Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zarema Gilazieva | -- | 3978 | 2022-06-01 11:18:02 | | | |
| 2 | Rita Xu | Meta information modification | 3978 | 2022-06-01 11:46:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gilazieva, Z.; Ponomarev, A.; Rizvanov, A.; Solovyeva, V. Mesenchymal Stem Cells and Their Extracellular Vesicles. Encyclopedia. Available online: https://encyclopedia.pub/entry/23658 (accessed on 07 February 2026).
Gilazieva Z, Ponomarev A, Rizvanov A, Solovyeva V. Mesenchymal Stem Cells and Their Extracellular Vesicles. Encyclopedia. Available at: https://encyclopedia.pub/entry/23658. Accessed February 07, 2026.
Gilazieva, Zarema, Aleksei Ponomarev, Albert Rizvanov, Valeriya Solovyeva. "Mesenchymal Stem Cells and Their Extracellular Vesicles" Encyclopedia, https://encyclopedia.pub/entry/23658 (accessed February 07, 2026).
Gilazieva, Z., Ponomarev, A., Rizvanov, A., & Solovyeva, V. (2022, June 01). Mesenchymal Stem Cells and Their Extracellular Vesicles. In Encyclopedia. https://encyclopedia.pub/entry/23658
Gilazieva, Zarema, et al. "Mesenchymal Stem Cells and Their Extracellular Vesicles." Encyclopedia. Web. 01 June, 2022.
Copy Citation
Mesenchymal stem cells (MSCs) are a major component of the tumor microenvironment (TME) and play an important role in tumor progression. MSCs remodel the extracellular matrix, participate in the epithelial–mesenchymal transition, promote the spread of metastases, and inhibit antitumor immune responses in the TME; however, there are also data pertaining to the antitumor effects of MSCs. MSCs activate the cell death mechanism by modulating the expression of proteins involved in the regulation of the cell cycle, angiogenesis receptors, and proapoptotic proteins. One of the main ways in which MSCs and TME interact is through the production of extracellular vesicles (EVs) by cells.
mesenchymal stem cells
extracellular vesicle
tumor microenvironment
intercellular communication
1. Introduction
Mesenchymal stem cells (MSCs) are a population of cells capable of self-renewal and differentiation into osteoblasts, chondrocytes, adipocytes, myocytes, and neurons [1][2]. MSCs can be obtained from various tissues, including bone marrow, adipose tissue, dental pulp, the placenta and the umbilical cord [3]. MSCs have been actively studied and used as a therapeutic tool for a long time, for many diseases, because they are able to undergo self-renewal, multipotency, and immunomodulation, in the absence of major histocompatibility complex (MHC) class II antigens [4][5]. Undoubtedly, MSCs play a special role in tumor progression; however, the mechanisms of action of MSCs on tumor cells, which include both anti-tumor and pro-tumor effects, are still being investigated.
MSCs are able to exhibit a pronounced tropism for areas of inflammation and tumor niches, where they become an integral part of the tumor stroma [6]. To demonstrate the tropism of MSCs for tumor niches, Nakamizo et al. used a mouse xenograft model of human glioma where MSCs, labeled with a fluorescent vital dye SP-DiI, were injected into the carotid artery. The cells were not found in healthy brain tissue, but were localized within areas of glioma, whereas fibroblasts did not show such specificity, and this indicated the MSCs were able to undertake selective migration to the area of tumor formation [7]. In addition, magnetic resonance imaging (MRI) was used to show MSC tropism in a rat orthotopic xenograft model of malignant glioma [8]. This tropism may be related to the monocyte chemoattractant protein 1 (MCP-1)/CC motif chemokine ligand 2 (CCL2) and stromal cell-derived factor-1 (SDF-1)/CXC motif chemokine ligand 12 (CXCL12) chemokines, which mediate MSC migration to CD133+ glioblastoma cells in vitro [9]. MSC tropism can be monitored using bioluminescence imaging, in which MSCs are modified with a luciferase gene. This method allows researchers to determine the migration and engraftment of MSCs in the area of inflammation or tumor formation [10].
The fact that MSCs have the capacity to migrate to tumor niches has been confirmed by a number of other studies. For example, human MSCs genetically modified with an oncolytic virus migrated to tumor cells and released viral particles, which then infected U87MG glioma cells in vitro. By using MSCs, it was possible to deliver 46 times more viral copies to the tumor area when compared with injecting a pure oncolytic virus [11]. The tropism of MSCs for tumor foci was also shown during systemic administration into xenograft mouse models with human mammary and thyroid gland tumors [12]. It is likely that MSCs, similar to immune cells, are able to migrate to inflammation foci due to chemotaxis [13]. Chemoattractants are mediators secreted by tumor cells, such as vascular endothelial growth factor (VEGF) and transforming growth factor (TGF) β1, as well as the cytokines interleukin (IL)-8 and neurotrophin-3 (NT-3) [14][15][16], which attract MSCs to the tumor microenvironment (TME).
Tumor cells can change the functional profile of MSCs from normal trophic to pro-tumor, using extracellular vesicles (EVs). This change leads to the fact that EVs from reprogrammed MSCs begin to affect other TME cells, such as fibroblasts, immune and endothelial cells [17]; however, there is evidence that MSCs also have the potential to exhibit antitumor activity, which is also mediated by EVs (Figure 1) [17].
