Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nydia Tejeda | -- | 1619 | 2022-05-24 01:43:16 | | | |
| 2 | Camila Xu | Meta information modification | 1619 | 2022-05-24 05:03:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tejeda, N.; Mei, K.; , . Wnt Signaling Triggers Macropinocytosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/23258 (accessed on 08 February 2026).
Tejeda N, Mei K, . Wnt Signaling Triggers Macropinocytosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/23258. Accessed February 08, 2026.
Tejeda, Nydia, Kuo-Ching Mei, . "Wnt Signaling Triggers Macropinocytosis" Encyclopedia, https://encyclopedia.pub/entry/23258 (accessed February 08, 2026).
Tejeda, N., Mei, K., & , . (2022, May 24). Wnt Signaling Triggers Macropinocytosis. In Encyclopedia. https://encyclopedia.pub/entry/23258
Tejeda, Nydia, et al. "Wnt Signaling Triggers Macropinocytosis." Encyclopedia. Web. 24 May, 2022.
Copy Citation
Membrane trafficking, including endocytosis and exocytosis, is very important in the interaction between cells and their environment. Endocytosis mediates the degradation of receptors, hence downregulating signaling pathways. The Wnt pathway is essential for cellular functions, such as cell fate determination, cell migration, cell polarity, neural patterning and organogenesis during embryonic development, including axis formation. Macropinocytosis is the large nonselective uptake of molecules such as nutrients and other macromolecules in the cellular environment.
macropinocytosis
Wnt signaling
membrane trafficking
V-ATPase
1. Membrane Trafficking, Lysosomes, V-ATPase, and Macropinocytosis in the Wnt Pathway
Membrane trafficking, including endocytosis and exocytosis, is very important in the interaction between cells and their environment. Endocytosis mediates the degradation of receptors, hence downregulating signaling pathways [1]. The Wnt pathway is essential for cellular functions, such as cell fate determination, cell migration, cell polarity, neural patterning and organogenesis during embryonic development, including axis formation [2]. The Wnt signaling pathway has been linked to cancer since its discovery. It was found that the overexpression or insertion of int1, a mouse gene identical to the Drosophila gene Wnt1, in the Wnt1 region of the genome lead to the formation of tumors [3][4]. Wnt signaling is very complex, belonging to large families of both ligands and receptors. In mammals, there are 19 Wnt ligands and 10 Fzd receptors, in addition to several other pathway activators. Wnt proteins range in length from 350 to 400 amino acids and are post-translationally modified by the O-acyltransferase Porcupine (PORCN), which palmitoylates Wnt proteins in single serine residues. This lipidation forms a binding motif for interacting with Wntless (WLS), which chaperones Wnt proteins to the plasma membrane for secretion. Once secreted, Wnt proteins signal in a paracrine manner, binding nearby receptor complexes [5]. The Wnt ligand binds to the receptors Frizzled and the LDL receptor-related protein 6 (Lrp6), leading to Lrp6 signalosome formation [6]. The Wnt pathway requires endocytosis of a signal receptor for signal transduction to occur [7]. These co-receptors recruit the β-catenin destruction complex containing Glycogen synthase kinase-3 (GSK3). In the absence of Wnt, GSK3 phosphorylates the transcription factor β-catenin, which is subsequently degraded [8] (Figure 1). However, in the presence of Wnt, the ligand binds to the receptors phosphorylating Lrp6, which prevents the complex from localizing into the cytoplasm to mark β-catenin for degradation [6]. Instead, the receptor complex is endocytosed into a vesicle. When activated, the Wnt pathway stabilizes β-catenin, allowing it to localize in the nucleus to interact with other transcriptional regulators, such as TCF/LEF1 (T cell factor/lymphoid enhancer factor family), to trigger the transcription of many different Wnt target genes important for cell fate determination and oncogenesis [9]. So, prior to the activation of β-catenin, the vesicles sequester GSK3, which is found in the same vesicle, into which the receptor complex is sequestered after the binding of the Wnt ligand. As GSK3 and the receptor complex are endocytosed into the vesicles, the endosomal sorting complexes required for transport (ESCRT) machinery move the vesicles into multivesicular bodies (MVB) [10]. As seen in Figure 2, the cell recognizes the decrease in cytoplasmic GSK3 levels, which stabilizes many different Wnt-stabilized-related proteins, such as Ras and PAK1, which trigger macropinocytosis through a pathway called Wnt Stabilization of Proteins (Wnt-STOP) [11][12]. This aberrant activation of the Wnt pathway is strongly implicated in the onset and progression of numerous types of cancer; therefore, this can have therapeutic advantages for cancer treatment, where multiple such targets have been identified with inhibitors acting at different steps of Wnt signaling pathway (Table 1). However, there are currently no FDA-approved specific Wnt-targeting drugs. The reasons for these poor therapeutic benefits are they often lack satisfactory efficacy, specificity, and safety. For instance, due to the crucial roles of Wnt/β-catenin signaling in many cellular functions, many targeted therapies demonstrated obvious side effects. These facts suggest that Wnt/β-catenin signaling-targeted therapies in cancers are still unable to provide a solid clinical translation [13].
