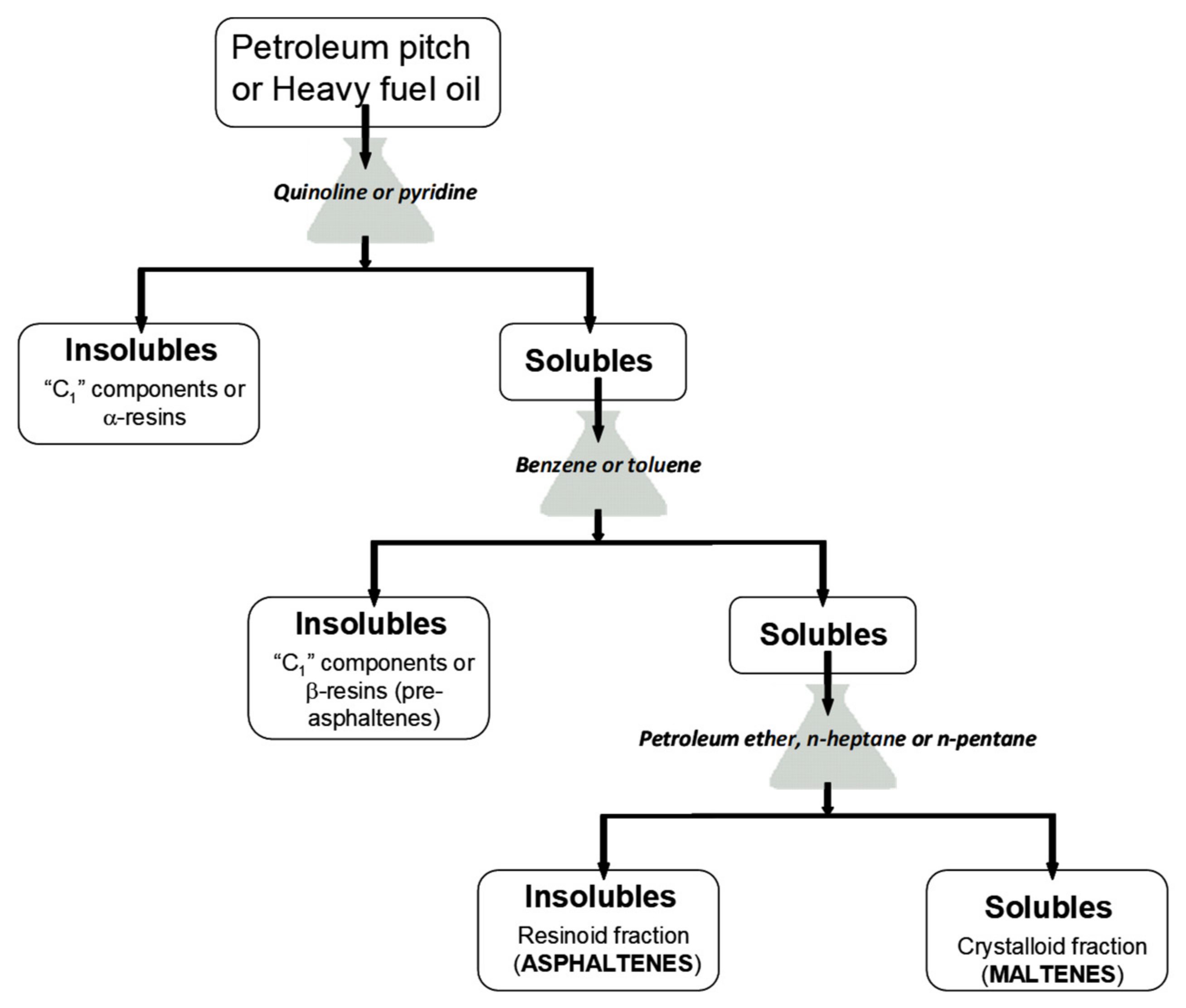
2. Solubility as a Characterization Technique
3. Mass Spectrometry (MS)
4. Size Exclusion Chromatography (SEC)
5. Raman Spectroscopy

6. FTIR Spectroscopy

References
- Wen, C.S.; Chilingarian, G.; Yen, T.F. Properties and structure of bitumens. In Bitumens, Asphalts and Tar Sands; Elsevier: Amsterdam, The Netherlands, 1978; Volume 7, pp. 155–190.
- Mullins, O.C.; Sheu, E.Y. (Eds.) Structures and Dynamics of Asphaltenes; Springer Science & Business Media: New York, NY, USA, 2013.