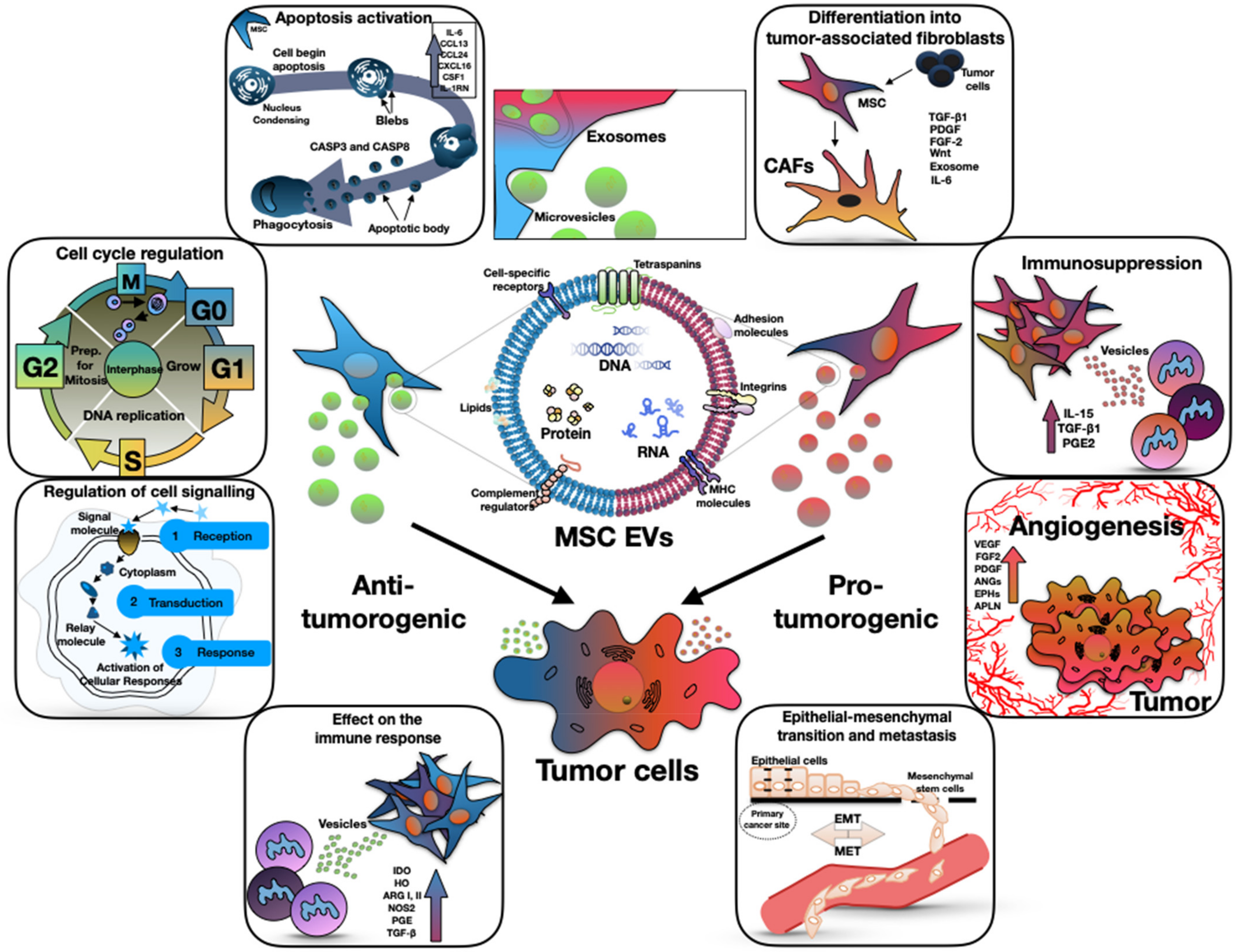
Figure 1. The dual (anti-tumorigenic and pro-tumorigenic) effect of mesenchymal stem cell derived extracellular vesicles on tumor progression. The central part of the figure shows the structure of the EVs. The left side of the figure shows the antitumor effect of EVs through the apoptosis activation, cell cycle regulation, effect on the immune response, and regulation of cell signaling. The right side of the figure shows the pro-tumor effect of EVs through the differentiation into tumor-associated fibroblasts, immunosuppression, angiogenesis and epithelial–mesenchymal transition.
2. Pro-Tumor Properties of Mesenchymal Stromal Cells
2.1. Differentiation into Tumor-Associated Fibroblasts
Tumor-associated fibroblasts (TAFs) in TMEs play a key role in tumor progression. TAFs are cells with a mesenchymal origin that are present in many invasive solid tumors and secrete large numbers of EVs. MSCs in the TME are able to differentiate into TAFs under the influence of various trophic factors, such as TGF-β and epidermal growth factor (EGF), secreted by tumor cells in large quantities [18][19]. In addition, the CXC motif chemokine receptor (CXCR) type 6 signaling pathway can stimulate the differentiation of MSCs into TAFs [20], whereas TGF-β can induce fibroblast differentiation into myofibroblasts by activating the TGF-β/SMAD family member 3 (SMAD3) signaling pathway, synthesis of α-smooth muscle actin (α-SMA) and basic fibroblast growth factor (FGF2) [21]. In a study by Miyazaki et al., in which a model of pancreatic cancer was reproduced, the origin of TAFs from MSCs was confirmed. Mice underwent transplantation with human MSCs extracted from adipose tissue and human pancreatic duct adenocarcinoma cells. It was shown that TAFs are derived from transplanted MSCs and are composed of heterogeneous TAF subpopulations [22]. MSCs can also differentiate into separate TAF subtypes depending on exposure to differing co-culture conditions [23].
TAFs allow the formation of a unique microenvironment that is important during tumor development by releasing fibronectin (FN) and collagen [24]. TAFs are able to stimulate tumor cell proliferation through the secretion of various growth factors, hormones, and cytokines, including hepatocyte growth factor (HGF), FGFs, SDF-1, and IL-6 [25]. SDF-1 activates CXCR4 synthesis and stimulates tumor cell proliferation [24][26]. TAFs also secrete chemokines such as insulin-like growth factor (IGF) 1 and 2, platelet-derived growth factor (PDGF), integrin-α11 (ITGA11), transmembrane heparan sulfate proteoglycan (syndecan-1 (SDC-1), CD138), and matrix metallopeptidase 2 (MMP2). These components have been shown to stimulate proliferation, migration and invasion of tumor cells, as well as increase tumor cell viability against the background of antitumor therapy, thus contributing towards the formation of resistant clones [27]. In in vitro experiments, TAFs have also increased the proliferation rates of neuroblastoma and colon cancer tumor cells [28][29].
Borriello et al. isolated cells from a primary human neuroblastoma tumor and characterized a population of TAFs secreting fibroblast activation protein α (αFAP) and fibroblast-specific protein 1 (FSP-1), which had similar phenotypic and functional characteristics to MSCs extracted from bone marrow [28]. Human neuroblastoma tumor analysis also revealed αFAP- and FSP-1-positive cells in the tumor stroma, which correlated with the presence of tumor-associated macrophages (TAMs). Presumably, the pro-oncogenic role of these TAFs might be mediated by activation of the signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated protein kinase (ERK) signaling pathways 1/2 [28].