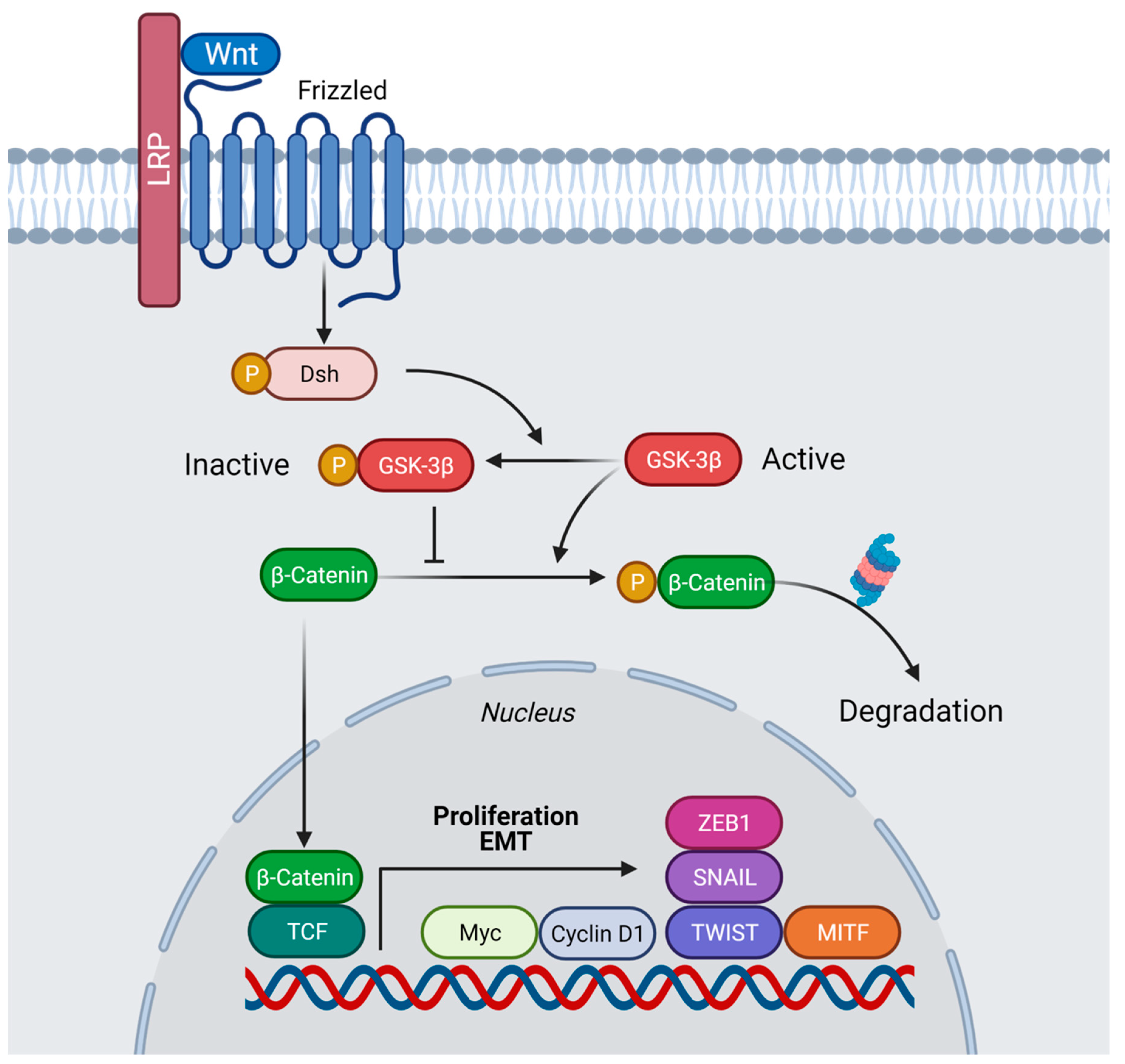 Figure 1. Model of the Wnt/β-catenin pathway in presence of Wnt ligand. Binding of Wnt to the receptors Frizzled (Fz) and Lrp6 leads to inhibition of β-catenin degradation. After stabilization, β-catenin is translocated into the nucleus and interacts with members of the TCF/Lef-1 family of transcription factors to co-activate expression of numerous oncogenes involved in proliferation and migration, in particular Cyclin D1 and c-myc, as well as other genes, including Twist, Snail, ZEB1, and MITF, thus, facilitating EMT. Created with BioRender.com (accessed on 29 April 2022).
Figure 1. Model of the Wnt/β-catenin pathway in presence of Wnt ligand. Binding of Wnt to the receptors Frizzled (Fz) and Lrp6 leads to inhibition of β-catenin degradation. After stabilization, β-catenin is translocated into the nucleus and interacts with members of the TCF/Lef-1 family of transcription factors to co-activate expression of numerous oncogenes involved in proliferation and migration, in particular Cyclin D1 and c-myc, as well as other genes, including Twist, Snail, ZEB1, and MITF, thus, facilitating EMT. Created with BioRender.com (accessed on 29 April 2022).