- Mullins, O.C.; Sheu, E.Y.; Hammami, A.; Marshall, A.G. Asphaltenes, Heavy Oils, and Petroleomics; Springer Science and Business Media: New York, NY, USA, 2007.
- Yen, T.F.; Chilingarian, G.V. Asphaltenes and Asphalts, 2: Part B; Elsevier: Amsterdam, The Netherlands, 2000.
- Speight, J.G. Petroleum Asphaltenes—Part 1: Asphaltenes, Resins and the Structure of Petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477.
- Mullins, O.C.; Martinez-Haya, B.; Marshall, A.G. Contrasting perspective on asphaltene molecular weight. Energy Fuels 2008, 22, 1765–1773.
- Badre, S.; Carla Goncalves, C.; Norinaga, K.; Gustavson, G.; Mullins, O.C. Molecular size and weight of asphaltene and asphaltene solubility fractions fromcoals, crude oils and bitumen. Fuel 2006, 85, 1–11.
- Kokal, S.L.; Selim, G.S. Asphaltenes: The Cholesterol of Petroleum. In Proceedings of the Middle East Oil Show, Manama, Bahrain, 11–14 March 1995.
- Herod, A.A.; Bartle, K.D.; Kandiyoti, R. Comment on a paper by Mullins, Martinez-Haya and Marshall “Contrasting perspective on asphaltene molecular weight. This comment vs the overview of Herod, A.A., Bartle, K.D. and Kandiyoti, R”. Energy Fuels 2008, 22, 4312–4317.
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Andrews, A.B.; Gross, L.; Pauchard, V.; Banerjee, S.; Mullins, O.C. Overview of asphaltene nanostructures and thermodynamic applications. Energy Fuels 2020, 34, 15082–15105.
- Greinke, R.A. Kinetics of petroleum pitch polymerization by gel permeation chromatography. Carbon 1986, 24, 677–686.
- Edwards, W.F.; Jin, L.; Thies, M.C. MALDI-TOF mass spectrometry: Obtaining reliable mass spectra for insoluble carbonaceous pitches. Carbon 2003, 41, 2761–2768.
- Russo, C.; Ciajolo, A.; Stanzione, F.; Tregrossi, A.; Oliano, M.M.; Carpentieri, A.; Apicella, B. Investigation on chemical and structural properties of coal- and petroleum-derived pitches and implications on physico-chemical properties (solubility, softening and coking). Fuel 2019, 245, 478–487.
- Marsh, H.; Cornford, C. Mesophase: The precursor to graphitizable carbon. In Petroleum Derived Carbons; American Chemical Society: Colombia, WA, USA, 1976; pp. 266–280.
- Edie, D.D. The effect of processing on the structure and properties of carbon fibers. Carbon 1998, 36, 345–362.
- Beauharnois, M.E.; Edie, D.D.; Thies, M.C. Carbon fibers from mixtures of AR and supercritically extracted mesophases. Carbon 2001, 39, 2101–2111.
- Wagner, M.H.; Jäger, H.; Letizia, I.; Wilhelmi, G. Quality assessment of binder pitches for carbon and graphite electrodes. Fuel 1988, 67, 792–797.
- Gargiulo, V.; Apicella, B.; Stanzione, F.; Tregrossi, A.; Millan, M.; Ciajolo, A.; Russo, C. Structural Characterization of Large Polycyclic Aromatic Hydrocarbons. Part 2: Solvent-Separated Fractions of Coal Tar Pitch and Naphthalene-Derived Pitch. Energy Fuels 2016, 30, 2574–2583.
- Ozel, M.Z.; Bartle, K.D. Production of Mesophase Pitch from Coal Tar and Petroleum Pitches using Supercritical Fluid Extraction. Turk. J. Chem. 2002, 26, 417–424.
- Chwastiak, S.; Lewis, I.C. Solubility of Mesophase Pitch. Carbon 1978, 16, 156–157.
- Gross, J.H. Mass Spectrometry: A Textbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006.
- Cotter, R.J. Time-of-Flight Mass Spectrometry; ACS Professional Reference Books; American Chemical Society: Colombia, WA, USA, 1997.
- Awad, H.; Khamis, M.M.; El-Aneed, A. Mass spectrometry, review of the basics: Ionization. Appl. Spectrosc. Rev. 2015, 50, 158–175.
- Vastola, F.J.; Mumma, R.O.; Pirone, A.J. Analysis of organic salts by laser ionization. Org. Mass Spectrom. 1979, 3, 101–104.
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301.