Various signaling pathways can be activated by tumor cell exosomes that act on MSCs. It has been shown that lymphocytic leukemia cell exosomes deliver microRNA miR-146a to bone marrow MSCs, where miR-146a mediates the transition of MSCs to TAFs by targeting ubiquitin-specific peptidase 16 (USP16) [30].
2.2. Immunosuppression
MSCs are not able to activate T cell immune responses, since the levels of MHCI and MHCII antigens are reduced on the surface of MSCs. Additionally, there is also no expression of CD40, CD80, CD86 and co-stimulatory molecules necessary for T cell activation [31]; however, MSCs secrete a wide range of regulatory molecules, including IL-15, TGF-β1, and prostaglandin E2 (PGE2), which allows them to suppress the immune response by inhibiting dendritic cell (DC) maturation and suppressing the functions of T cells, B cells, and natural killer cells (NK cells), leading to a decrease in the secretion of soluble immune mediators and inhibition of NK-mediated cytotoxicity. The immunosuppressive properties of MSCs can also enhance tumor necrosis factor α (TNF-α), IL-1β, IL-6, and interferon (IFN) γ, which are involved in the development of antitumor immune responses and angiogenesis [32]. In some types of tumors, where the inflammatory microenvironment predominates, MSCs can directly interact with immune cells, which also leads to a weakening of antitumor immune responses [33]. For example, MSCs are able to inhibit T cell activity, either by suppressing their proliferation or, in the case of activated T cells, by inducing apoptosis. Inhibition of T cell proliferation can also be enhanced by various mechanisms, such as IFN-γ-mediated activation of the programmed cell death receptor ligand 1 (PD-L1) [34] or STRO-1 synthesis [35].
In silico analysis showed a positive association between MSC-associated genes and PD-L1 expression in various types of breast cancer. The conditioned medium (CM) of MSCs not only induced a phenotype switch, but also stimulated PD-L1 expression at the protein level through the secretion of various cytokines, especially CCL5. Treatment of MSCs with the cytokine inhibitor pirfenidone showed a significant decrease in CCL5 secretion and, hence, PD-L1 expression in breast cancer cells [36].
Presumably, the source of origin of MSCs may influence their immunosuppressive properties. For example, MSCs from adipose tissue and bone marrow inhibited lymphocytes but only when they were in close contact [37], while MSCs from Wharton’s jelly suppressed peripheral blood lymphocyte proliferation without the need for close contact [38].
Djouad et al. showed that the immunosuppressive effects of MSCs resulted in a higher incidence of melanoma in a mouse model with allogeneic melanoma cell transplantation [39]. Based on the known immunosuppressive properties of MSCs, it can be assumed that MSCs are able to suppress graft-versus-tumor and graft-versus-host reactions [40][41].
2.3. Angiogenesis Induction
A large number of pro-angiogenic factors, including VEGF, FGF2, PDGF, angiopoietins (ANGs), ephrins (EPHs), apelin (APLN) and their related receptors and chemokines, are known to promote tumor neovascularization [42]. These factors are often synthesized simultaneously, effectively interacting at different stages of tumor angiogenesis.
MSCs can stimulate tumor neoangiogenesis through the production and secretion of multiple angiogenesis factors such as ANGs, EGF, galectin-1 (GAL-1), IGF-1, keratinocyte growth factor (KGF), VEGF, TGF-β, IL-6, and macrophage inflammatory protein (MIP) 2. In addition, MSCs are directly involved in the recruitment of endothelial cells, thereby contributing to the formation of new tumor blood vessels [43][44]. Furthermore, studies have shown that MSCs can differentiate into endothelial cells, which also supports tumor vascularization [20].
In a xenograft model of mice using human hepatocellular carcinoma, it was found that human bone marrow MSCs were able to enhance tumor angiogenesis due to the action of TGF-β1, the secretion of which increased in tumor cells following the intravenous administration of MSCs. Modulation of the TGF-β1/SMAD signaling pathway and its interaction with VEGF may partly explain the complex role of MSCs in tumor progression [45].
Another study that demonstrated the effect of MSCs on angiogenesis was conducted by Huang et al. Using a xenograft model, with human colorectal cancer cells, MSCs and their cell mixture were injected subcutaneously into immunocompromised mice. Mixing various colorectal cancer cells with MSCs increased the tumor growth rate and angiogenesis to a greater extent than mixing with carcinoma-associated fibroblasts or normal colonic fibroblasts. The secretion of IL-6 from MSCs increased the secretion of endothelin-1 (ET-1) in cancer cells, which induced the activation of protein kinase B α (AKT) and ERK in endothelial cells, thereby increasing their ability to recruit and sustain angiogenesis in the tumor [46].
Batlle et al. found that inhibition of p38α kinase, a negative regulator of the MSC angiogenic program in the perivascular spaces, enhanced angiogenesis in human and mouse colon tumors and correlated with increased carcinogenesis [47]. It was also shown that p38α regulated the acquisition of endothelial-like phenotype of MSCs in colon tumors [47].
2.4. Epithelial–Mesenchymal Transition and Metastasis
Epithelial–mesenchymal transition (EMT) is the process by which epithelial cells change from an epithelial phenotype to a mesenchymal phenotype, and this occurs during embryonic development, wound healing, and pathological processes such as fibrosis and tumor progression [48]. Karnoub et al. found that human bone marrow MSCs, when co-administered subcutaneously with MCF-7 breast cancer cells, increased the metastatic potential of MCF-7 tumor cells in a xenograft mouse model with breast cancer [49]. MCF-7 cells stimulated de novo synthesis of CCL5 in MSCs, which then, using paracrine signaling, acted on tumor cells, enhancing their motility, invasion, and metastasis [49]. It was also shown that adipose tissue MSCs, when co-cultivated on Matrigel with MCF-7 cells, induced the formation of tumor spheres in vitro and promoted tumor growth in vivo in a xenograft mouse model with breast cancer. Tumor sphere formation by MCF-7 cells and MSCs has been associated with the induction of stem-like properties that mediate EMT [50].
The co-administration of adipose tissue MSCs and MDA-PCa-118b prostate cancer cells subcutaneously in mice increased tumor growth [51]. In addition, it was found that bone marrow MSCs stimulated the proliferation, migration and invasion of PC3 prostate cancer cells in vitro, and the described effect was then inhibited by blocking TGF-β [52]. In the early stages of carcinogenesis, TGF-β exhibited an immunosuppressive effect on tumor cells, by inhibiting cell proliferation, while at later stages it induced EMT, thus promoting tumor metastasis [53].