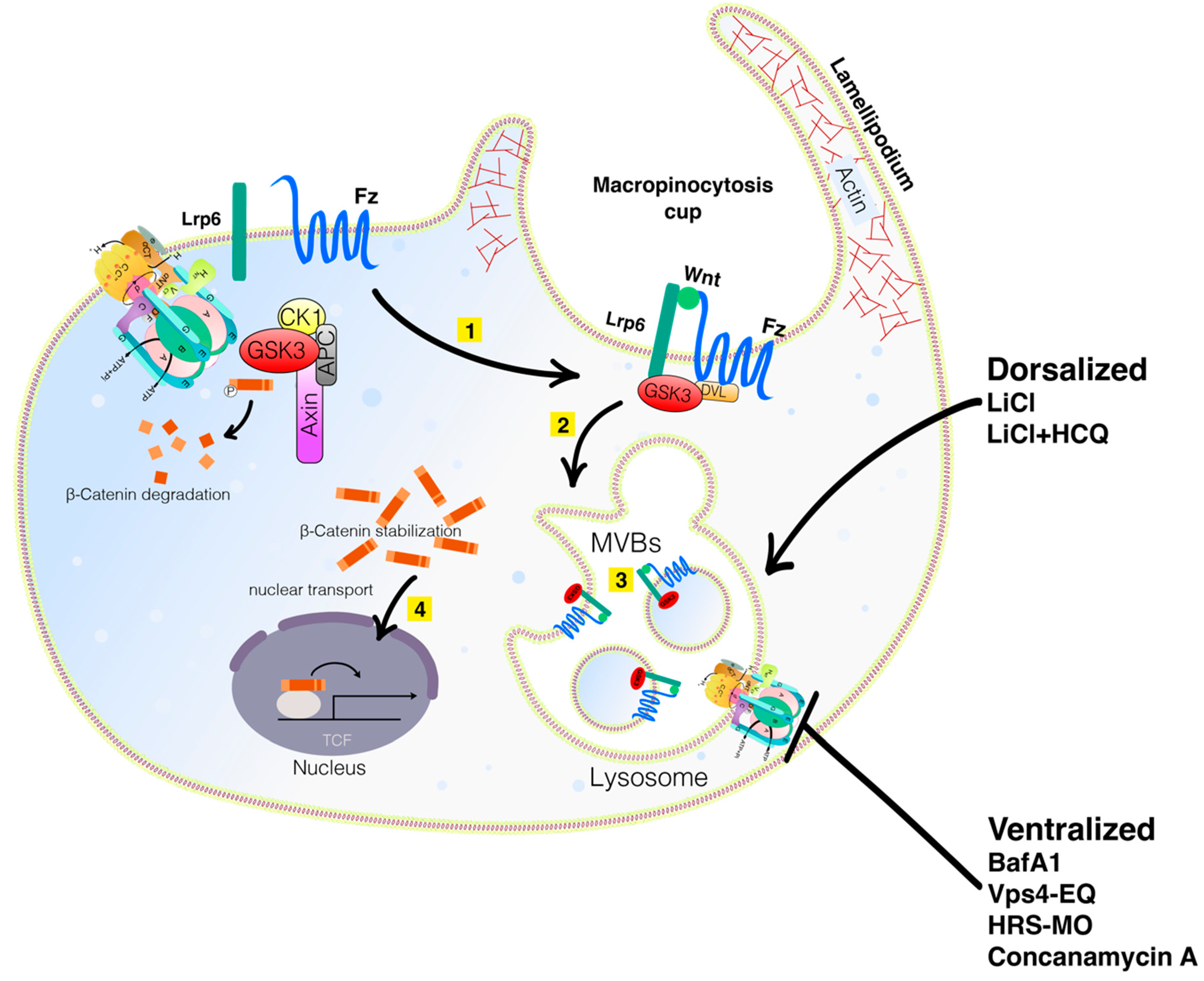 Figure 2. Wnt signaling involves macropinocytosis, V-ATPase, MVBs, membrane trafficking, and lysosomes. Sequestration of GSK3 is a vital step in the activation of the canonical Wnt pathway. When the Wnt ligands bind to the Fz receptor and the Lrp6 co-receptors (Step 1 in yellow), GSK3 is translocated into the membrane. It is then internalized into an early endosome and subsequently into MVBs (Step 2). The sequestration of GSK3 and the destruction complex activate the Wnt pathway (Step 3). Lysosomal activity is critical for dorsal development. Mimicking Wnt signaling with LiCl can dorsalize embryos, an effect that is even more pronounced with LiCl plus HCQ. Inhibiting lysosomal activity with BafA1 or Concanamycin A or interfering with the MVB formation with VPS4-EQ or HRS-MO ventralizes embryos. Wnt and cell adhesion are often active in the same processes and crosstalk between them exists by reciprocal regulation and sharing of components. Knowing how Wnt signaling and cell adhesion cooperate will improve the understanding of embryonic development decisions and carcinomas. Diagram based on findings reported in Tejeda—Muñoz et al., 2022, with permission from Proceeding of the National Academy of Science and Creative Commons.
Figure 2. Wnt signaling involves macropinocytosis, V-ATPase, MVBs, membrane trafficking, and lysosomes. Sequestration of GSK3 is a vital step in the activation of the canonical Wnt pathway. When the Wnt ligands bind to the Fz receptor and the Lrp6 co-receptors (Step 1 in yellow), GSK3 is translocated into the membrane. It is then internalized into an early endosome and subsequently into MVBs (Step 2). The sequestration of GSK3 and the destruction complex activate the Wnt pathway (Step 3). Lysosomal activity is critical for dorsal development. Mimicking Wnt signaling with LiCl can dorsalize embryos, an effect that is even more pronounced with LiCl plus HCQ. Inhibiting lysosomal activity with BafA1 or Concanamycin A or interfering with the MVB formation with VPS4-EQ or HRS-MO ventralizes embryos. Wnt and cell adhesion are often active in the same processes and crosstalk between them exists by reciprocal regulation and sharing of components. Knowing how Wnt signaling and cell adhesion cooperate will improve the understanding of embryonic development decisions and carcinomas. Diagram based on findings reported in Tejeda—Muñoz et al., 2022, with permission from Proceeding of the National Academy of Science and Creative Commons.Table 1. Wnt/β-catenin signaling inhibitors.