- Apicella, B.; Carpentieri, A.; Alfè, M.; Barbella, R.; Tregrossi, A.; Pucci, P.; Ciajolo, A. Mass spectrometric analysis of large PAH in a fuel-rich ethylene flame. Proc. Combust. Inst. 2007, 31, 547–553.
- Rizzi, A.; Cosmina, P.; Flego, C.; Montanari, L.; Seraglia, R.; Traldi, P. Laser desorption/ionization techniques in the characterization of high molecular weight oil fractions Part 1. Asphaltenes. J. Mass Spectrom. 2006, 41, 1232–1237.
- Comisarow, M.B.; Marshall, A.G. Fourier Transform Ion Cyclotron Resonance Spectroscopy. Chem. Phys. Lett. 1974, 25, 282–283.
- Robb, D.B.; Covey, T.R.; Bruins, A.P. Atmospheric pressure photoionization: An ionization method for liquid chromatography-mass spectrometry. Anal. Chem. 2000, 72, 3653–3659.
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418.
- Acevedo, S.; Gutierrez, L.B.; Negrin, G.; Pereira, J.C.; Mendez, B.; Delolme, F.; Dessalces, G.; Broseta, D. Molecular weight of petroleum asphaltenes: A comparison between mass spectrometry and vapor pressure osmometry. Energy Fuels 2005, 19, 1548–1560.
- Al-Muhareb, E.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. Characterization of petroleum asphaltenes by size exclusion chromatography, UV-fluorescence and mass spectrometry. Pet. Sci. Technol. 2007, 25, 81–91.
- Hortal, A.R.; Martinez-Haya, B.; Lobato, M.D.; Pedrosa, J.M.; Lago, S. On the determination of molecular weight distributions of asphaltenes and their aggregates in laser desorption ionization experiments. J. Mass Spectrom. 2006, 41, 960–968.
- Tanaka, R.; Sato, S.; Takanohashi, T.; Hunt, J.E.; Winans, R.E. Analysis of the molecular weight distribution of petroleum asphaltenes using laser desorption-mass spectrometry. Energy Fuels 2004, 18, 1405–1413.
- Apicella, B.; Ciajolo, A.; Carpentieri, A.; Popa, C.; Russo, C. Characterization Techniques Coupled with Mathematical Tools for Deepening Asphaltene Structure. Fuels 2022, 3, 75–84.
- Groenzin, H.; Mullins, O.C. Asphaltenes molecular size and structure. J. Phys. Chem. A 1999, 103, 11237.
- Groenzin, H.; Mullins, O.C. Molecular size and structure of asphaltenes from various sources. Energy Fuels 2000, 14, 677–684.
- Pomerantz, A.E.; Hammond, M.R.; Morrow, A.L.; Mullins, O.C.; Zare, R.N. Two-step laser mass spectrometry of asphaltenes. J. Am. Chem. Soc. 2008, 130, 7216.
- McKenna, A.M.; Marshall, A.G.; Rodgers, R.P. Heavy Petroleum Composition. 4. Asphaltene Compositional Space. Energy Fuels 2013, 27, 1257–1267.
- Apicella, B.; Alfè, M.; Ciajolo, A. Mass Spectrometric Advances in the Analysis of Large Aromatic Fractions of Heavy Fuel Oils and Carbon Particulates. Combust. Sci. Technol. 2010, 182, 640–652.
- Apicella, B.; Alfè, M.; Amoresano, A.; Galano, E.; Ciajolo, A. Advantages and limitations of Laser desorption/ionization Mass Spectrometric techniques in the chemical characterization of complex carbonaceous materials. Int. J. Mass Spectrom. 2010, 295, 98–102.
- Hortal, A.R.; Hurtado, P.; Martinez-Haya, B.M.; Mullins, O.C. Molecular-weight distributions of coal and petroleum asphaltenes from laser desorption/ionization experiments. Energy Fuels 2007, 21, 2863–2868.
- Merdrignac, I.; Espinat, D. Physicochemical characterization of petroleum fractions: The state of the art. Oil Gas Sci. Technol.–Rev. IFP 2007, 62, 7–32.
- Islas, C.A.; Suelves, I.; Millan, M.; Apicella, B.; Herod, A.A.; Kandiyoti, R. Matching average masses of pitch fractions of narrow polydispersity, derived from matrix-assisted laser desorption ionisation time-of-flight mass spectrometry, with the polystyrene calibration of SEC. J. Sep. Sci. 2003, 26, 1422–1428.
- Karaka, F.; Islas, C.A.; Millan, M.; Behrouzi, M.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. The calibration of size exclusion chromatography columns: Molecular mass distributions of heavy hydrocarbon liquids. Energy Fuels 2004, 18, 778–788.