Lacerda et al. found that the co-administration of human bone marrow MSCs subcutaneously with SUM149 breast cancer cells increased the invasion and metastasis of the SUM149 cells in a xenograft model of mice with breast cancer [54]. Primary tumors injected together with MSCs were characterized by a higher level of EGF receptor synthesis and contributed towards the development of metastases following tumor removal. This effect was abolished by treatment with the EGF receptor inhibitor—erlotinib [54]. These results point to a role for MSCs in tumor progression and metastasis, possibly through the induction of EMT in primary tumor cells.
After subcutaneous co-injection with B16 mouse melanoma cells, MSCs were able to occupy perivascular sites in tumors and enhanced the metastasis of B16 cells to the lungs. MSCs activated an EMT-like profile in the B16 cells, increasing their mobility and invasiveness. These effects were abolished by blocking MET protein phosphorylation in B16 cells using small molecule inhibitors. MSCs also activated an EMT-like profile in human melanoma cells at various stages of progression. EMT activation in human cells has been associated with increased levels of phosphorylated (p) STAT1 and pSTAT3. Both murine and human melanoma cells are able to activate an EMT-like program and acquire metastatic features through activation of various pathways by MSC secretome [55].
Interestingly, in a study by Takigawa et al., the proliferation and migration of KM12SM human colon tumor cells increased following co-cultivation with MSCs. Expression of genes associated with EMT, such as FN, secreted protein acidic and rich in cysteine (SPARC), and GAL-1, were increased by direct co-cultivation with MSCs. Thus, MSCs induced EMT in colon cancer cells through direct intercellular contact and, therefore, may play an important role in colon cancer metastasis [56].
Human MSCs can promote hepatocellular carcinoma growth via activation of the mitogen-activated protein kinase (MAPK) signaling pathway, promoting metastasis in vivo via EMT. RNA sequencing showed an overexpression of α5 integrin (ITGA5) in the MSC-treated hepatocellular carcinoma cells. ITGA5 siRNA blocked the MSC-induced migration and invasion of hepatocellular carcinoma cells, whilst ITGA5 overexpression promoted tumor cell migration and invasion, indicating that ITGA5 expression is associated with MSC-induced tumor metastasis [57].
3. Antitumor Properties of Mesenchymal Stromal Cells
3.1. Apoptosis Induction
Sun et al. found that in a xenograft mouse model with breast cancer, systemic administration of MSCs from adipose tissue or umbilical cord blood inhibited metastasis and reduced the growth rate of MDA-MB-231 tumor cells [58]. The authors also showed that transplantation of MSCs at an early stage of tumor development, prior to detectable clinical symptoms, did not contribute to the growth and metastasis of tumor cells [58]. Therefore, tumor inhibition could be associated with the degradation of poly-(ADP-ribose) polymerase (PARP) due to the activation of caspase (CASP) 3, which induces apoptosis, and the expression of the p21 gene [58][59].
Atsuta et al. found that when MSCs interacted with multiple myeloma (MM) cells, tumor cell proliferation was inhibited through Fas-mediated apoptosis (Fas and Fas-L are co-synthesized on MSCs, which, when co-cultivated, can reduce the proliferation of MM cells), and apoptosis was induced due to the activation of CASP3 and CASP8 [60]. Cord blood MSCs have inhibited the growth of H1299 lung cancer and A375 melanoma cells in vitro. At the same time, the synthesis of kinases, including AKT, phosphoinositide 3-kinase (PI3K), ERK, STAT3, and the mammalian target of rapamycin (mTOR), were significantly reduced in both tumor cell types when co-cultivated with MSCs [61][62]. Cord blood MSCs have also shown an inhibitory effect on ovarian cancer cells CAOV-3 in co-culture, where increases in the number of apoptotic CAOV-3 cells alongside decreased proliferation were noted [63].
The antitumor activity of particular MSCs may also depend on cell origin. For example, in a xenograft model of mice with human hepatocarcinoma, it was shown that when MSCs from bone marrow and umbilical cord blood were injected, the tumor size decreased, but MSCs from umbilical cord blood had a more pronounced antitumor effect than MSCs from bone marrow [64]. In addition, it was found that MSCs from various sources (adipose tissue, bone marrow, and umbilical cord blood) when co-cultivated with ovarian cancer cells (OVCAR3, CAOV-3, IGROV3, and SKOV3), caused a significant decrease in synthesis levels of tumor markers, such as tumor antigen CA-125, lactate dehydrogenase (LDH) and β-subunit of human chorionic gonadotropin (hCG) in tumor cells, and also suppressed cell proliferation in vitro. A similar effect was observed when ovarian cancer cells were cultivated with CM from MSCs [65]. It was found that the CM from adipose tissue MSCs suppressed MCF-7 breast cancer cell growth by activating the STAT1 signaling pathway with IFN-β [66].
Coccè et al. showed that MSCs, their lysate, and secretions, inhibited proliferation of malignant pleural mesothelioma cells. MSC lysates also induced apoptosis of tumor cells. In addition, MSCs integrated with tumor cells and had a significant inhibitory effect on the proliferation of malignant pleural mesothelioma cells [67]. Aslam et al. showed that MSC lysates (Wharton’s Jelly and bone marrow) induced a general inhibitory effect on the proliferation of glioma cells and fibroblasts [68].
MSCs obtained from the placental decidual parietal membrane modulated the expression of various proapoptotic genes, as well as oncogenes, in MDA231 human breast cancer cells. The MDA231 cells showed a significant reduction in the proliferation, migration and invasion potential after they were treated with MSCs [69].
Thus, a large number of studies confirm that MSCs can induce apoptosis through various mechanisms; however, the use of MSCs in therapy requires further study of their effect on tumor cells in order to identify specific mechanisms and select certain conditions for their interaction with tumor cells.
3.2. Regulation of Cell Signaling
PI3K/AKT and WNT/β-catenin signaling pathways control cell survival, proliferation, growth, migration, and metabolism. Numerous studies have described the need for AKT signaling for tumor cell migration, invasion, and survival. The WNT signaling pathway is also associated with the development of carcinomas of the breast, liver, colon, skin, stomach, and ovaries [70].