| Wnt/β-catenin Pathway Inhibitors | Name |
|---|---|
| Repressor targeting Wnt ligand | sFRP1 (FRP, SARP2, FrzA) SFRP1, sFRP2 (SARP1) SFRP2 sFRP3 (FrzB, Fritz) FRZB, sFRP4 (FrzB-2) SFRP4, sFRP5 (SARP3) SFRP5 Sizzled, Sizzled2, Crescent, WIF-1, Tiki, Cerberus, Notum, Coco, Dkk-3 (REIC) (DKK3), Soggy (DKKL2), Ipafricept, OMP-18R5, F2.A, IGFBP4, Fz7-21, OTSA-101, Gpr177, Wise, 90γ-OTSA-101, OMP-54F28 |
| Repressor targeting Lrps | Dkk-1 (DKK1), Sost, Dkk-2 (DKK2), Dkk-4 (DKK4) |
| Repressor targeting Fzl | sFRP1 (FRP, SARP2, FrzA) SFRP1 (inhibits at high concentrations), IGFBP4, OTSA101, OMP-18R5, OMP-54F28 |
| PORC inhibitors | WNT974, CGX1321, IWP-2, ETC-159, RXC004, GNF-6231, 90γ-OTSA-101, LGK974 |
| β-catenin/TCF inhibitors | PFK115-584, CGP049090, CWP291, FL3, ZINC02092166, NC043, iCRT14 |
| CBP/ β-catenin binding inhibitors | PRI-724, ICG001, GNE-781, JW67, JW74, NLS-StAx-h, INT-01 |
| DVL inhibitors | FJ9, NSC668036, 3289-8625, Niclosamide, J01-017a, sulindac, LM02 |
| Repressor targeting Axin | Tankyrase inhibitors; XAV939, IWR-1, NVP-TNKS656, LZZ-02, JW74, WIKI14, K-756, G007-LK, G244-LM, FL3 |
| β-catenin inhibitors | COX inhibitors; Aspirin, Celecoxib, Sulindac, 1,25(OH)2D35R, SM08502, PKF115-584, PKF118-310, SAH-BCL9 |
| Repressor targeting CKI | Pyrivinium |
| Repressor targeting GSK3β | Genistein |
| TCF/LEF inhibitors | TNIK inhibitor, NCB-0846, PKF115-584, CGP049090 |
| Repressor targeting DKK | DKN-01 |
| Regulates alternative splicing of TCF inhibitors | SAM68, OMP-54F28 |
Endocytosis is known to play a role in cancer by causing a loss of cell adhesion or morphological polarity, which can lead to the malignant transformation of cells [14][15][16]. For example, the small GTPases Rab proteins, which regulate vesicle transport, protein trafficking, membrane targeting and fusion, also mediate vesicle dynamics, which can work with oncogenic signaling pathways to increase tumor formation [17][18][19][20]. The dysregulated expression of oncogenic Rabs with regard to protein levels or activities, such as Rab1, Rab25, and Rab35, increases proliferation, invasion, and migration through the activation of different signaling pathways. The overexpression of Rab proteins such as Rab3d is seen in breast and lung cancer [21]. Rab2A also facilitates Erk1/2 activation, leading to Zeb1 upregulation and β-catenin nuclear translocation, which promotes tumor initiation. In addition, Wnt signaling is also known to increase endocytosis, which can also implicate a possible connection between Wnt signaling and cancer [22].
2. Wnt Signaling Triggers Macropinocytosis
Recent investigations have shown that the increase in endocytosis in Wnt signaling utilizes macropinocytosis. Pinocytosis (Gr., pinein, to drink) is a clathrin-independent endocytic mechanism first described by Warren Lewis (1931). The term macropinocytosis is currently used to designate actin-driven pinocytic vesicles larger than 200 nm. Macropinocytosis is the large nonselective uptake of molecules such as nutrients and other macromolecules in the cellular environment [23]. The macropinosomes that allow the uptake of water and other molecules have membranes derived from the cell plasma membrane’s actin-rich regions, called ruffles, that undergo protrusive movements to allow the vesicle to close and internalize its contents, which are either transported to the lysosome for degradation or to the cell surface [24]. Wnt signaling utilizes macropinocytosis to transport the contents from the cell surface in MVBs to the lysosomes in the cell for degradation [25].