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the Molecular Structures of Asphaltenes by Atomic Force Microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876.
- Cristadoro, A.; Kulkarni, S.U.; Burgess, W.A.; Cervo, E.G.; Räder, H.J.; Müllen, K.; Bruce, D.A.; Thies, M.C. Structural characterization of the oligomeric constituents of petroleum pitches. Carbon 2009, 47, 2358–2370.
- Burgess, W.A.; Pittman, J.J.; Marcus, R.K.; Thies, M.C. Structural identification of the monomeric constituents of petroleum pitch. Energy Fuels 2010, 24, 4301–4311.
- Zhang, W.; Andersson, J.T.; Rader, H.J.; Mullen, K. Molecular characterization of large polycyclic aromatic hydrocarbons in solid petroleum pitch and coal tar pitch by high resolution MALDI ToF MS and insights from ion mobility separation. Carbon 2015, 95, 672–680.
- Sadao, M.; Barth, H.G. Size Exclusion Chromatography; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013.
- Kandiyoti, R.; Herod, A.; Bartle, K.D.; Morgan, T.J. Solid Fuels and Heavy Hydrocarbon Liquids: Thermal Characterization and Analysis; Elsevier: Amsterdam, The Netherlands, 2016.
- Herod, A.A.; Bartle, K.D.; Kandiyoti, R. Characterization of heavy hydrocarbons by chromatographic and mass spectrometric methods: An overview. Energy Fuels 2007, 21, 2176–2203.
- Morgan, T.J.; Georg, A.; Alvarez, P.; Herod, A.A.; Millan, M.; Kandiyoti, R. Isolation of size exclusion chromatography elution-fractions of coal and petroleum-derived samples and analysis by laser desorption mass spectrometry. Energy Fuels 2009, 23, 6003–6014.
- Zhao, P.; Yang, M.; Fan, W.; Wang, X.; Tang, F.; Yang, C.; Dou, X.; Li, S.; Wang, Y.; Cao, Y. Facile One-Pot Conversion of Petroleum Asphaltene to High Quality Green Fluorescent Graphene Quantum Dots and Their Application in Cell Imaging. Part. Part. Syst. Charact. 2016, 33, 635–644.
- Karaca, F.; Morgan, T.J.; George, A.; Bull, I.D.; Herod, A.A.; Millan, M.; Kandiyoti, R. Molecular mass ranges of coal tar pitch fractions by mass spectrometry and size-exclusion chromatography. Rapid Commun. Mass Spectrom. 2009, 23, 2087–2098.
- Gargiulo, V.; Apicella, B.; Alfè, M.; Russo, C.; Stanzione, F.; Tregrossi, A.; Amoresano, A.; Millan, M.; Ciajolo, A. Structural Characterization of Large Polycyclic Aromatic Hydrocarbons. Part 1: The Case of Coal Tar Pitch and Naphthalene-Derived Pitch. Energy Fuels 2015, 29, 5714–5722.
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095.
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414.
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126.
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281.
- Bouhadda, Y.; Bormann, D.; Sheu, E.; Bendedouch, D.; Krallafa, A.; Daaou, M. Characterization of Algerian Hassi-Messaoud asphaltene structure using Raman spectrometry and X-ray diffraction. Fuel 2007, 86, 1855–1864.
- Dumont, M.; Chollon, G.; Dourges, M.A.; Pailler, R.; Bourrat, X.; Naslain, R.; Bruneel, J.L.; Couzi, M. Chemical, microstructural and thermal analyses of a naphthalene-derived mesophase pitch. Carbon 2002, 40, 1475–1486.
- Ferrari, A.C.; Robertson, J. Origin of the 1150−cm−1 Raman mode in nanocrystalline diamond. Phys. Rev. B 2001, 63, 121405.
- Mallet-Ladeira, P.; Puech, P.; Weisbecker, P.; Vignoles, G.L.; Monthioux, M. Behavior of Raman D band for pyrocarbons with crystallite size in the 2–5 nm range. Appl. Phys. A 2014, 114, 759–763.
- Puech, P.; Plewa, J.M.; Mallet-Ladeira, P.; Monthioux, M. Spatial confinement model applied to phonons in disordered graphene-based carbons. Carbon 2016, 105, 275–281.
- Casiraghi, C.; Piazza, F.; Ferrari, A.C.; Grambole, D.; Robertson, J. Bonding in hydrogenated diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2005, 14, 1098–1102.