Intravenous administration of MSCs effectively inhibited tumor cell proliferation via inhibition of the AKT pathway in a model of Kaposi’s sarcoma [71]. Moreover, cord blood stem cells activated the synthesis of phosphatase PTEN (phosphatase and tensin homolog deleted on chromosome 10) in glioma cells, which led to the suppression of signal transduction along the AKT pathway [72].
In addition to inhibiting the PI3K/AKT signaling pathway, MSCs can also suppress the WNT/β-catenin signaling pathway by inducing Dickkopf-related protein 1 (DKK-1) synthesis. In vitro experiments showed β-catenin levels decreased in human carcinoma cells (hepatocellular carcinomas H7402 and HepG2, breast carcinomas MCF-7, hematopoietic carcinomas K562 and HL60) under the influence of DKK-1 secreted by MSCs. Suppression of DKK-1 activity with neutralizing antibodies or small interfering RNAs (siRNAs) resulted in a weakening of the inhibitory effect of MSCs on tumor cell proliferation [73][74][75]. The study’s authors showed that MSCs can also affect the STAT3 pathway in breast cancer cells. CM from human umbilical cord MSCs suppressed the activation of STAT3 signaling and tumor growth [76].
Thus, the regulation of signaling pathways by MSCs can lead to tumor inhibition and the study of these properties of MSCs can provide new clues to the MSC-mediated mechanism of disease prevention.
3.3. Cell Cycle Regulation
MSCs secrete many cytokines that can temporarily induce cell cycle arrest in tumor cells in the G0/G1 phase by reducing the synthesis of cyclins A, E, and D2, as well as p27KIP1 [77][78]. Lu et al. found that adipose tissue MSCs and CM from these cells suppressed the growth of pancreatic adenocarcinoma [59]. In addition, CM from MSCs stimulated tumor cell necrosis after G0/G1 phase arrest in the absence of apoptosis, and MSCs modulated the expression of p21 gene, which is a negative regulator of the tumor cell cycle [59].
In another experiment, the co-cultivation of chronic myelogenous leukemia K562 cells with bone marrow or umbilical cord blood MSCs resulted in the accumulation of tumor cells in the G0/G1 phase and a slowdown in the transition to the S phase [79][80]. This regulation of the cell cycle may be caused by the secretion of cytokines such as IL-6 and IL-8 [80].
Sarmadi et al. showed that human umbilical cord MSCs affected the proliferation of leukemic cells—the growth of tumor cells was stopped at the G0/G1 phase. This result may be associated with the impaired expression of genes encoding cell cycle regulators, such as cell division protein kinase 6 (CDK6), cyclin E2 (CCNE2), cyclin-dependent kinase inhibitor (CDKN) 1A, CDKN2A, dumbbell former 4 protein (DBF4), mouse double minute 2 homolog (MDM2), proteasome activator complex subunit 3 (PSME3), and G0/G1 switch 2 (G0S2) [81].
In contrast, many in vivo studies have shown that the combined administration of MSCs and tumor cells resulted in more active tumor growth than when only tumor cells were administered; however, the exact mechanisms by which the MSCs are able to induce cell cycle arrest are not fully understood. Possibly, the slowing down or stopping of the cell cycle can be induced in certain types of tumor cells and under certain conditions of co-cultivation (type of medium, cell concentration or time of co-cultivation).
3.4. Effect on the Immune Response
It is known that MSCs can suppress immune responses; however, there are data reporting the opposite effects of MSCs too. For example, it has been shown that MSCs can stimulate peripheral blood mononuclear cells [82][83]. Kawabata et al. showed that rat umbilical cord MSCs attenuated mammary tumor growth by enhancing the host’s antitumor immune response. Immunohistochemical analysis showed that the majority of infiltrating lymphocytes in tumors from MSC-treated rats were CD3+ T cells. In addition, the MSC treatment significantly increased the infiltration of CD8+ and CD4+ T cells and NK cells throughout the tumor tissue [84] and it should be noted that the presence of an immune infiltrate is usually associated with a good prognosis. Ohlsson et al. found that the co-administration of tumor cells and MSCs to rats induced increased infiltration of granulocytes and monocytes in vivo, as opposed to the administration of either tumor cells or MSCs alone [85]. The authors used a preformed gelatin matrix containing colon carcinoma cells and MSCs, which was then subcutaneously transplanted into rats to control tumor growth and a subsequent inflammatory response. MSCs suppressed the proliferation of tumor cells and infiltration by both granulocytes and macrophages was much higher in rats that were simultaneously transplanted with a mixture of tumor cells and MSCs than in rats injected with tumor cells without MSCs [85].
According to the literature, it is possible to influence MSCs by blocking their immunosuppressive properties. For example, inhibition of indoleamine-pyrrole 2,3-dioxygenase (IDO), heme oxygenase (HO), arginase (ARG) I and ARGII, nitric oxide synthase 2 (NOS2), PGE2 and the TGF-β, PD-1-PD signaling pathway -L1 may lead to overcoming immunosuppression in TME [86][87][88][89][90].
Thus, the effect on the immune system and its stimulation can be used as one of the options for antitumor therapy; however, a large amount of data on the immunosuppressive properties of MSCs has cast doubt on the possibility of their use for such therapy. Therefore, it is relevant to understand the mechanisms of the influences of MSCs on the immune system, since the knowledge gained can accelerate the development of this direction.
References
- Chulpanova, D.S.; Solovyeva, V.V.; James, V.; Arkhipova, S.S.; Gomzikova, M.O.; Garanina, E.E.; Akhmetzyanova, E.R.; Tazetdinova, L.G.; Khaiboullina, S.F.; Rizvanov, A.A. Human Mesenchymal Stem Cells Overexpressing Interleukin 2 Can Suppress Proliferation of Neuroblastoma Cells in Co-Culture and Activate Mononuclear Cells In Vitro. Bioengineering 2020, 7, 59.
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024.
- Solovyeva, V.V.; Salafutdinov, I.I.; Tazetdinova, L.G.; Khaiboullina, S.F.; Masgutov, R.F.; Rizvanov, A.A. Genetic Modification of Adipose Derived Stem Cells with Recombinant Plasmid DNA pBud-VEGF-FGF2 Results in Increased of IL-8 and MCP-1 Secretion. J. Pure Appl. Microbiol. 2014, 8, 523–528.
- Malhotra, P.; Shukla, M.; Meena, P.; Kakkar, A.; Khatri, N.; Nagar, R.K.; Kumar, M.; Saraswat, S.K.; Shrivastava, S.; Datt, R.; et al. Mesenchymal stem cells are prospective novel off-the-shelf wound management tools. Drug Deliv. Transl. Res. 2022, 12, 79–104.