Colorectal carcinoma cells (CRC) are known for their increased nuclear β-catenin when APC, a destruction complex protein, is mutated. Furthermore, studies have demonstrated that CRC SW480 cells have robust macropinocytosis which, interestingly, is required for Wnt signaling. This was demonstrated by the decrease in nuclear β-catenin when colorectal cancer cells were treated with macropinocytosis inhibitors such as EIPA [25]. Additionally, mutations in Axin, another component of the destruction complex, increased macropinocytosis [25]. Furthermore, this research shows that macropinocytosis is important for Wnt signaling since nuclear β-catenin accumulation is a marker for an active Wnt signal, which is reduced when cell drinking is inhibited by derivatives of the diuretic amiloride.
In a different study, by expanding the multivesicular body (MVB) compartment using low doses of the lysosomotropic agent Hydroxychloroquine (HCQ), a strong potentiation of Wnt signaling by LiCl injection in the Xenopus embryo was observed, an effect that could be blocked by inhibiting macropinocytosis [26]. Blocking lysosome acidification by V-ATPase via a brief pulse with Bafilomycin A1 (BafA1) at the 32-cell stage inhibited the induction of the primary embryonic axis. The inductive activity of the dorsal determinant Huluwa (Hwa) [27] was also blocked by interfering with lysosome acidification or the MVB-forming ESCRT machinery. These results show that the cell biology of lysosomes plays a fundamental role in vertebrate development; not only linking Wnt signal transduction and membrane trafficking, but also showing that lysosomes/MVBs are required for the activation of the Wnt signal [28].
References
- Di Guglielmo, G.M.; Le Roy, C.; Goodfellow, A.F.; Wrana, J.L. Distinct Endocytic Pathways Regulate TGF-β Receptor Signalling and Turnover. Nat. Cell Biol. 2003, 5, 410–421.
- Komiya, Y.; Habas, R. Wnt Signal Transduction Pathways. Organogenesis 2008, 4, 68–75.
- Cabrera, C.V.; Alonso, M.C.; Johnston, P.; Phillips, R.G.; Lawrence, P.A. Phenocopies Induced with Antisense RNA Identify the Wingless Gene. Cell 1987, 50, 659–663.
- Nusse, R.; Varmus, H.E. Many Tumors Induced by the Mouse Mammary Tumor Virus Contain a Provirus Integrated in the Same Region of the Host Genome. Cell 1982, 31, 99–109.
- Takada, S.; Fujimori, S.; Shinozuka, T.; Takada, R.; Mii, Y. Differences in the secretion and transport of Wnt proteins. J. Biochem. 2017, 161, 1–7.
- Bilić, J.; Huang, Y.-L.; Davidson, G.; Zimmermann, T.; Cruciat, C.-M.; Bienz, M.; Niehrs, C. Wnt Induces LRP6 Signalosomes and Promotes Dishevelled-Dependent LRP6 Phosphorylation. Science 2007, 316, 1619–1622.
- Blitzer, J.T.; Nusse, R. A Critical Role for Endocytosis in Wnt Signaling. BMC Cell Biol. 2006, 7, 28.
- Stamos, J.L.; Weis, W.I. The β-Catenin Destruction Complex. Cold Spring Harb. Perspect. Biol. 2012, 5, a007898.
- Behrens, J.; von Kries, J.P.; Kühl, M.; Bruhn, L.; Wedlich, D.; Grosschedl, R.; Birchmeier, W. Functional Interaction of β-Catenin with the Transcription Factor LEF-1. Nature 1996, 382, 638–642.
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.-L.; Fuentealba, L.C.; Vorwald, P.P.; Gumper, I.; Sabatini, D.D.; De Robertis, E.M. Wnt Signaling Requires Sequestration of Glycogen Synthase Kinase 3 inside Multivesicular Endosomes. Cell 2010, 143, 1136–1148.
- Acebron, S.P.; Karaulanov, E.; Berger, B.S.; Huang, Y.-L.; Niehrs, C. Mitotic Wnt Signaling Promotes Protein Stabilization and Regulates Cell Size. Mol. Cell 2014, 54, 663–674.