- Buijnsters, J.G.; Gago, R.; Jiḿnez, I.; Camero, M.; Agulló-Rueda, F.; Gómez-Aleixandre, C. Hydrogen quantification in hydrogenated amorphous carbon films by infrared, Raman, and X-ray absorption near edge spectroscopies. J. Appl. Phys. 2009, 105, 093510.
- Riedeman, J.S.; Kadasala, N.R.; Wei, A.; Kenttämaa, H.I. Characterization of Asphaltene Deposits by Using Mass Spectrometry and Raman Spectroscopy. Energy Fuels 2016, 30, 805–809.
- Öner, F.O.; Yürüm, A.; Yürüma, Y. Structural characterization of semicokes produced from the pyrolysis of petroleum pitches. J. Anal. Appl. Pyrolysis 2015, 111, 15–26.
- Chen, K.; Zhang, H.; Ibrahim, U.K.; Xue, W.; Liu, H.; Guo, A. The quantitative assessment of coke morphology based on the Raman spectroscopic characterization of serial petcokes. Fuel 2019, 246, 60–68.
- Kostecki, R.; Tran, T.; Song, X.; Kinoshita, K.; McLarnon, F. Raman Spectroscopy and Electron Microscopy of Heat-Treated Petcokes for Lithium-Intercalation Electrodes. J. Electrochem. Soc. 1997, 144, 3111.
- Dischler, B.; Bubenzer, A.; Koidl, P. Bonding in hydrogenated hard carbon studied by optical spectroscopy. Solid State Comun. 1983, 48, 105–108.
- Centrone, A.; Brambilla, L.; Renouard, T.; Gherghel, L.; Mathis, C.; Mullen, K.; Zerbi, G. Structure of new carbonaceous material. The role of vibrational spectroscopy. Carbon 2005, 43, 1593–1609.
- Calemma, V.; Iwanski, P.; Nali, M.; Scotti, R.; Montanari, L. Structural Characterization of Asphaltenes of Different Origins. Energy Fuels 1995, 9, 225–230.
- Wilt, B.K.; Welch, W.T. Determination of Asphaltenes in Petroleum Crude Oils by Fourier Transform Infrared Spectroscopy. Energy Fuels 1998, 12, 1008–1012.
- Aske, N.; Kallevik, H.; Sjoblom, J. Determination of Saturate, Aromatic, Resin, and Asphaltenic (SARA) Components in Crude Oils by Means of Infrared and Near-Infrared Spectroscopy. Energy Fuels 2001, 15, 1304–1312.
- Guillén, M.D.; Iglesias, M.J.; Domínguez, A.; Blanco, C.G. Fourier transform infrared study of coal tar pitches. Fuel 1995, 74, 1595–1598.
- Guillén, M.D.; Iglesias, M.J.; Domínguez, A.; Blanco, C.G. Semiquantitative FTIR Analysis of a Coal Tar Pitch and Its Extracts and Residues in Several Organic Solvents. Energy Fuels 1992, 6, 518–525.
- Alcañiz-Monge, J.; Cazorla-Amorós, D.; Linares-Solano, A. Characterisation of coal tar pitches by thermal analysis, infrared spectroscopy and solvent fractionation. Fuel 2001, 80, 41–48.
- Tzeng, S.S.; Pan, J.H. Oxidative stabilization of petroleum pitch at high pressure and its effects on the microstructure and carbon yield after carbonization/graphitization. Mater. Chem. Phys. 2002, 74, 214–221.
- Menendez, J.A.; Pis, J.J.; Alvarez, R.; Barriocanal, C.; Canga, C.S.; Diez, M.A. Characterization of Petcoke as an Additive in Metallurgical Cokemaking. Influence on Metallurgical Coke Quality. Energy Fuels 1997, 11, 379–384.
- Ristein, J.; Stief, R.T.; Ley, L.; Beyer, W. A comparative analysis of a-C:H by infrared spectroscopy and mass selected thermal effusion. J. Appl. Phys. 1998, 84, 3836–3847.
- Jacob, W.; Unger, M. Experimental determination of the absorption strength of C–H vibrations for infrared analysis of hydrogenated carbon films. Appl. Phys. Lett. 1996, 68, 475–477.
- Russo, C.; Stanzione, F.; Tregrossi, A.; Ciajolo, A. Infrared spectroscopy of some carbon based materials relevant in combustion: Qualitative and quantitative analysis of hydrogen. Carbon 2014, 74, 127–138.
- Russo, C.; Tregrossi, A.; Ciajolo, A. Dehydrogenation and growth of soot in premixed flames. Proc. Comb. Inst. 2015, 35, 1803–1809.