- Lou, S.Y.; Duan, Y.T.; Nie, H.Z.; Cui, X.J.; Du, J.L.; Yao, Y.F. Mesenchymal stem cells: Biological characteristics and application in disease therapy. Biochimie 2021, 185, 9–21.
- Melzer, C.; Yang, Y.; Hass, R. Interaction of MSC with tumor cells. Cell Commun. Signal. CCS 2016, 14, 20.
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J.; et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005, 65, 3307–3318.
- Cao, M.H.; Mao, J.J.; Duan, X.H.; Lu, L.J.; Zhang, F.; Lin, B.L.; Chen, M.W.; Zheng, C.S.; Zhang, X.; Shen, J. In vivo tracking of the tropism of mesenchymal stem cells to malignant gliomas using reporter gene-based MR imaging. Int. J. Cancer 2018, 142, 1033–1046.
- Pavon, L.F.; Sibov, T.T.; de Souza, A.V.; da Cruz, E.F.; Malheiros, S.M.F.; Cabral, F.R.; de Souza, J.G.; Boufleur, P.; de Oliveira, D.M.; de Toledo, S.R.C.; et al. Tropism of mesenchymal stem cell toward CD133(+) stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res. Ther. 2018, 9, 310.
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using In Vivo Bioluminescent Imaging. Stem Cells 2009, 27, 2614–2623.
- Sonabend, A.M.; Ulasov, I.V.; Tyler, M.A.; Rivera, A.A.; Mathis, J.M.; Lesniak, M.S. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells 2008, 26, 831–841.
- Kalimuthu, S.; Zhu, L.; Oh, J.M.; Gangadaran, P.; Lee, H.W.; Baek, S.H.; Rajendran, R.L.; Gopal, A.; Jeong, S.Y.; Lee, S.W.; et al. Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin. Int. J. Med. Sci. 2018, 15, 1051–1061.
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912.
- Ringe, J.; Strassburg, S.; Neumann, K.; Endres, M.; Notter, M.; Burmester, G.R.; Kaps, C.; Sittinger, M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007, 101, 135–146.
- Kim, S.M.; Oh, J.H.; Park, S.A.; Ryu, C.H.; Lim, J.Y.; Kim, D.S.; Chang, J.W.; Oh, W.; Jeun, S.S. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells 2010, 28, 2217–2228.
- Klopp, A.H.; Spaeth, E.L.; Dembinski, J.L.; Woodward, W.A.; Munshi, A.; Meyn, R.E.; Cox, J.D.; Andreeff, M.; Marini, F.C. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007, 67, 11687–11695.
- Lindoso, R.S.; Collino, F.; Vieyra, A. Extracellular vesicles as regulators of tumor fate: Crosstalk among cancer stem cells, tumor cells and mesenchymal stem cells. Stem Cell Investig. 2017, 4, 75.
- Jotzu, C.; Alt, E.; Welte, G.; Li, J.; Hennessy, B.T.; Devarajan, E.; Krishnappa, S.; Pinilla, S.; Droll, L.; Song, Y.H. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell. Oncol. 2011, 34, 55–67.
- Pietras, K.; Ostman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331.
- Lee, H.Y.; Hong, I.S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017, 108, 1939–1946.
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010, 70, 9621–9630.
- Miyazaki, Y.; Oda, T.; Inagaki, Y.; Kushige, H.; Saito, Y.; Mori, N.; Takayama, Y.; Kumagai, Y.; Mitsuyama, T.; Kida, Y.S. Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Sci. Rep. 2021, 11, 1–12.
- Miyazaki, Y.; Oda, T.; Mori, N.; Kida, Y.S. Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer-associated fibroblastsin vitro. FEBS Open Bio 2020, 10, 2268–2281.
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. (Landmark Ed.) 2010, 15, 166–179.
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337.
- Sullivan, R.; Maresh, G.; Zhang, X.; Salomon, C.; Hooper, J.; Margolin, D.; Li, L. The Emerging Roles of Extracellular Vesicles As Communication Vehicles within the Tumor Microenvironment and Beyond. Front. Endocrinol. 2017, 8, 194.
- Peddareddigari, V.G.; Wang, D.; Dubois, R.N. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2010, 3, 149–166.
- Borriello, L.; Nakata, R.; Sheard, M.A.; Fernandez, G.E.; Sposto, R.; Malvar, J.; Blavier, L.; Shimada, H.; Asgharzadeh, S.; Seeger, R.C.; et al. Cancer-Associated Fibroblasts Share Characteristics and Protumorigenic Activity with Mesenchymal Stromal Cells. Cancer Res. 2017, 77, 5142–5157.
- Nakagawa, H.; Liyanarachchi, S.; Davuluri, R.V.; Auer, H.; Martin, E.W., Jr.; de la Chapelle, A.; Frankel, W.L. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene 2004, 23, 7366–7377.
- Yang, Y.L.; Li, J.; Geng, Y.H. Exosomes derived from chronic lymphocytic leukaemia cells transfer miR-146a to induce the transition of mesenchymal stromal cells into cancer-associated fibroblasts. J. Biochem. 2020, 168, 491–498.
- Bassi, E.J.; Aita, C.A.; Camara, N.O. Immune regulatory properties of multipotent mesenchymal stromal cells: Where do we stand? World J. Stem Cells 2011, 3, 1–8.
- Kansy, B.A.; Dissmann, P.A.; Hemeda, H.; Bruderek, K.; Westerkamp, A.M.; Jagalski, V.; Schuler, P.; Kansy, K.; Lang, S.; Dumitru, C.A.; et al. The bidirectional tumor--mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res. 2014, 5, 95.
- O’Malley, G.; Heijltjes, M.; Houston, A.M.; Rani, S.; Ritter, T.; Egan, L.J.; Ryan, A.E. Mesenchymal stromal cells (MSCs) and colorectal cancer: A troublesome twosome for the anti-tumour immune response? Oncotarget 2016, 7, 60752–60774.
- Sheng, H.; Wang, Y.; Jin, Y.; Zhang, Q.; Zhang, Y.; Wang, L.; Shen, B.; Yin, S.; Liu, W.; Cui, L.; et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008, 18, 846–857.