- Dobrowolski, R.; Vick, P.; Ploper, D.; Gumper, I.; Snitkin, H.; Sabatini, D.D.; De Robertis, E.M. Presenilin Deficiency or Lysosomal Inhibition Enhances Wnt Signaling through Relocalization of GSK3 to the Late-Endosomal Compartment. Cell Rep. 2012, 2, 1316–1328.
- Morris, A.; Pagare, P.P.; Li, J.; Zhang, Y. Drug discovery efforts toward inhibitors of canonical Wnt/β-catenin signaling pathway in the treatment of cancer: A composition-of-matter review (2010–2020). Drug Discov. Today 2021, 27, 1115–1127.
- Mosesson, Y.; Mills, G.B.; Yarden, Y. Derailed Endocytosis: An Emerging Feature of Cancer. Nat. Rev. Cancer 2008, 8, 835–850.
- Tejeda-Muñoz, N.; De Robertis, E.M. Wnt, GSK3, and Macropinocytosis. Subcell. Biochem. 2022, 98, 169–187.
- Colozza, G.; Jami-Alahmadi, Y.; Dsouza, A.; Tejeda-Muñoz, N.; Albrecht, L.V.; Sosa, E.A.; Wohlschlegel, J.A.; De Robertis, E.M. Wnt-inducible Lrp6-APEX2 interacting proteins identify ESCRT machinery and Trk-fused gene as components of the Wnt signaling pathway. Sci. Rep. 2020, 10, 21555.
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525.
- Chavrier, P.; Goud, B. The Role of Arf and Rab Gtpases in Membrane Transport. Curr. Opin. Cell 1999, 11, 466–475.
- Pereira-Leal, J.B.; Seabra, M.C. The Mammalian Rab Family of Small GTPases: Definition of Family and Subfamily Sequence Motifs Suggests a Mechanism for Functional Specificity in the Ras Superfamily. J. Mol. Biol. 2000, 301, 1077–1087.
- Tzeng, H.-T.; Wang, Y.-C. Rab-Mediated Vesicle Trafficking in Cancer. J. Biomed. Sci. 2016, 23, 70.
- Gopal Krishnan, P.D.; Golden, E.; Woodward, E.A.; Pavlos, N.J.; Blancafort, P. Rab GTPases: Emerging Oncogenes and Tumor Suppressive Regulators for the Editing of Survival Pathways in Cancer. Cancers 2020, 12, 259.
- Tejeda-Muñoz, N.; Albrecht, L.V.; Bui, M.H.; De Robertis, E.M. Wnt Canonical Pathway Activates Macropinocytosis and Lysosomal Degradation of Extracellular Proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 10402–10411.
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An Endocytic Pathway for Internalising Large Gulps. Immunol. Cell Biol. 2011, 89, 836–843.
- Swanson, J.A.; King, J.S. The Breadth of Macropinocytosis Research. Philos. Trans. R. Soc. 2018, 374, 20180146.
- Albrecht, L.V.; Tejeda-Muñoz, N.; Bui, M.H.; Cicchetto, A.C.; Di Biagio, D.; Colozza, G.; Schmid, E.; Piccolo, S.; Christofk, H.R.; De Robertis, E.M. GSK3 Inhibits Macropinocytosis and Lysosomal Activity through the Wnt Destruction Complex Machinery. Cell Rep. 2020, 32, 107973.
- Tejeda-Munoz, N.; De Robertis, E.M. Lysosomes are required for early dorsal signaling in the Xenopus embryo. Proc. Natl. Acad. Sci. USA 2022, 119, e2201008119.
- Yan, L.; Chen, J.; Zhu, X.; Sun, J.; Wu, X.; Shen, W.; Zhang, W.; Tao, Q.; Meng, A. Maternal Huluwa dictates the embryonic body axis through β-catenin in vertebrates. Science 2018, 23, 362.
- Tejeda-Muñoz, N.; Morselli, M.; Moriyama, Y.; Sheladiya, P.; Pellegrini, M.; De Robertis, E.M. Canonical Wnt Signaling Induces Focal Adhesion and Integrin Beta-1 Endocytosis. iScience 2022, 25, 104123.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
24 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