- Nasef, A.; Zhang, Y.Z.; Mazurier, C.; Bouchet, S.; Bensidhoum, M.; Francois, S.; Gorin, N.C.; Lopez, M.; Thierry, D.; Fouillard, L.; et al. Selected Stro-1-enriched bone marrow stromal cells display a major suppressive effect on lymphocyte proliferation. Int. J. Lab. Hematol. 2009, 31, 9–19.
- Es, H.A.; Bigdeli, B.; Zhand, S.; Aref, A.R.; Thiery, J.P.; Warkiani, M.E. Mesenchymal stem cells induce PD-L1 expression through the secretion of CCL5 in breast cancer cells. J. Cell. Physiol. 2021, 236, 3918–3928.
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129.
- Zhou, C.; Yang, B.; Tian, Y.; Jiao, H.; Zheng, W.; Wang, J.; Guan, F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011, 272, 33–38.
- Djouad, F.; Plence, P.; Bony, C.; Tropel, P.; Apparailly, F.; Sany, J.; Noel, D.; Jorgensen, C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003, 102, 3837–3844.
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Gotherstrom, C.; Hassan, M.; Uzunel, M.; Ringden, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441.
- Ning, H.; Yang, F.; Jiang, M.; Hu, L.; Feng, K.; Zhang, J.; Yu, Z.; Li, B.; Xu, C.; Li, Y.; et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: Outcome of a pilot clinical study. Leukemia 2008, 22, 593–599.
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. CMLS 2020, 77, 1745–1770.
- Lin, L.; Sun, W.; Wang, L. Effects of mesenchymal stem cells on angiogenesis of cervical cancer HeLa cancer cell line HeLa in vivo. Zhonghua Yi Xue Za Zhi 2015, 95, 1175–1178.
- Zhang, T.; Lee, Y.W.; Rui, Y.F.; Cheng, T.Y.; Jiang, X.H.; Li, G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res. 2013, 4, 70.
- Li, G.C.; Zhang, H.W.; Zhao, Q.C.; Sun, L.I.; Yang, J.J.; Hong, L.; Feng, F.; Cai, L. Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor beta1. Oncol. Lett. 2016, 11, 1089–1094.
- Huang, W.H.; Chang, M.C.; Tsai, K.S.; Hung, M.C.; Chen, H.L.; Hung, S.C. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 2013, 32, 4343–4354.
- Batlle, R.; Andres, E.; Gonzalez, L.; Llonch, E.; Igea, A.; Gutierrez-Prat, N.; Berenguer-Llergo, A.; Nebreda, A.R. Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38 alpha through TGF-beta and JNK signaling. Nat. Commun. 2019, 10, 1–18.
- Christodoulou, I.; Goulielmaki, M.; Devetzi, M.; Panagiotidis, M.; Koliakos, G.; Zoumpourlis, V. Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Res. 2018, 9, 336.
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563.
- Chen, Y.; He, Y.; Wang, X.; Lu, F.; Gao, J. Adiposederived mesenchymal stem cells exhibit tumor tropism and promote tumorsphere formation of breast cancer cells. Oncol. Rep. 2019, 41, 2126–2136.
- Prantl, L.; Muehlberg, F.; Navone, N.M.; Song, Y.H.; Vykoukal, J.; Logothetis, C.J.; Alt, E.U. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate 2010, 70, 1709–1715.
- Ye, H.; Cheng, J.; Tang, Y.; Liu, Z.; Xu, C.; Liu, Y.; Sun, Y. Human bone marrow-derived mesenchymal stem cells produced TGFbeta contributes to progression and metastasis of prostate cancer. Cancer Investig. 2012, 30, 513–518.
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal Modulators of TGF-beta in Cancer. J. Clin. Med. 2017, 6, 7.
- Lacerda, L.; Debeb, B.G.; Smith, D.; Larson, R.; Solley, T.; Xu, W.; Krishnamurthy, S.; Gong, Y.; Levy, L.B.; Buchholz, T.; et al. Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model. Breast Cancer Res. BCR 2015, 17, 42.
- De Souza, L.E.B.; Ferreira, F.U.; Thome, C.H.; Brand, H.; Orellana, M.D.; Faca, V.M.; Fontes, A.M.; Covas, D.T. Human and mouse melanoma cells recapitulate an EMT-like program in response to mesenchymal stromal cells secretome. Cancer Lett. 2021, 501, 114–123.
- Takigawa, H.; Kitadai, Y.; Shinagawa, K.; Yuge, R.; Higashi, Y.; Tanaka, S.; Yasui, W.; Chayama, K. Mesenchymal Stem Cells Induce Epithelial to Mesenchymal Transition in Colon Cancer Cells through Direct Cell-to-Cell Contact. Neoplasia 2017, 19, 429–438.
- Chen, J.; Ji, T.; Wu, D.; Jiang, S.; Zhao, J.; Lin, H.; Cai, X.J. Human mesenchymal stem cells promote tumor growth via MAPK pathway and metastasis by epithelial mesenchymal transition and integrin alpha 5 in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 425.
- Sun, B.; Roh, K.H.; Park, J.R.; Lee, S.R.; Park, S.B.; Jung, J.W.; Kang, S.K.; Lee, Y.S.; Kang, K.S. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy 2009, 11, 289–298.
- Lu, Y.R.; Yuan, Y.; Wang, X.J.; Wei, L.L.; Chen, Y.N.; Cong, C.; Li, S.F.; Long, D.; Tan, W.D.; Mao, Y.Q.; et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol. 2008, 7, 245–251.
- Atsuta, I.; Liu, S.; Miura, Y.; Akiyama, K.; Chen, C.; An, Y.; Shi, S.; Chen, F.M. Mesenchymal stem cells inhibit multiple myeloma cells via the Fas/Fas ligand pathway. Stem Cell Res. 2013, 4, 111.
- Chai, L.; Bai, L.; Li, L.; Chen, F.; Zhang, J. Biological functions of lung cancer cells are suppressed in co-culture with mesenchymal stem cells isolated from umbilical cord. Exp. Ther. Med. 2018, 15, 1076–1080.
- Wang, W.; Li, L.; Chen, F.; Yang, Y. Umbilical cordderived mesenchymal stem cells can inhibit the biological functions of melanoma A375 cells. Oncol. Rep. 2018, 40, 511–517.
- Li, X.; Li, Z. Effects of human umbilical cord mesenchymal stem cells on co-cultured ovarian carcinoma cells. Microsc. Res. Tech. 2019, 82, 898–902.
- Alshareeda, A.T.; Alsowayan, B.; Almubarak, A.; Alghuwainem, A.; Alshawakir, Y.; Alahmed, M. Exploring the Potential of Mesenchymal Stem Cell Sheet on The Development of Hepatocellular Carcinoma In Vivo. J. Vis. Exp. 2018, 139, 1–6.
- Khalil, C.; Moussa, M.; Azar, A.; Tawk, J.; Habbouche, J.; Salameh, R.; Ibrahim, A.; Alaaeddine, N. Anti-proliferative effects of mesenchymal stem cells (MSCs) derived from multiple sources on ovarian cancer cell lines: An in-vitro experimental study. J. Ovarian Res. 2019, 12, 70.
- Ryu, H.; Oh, J.E.; Rhee, K.J.; Baik, S.K.; Kim, J.; Kang, S.J.; Sohn, J.H.; Choi, E.; Shin, H.C.; Kim, Y.M.; et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-beta and suppress the growth of MCF-7 human breast cancer cells. Cancer Lett. 2014, 352, 220–227.
- Cocce, V.; La Monica, S.; Bonelli, M.; Alessandri, G.; Alfieri, R.; Lagrasta, C.A.; Madeddu, D.; Frati, C.; Flammini, L.; Lisini, D.; et al. Inhibition of Human Malignant Pleural Mesothelioma Growth by Mesenchymal Stromal Cells. Cells 2021, 10, 1467.
- Aslam, N.; Abusharieh, E.; Abuarqoub, D.; Alhattab, D.; Jafar, H.; Alshaer, W.; Masad, R.J.; Awidi, A.S. An In Vitro Comparison of Anti-Tumoral Potential of Wharton’s Jelly and Bone Marrow Mesenchymal Stem Cells Exhibited by Cell Cycle Arrest in Glioma Cells (U87MG). Pathol. Oncol. Res. 2021, 27, 584710.
- Basmaeil, Y.; Bahattab, E.; Al Subayyil, A.; Kulayb, H.B.; Alrodayyan, M.; Abumaree, M.; Khatlani, T. Decidua Parietalis Mesenchymal Stem/Stromal Cells and Their Secretome Diminish the Oncogenic Properties of MDA231 Cells In Vitro. Cells 2021, 10, 3493.
- Rhee, K.J.; Lee, J.I.; Eom, Y.W. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033.
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247.
- Dasari, V.R.; Kaur, K.; Velpula, K.K.; Gujrati, M.; Fassett, D.; Klopfenstein, J.D.; Dinh, D.H.; Rao, J.S. Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS ONE 2010, 5, e10350.
- Qiao, L.; Xu, Z.; Zhao, T.; Zhao, Z.; Shi, M.; Zhao, R.C.; Ye, L.; Zhang, X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008, 18, 500–507.
- Qiao, L.; Xu, Z.L.; Zhao, T.J.; Ye, L.H.; Zhang, X.D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008, 269, 67–77.
- Zhu, Y.; Sun, Z.; Han, Q.; Liao, L.; Wang, J.; Bian, C.; Li, J.; Yan, X.; Liu, Y.; Shao, C.; et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 2009, 23, 925–933.
- He, N.; Kong, Y.; Lei, X.; Liu, Y.; Wang, J.; Xu, C.; Wang, Y.; Du, L.; Ji, K.; Wang, Q.; et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis. 2018, 9, 1026.
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827.
- Ramasamy, R.; Lam, E.W.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: Impact on in vivo tumor growth. Leukemia 2007, 21, 304–310.
- Fathi, E.; Farahzadi, R.; Valipour, B.; Sanaat, Z. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS ONE 2019, 14, e0215678.
- Fonseka, M.; Ramasamy, R.; Tan, B.C.; Seow, H.F. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) inhibit the proliferation of K562 (human erythromyeloblastoid leukaemic cell line). Cell Biol. Int. 2012, 36, 793–801.
- Sarmadi, V.H.; Ahmadloo, S.; Boroojerdi, M.H.; John, C.M.; Al-Graitte, S.J.R.; Lawal, H.; Maqbool, M.; Hwa, L.K.; Ramasamy, R. Human Mesenchymal Stem Cells-mediated Transcriptomic Regulation of Leukemic Cells in Delivering Anti-tumorigenic Effects. Cell Transpl. 2020, 29, 1–13.
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513.
- Papait, A.; Vertua, E.; Magatti, M.; Ceccariglia, S.; De Munari, S.; Silini, A.R.; Sheleg, M.; Ofir, R.; Parolini, O. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells 2020, 9, 127.
- Kawabata, A.; Ohta, N.; Seiler, G.; Pyle, M.M.; Ishiguro, S.; Zhang, Y.Q.; Becker, K.G.; Troyer, D.; Tamura, M. Naive rat umbilical cord matrix stem cells significantly attenuate mammary tumor growth through modulation of endogenous immune responses. Cytotherapy 2013, 15, 586–597.
- Ohlsson, L.B.; Varas, L.; Kjellman, C.; Edvardsen, K.; Lindvall, M. Mesenchymal progenitor cell-mediated inhibition of tumor growth in vivo and in vitro in gelatin matrix. Exp. Mol. Pathol. 2003, 75, 248–255.
- Poggi, A.; Varesano, S.; Zocchi, M.R. How to Hit Mesenchymal Stromal Cells and Make the Tumor Microenvironment Immunostimulant Rather Than Immunosuppressive. Front. Immunol. 2018, 9, 262.
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005, 35, 1482–1490.
- Chinnadurai, R.; Copland, I.B.; Patel, S.R.; Galipeau, J. IDO-Independent Suppression of T Cell Effector Function by IFN-gamma-Licensed Human Mesenchymal Stromal Cells. J. Immunol. 2014, 192, 1491–1501.
- Cheng, C.C.; Guan, S.S.; Yang, H.J.; Chang, C.C.; Luo, T.Y.; Chang, J.; Ho, A.S. Blocking heme oxygenase-1 by zinc protoporphyrin reduces tumor hypoxia-mediated VEGF release and inhibits tumor angiogenesis as a potential therapeutic agent against colorectal cancer. J. Biomed. Sci. 2016, 23, 18.
- Akhurst, R.J.; Hata, A. Targeting the TGF beta signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
827
Revisions:
2 times
(View History)
Update Date:
01 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




