Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jose Carugno | -- | 2050 | 2022-05-23 11:58:27 | | | |
| 2 | Dean Liu | Meta information modification | 2050 | 2022-05-24 03:51:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Carugno, J.; Watrowski, R.; Vitale, S.G.; Barra, F.; D'alterio, M.N.; Sathyapalan , T.; , .; Reyes-Muñoz, E.; Lin, L.; Angioni, S. Abnormal Uterine Bleeding in Perimenopausal Women. Encyclopedia. Available online: https://encyclopedia.pub/entry/23239 (accessed on 08 February 2026).
Carugno J, Watrowski R, Vitale SG, Barra F, D'alterio MN, Sathyapalan T, et al. Abnormal Uterine Bleeding in Perimenopausal Women. Encyclopedia. Available at: https://encyclopedia.pub/entry/23239. Accessed February 08, 2026.
Carugno, Jose, Rafał Watrowski, Salvatore Giovanni Vitale, Fabio Barra, Maurizio Nicola D'alterio, Thozhukat Sathyapalan , , Enrique Reyes-Muñoz, Li-Te Lin, Stefano Angioni. "Abnormal Uterine Bleeding in Perimenopausal Women" Encyclopedia, https://encyclopedia.pub/entry/23239 (accessed February 08, 2026).
Carugno, J., Watrowski, R., Vitale, S.G., Barra, F., D'alterio, M.N., Sathyapalan , T., , ., Reyes-Muñoz, E., Lin, L., & Angioni, S. (2022, May 23). Abnormal Uterine Bleeding in Perimenopausal Women. In Encyclopedia. https://encyclopedia.pub/entry/23239
Carugno, Jose, et al. "Abnormal Uterine Bleeding in Perimenopausal Women." Encyclopedia. Web. 23 May, 2022.
Copy Citation
Abnormal uterine bleeding (AUB) is a frequent symptom in perimenopausal women. It is defined as uterine bleeding in which the duration, frequency, or amount of bleeding is considered excessive and negatively affects the woman’s quality of life (QoL) and psychological well-being.
abnormal uterine bleeding
hysteroscopy
perimenopause
1. Abnormal Uterine Bleeding (AUB) in Perimenopausal Women
Perimenopause is the period between the first symptoms of diminished ovarian function, usually beginning in the early forties, lasting up to two years after the Final Menstrual Period (FMP). The variety and inconsistency of perimenopause definitions imply that a sharp distinction between “premenopausal”, “perimenopausal”, and “postmenopausal” AUB is difficult. In 1996, the World Health Organization defined perimenopause as “the period immediately prior to menopause (when the endocrinological, biological and clinical features of menopause begin) and the first year after menopause” [1]. In contrast, the term menopausal transition should be reserved for “that period of time before FMP when variability in the menstrual cycle is usually increased” [1]. Still, some studies arbitrarily set lower and upper limits of perimenopause, e.g., between 40 and 54 [2] or 42 and 52 [3] years of age or from four years before to 12 months after FMP [4].
Researchers define the “perimenopausal AUB” as any abnormal menstrual bleeding during menopausal transition or within the first year after menopause (except for cyclic bleeding in women using hormonal replacement therapy) [1][3][5]. AUB is the leading cause of approximately one-third of all outpatient gynecological visits, particularly in the perimenopausal period [3][4][5]. More than 90% of women experience at least one episode of AUB, and 78% of them at least three episodes of AUB during their transition to menopause [3]. The popular classification of nongestational causes of AUB was introduced by FIGO (International Federation of Gynecology and Obstetrics) in 2011 and revised in 2018 (Figure 1) [6][7][8]. The causes of nongestational AUB have been classified into nine categories arranged according to the acronym PALM-COEIN, including the structural causes (“PALM”): polyp, adenomyosis, leiomyoma, malignancy/hyperplasia, and non-structural causes (“COEIN”): coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and “not otherwise classified” [6][7]. The contribution of individual PALM-COEIN categories to the spectrum of AUB causes changes with age; nevertheless, endometrial polyps and fibroids remain the most common structural causes of AUB in perimenopause [9][10][11]. In the fourth decade of life, the impact of myomas and adenomyosis as AUB causes increases. Likewise, the highest incidence of endometrial polyps is reported in women aged 40–44 years [12]. Notably, the rate of structural pathologies (fibroids, polyps, adenomyosis) coexisting with each other or with uterine malignancies also increases with age [13][14]. Additionally, various local or systemic conditions, and hormonal or non-hormonal medications (tamoxifen, oral anticoagulants), can trigger uterine bleeding. Hematologic dysfunctions are also a frequent cause of AUB in perimenopausal women, being reported as the cardinal symptom in 32–100% of women with von Willebrand factor deficiency (relevant to 0.5–1% of the general population), in 5–98% of women with platelet dysfunction, and 35–70% of women with rare factor deficiencies [15]. Although the annual probability of spontaneous conception is about 10% by age 40–44, falling to 3% by age 45–49, the percentage of women fulfilling their reproductive goals in their fourth decade is continuously rising [16]. However, 84% of pregnancies in women over 48 years end in first-trimester miscarriage, and the rate of ectopics in women over 44 years rises to 7% [17]. Therefore, up to 12 months after FMP, excluding pregnancy is a mandatory part of the AUB diagnostic workup.
2. Initial Diagnostic Evaluation of AUB
The self-reported perception of AUB by the woman is the first step to determine its impact on QoL. It is well known that the patient’s subjective estimation does not always correlate with the objective amount of blood loss [18]. According to Munro et al. [6], menstrual bleeding exceeding 80 mL, as well as any intermenstrual and postcoital bleeding, should be considered abnormal. Nevertheless, about 14% of patients with mild to moderate blood loss consider their bleeding as heavy, and 40% of women with excessive blood loss consider their bleeding as standard [19]. In those cases, Pictorial Blood Assessment Charts can be helpful for the semiquantitative determination of AUB [20].
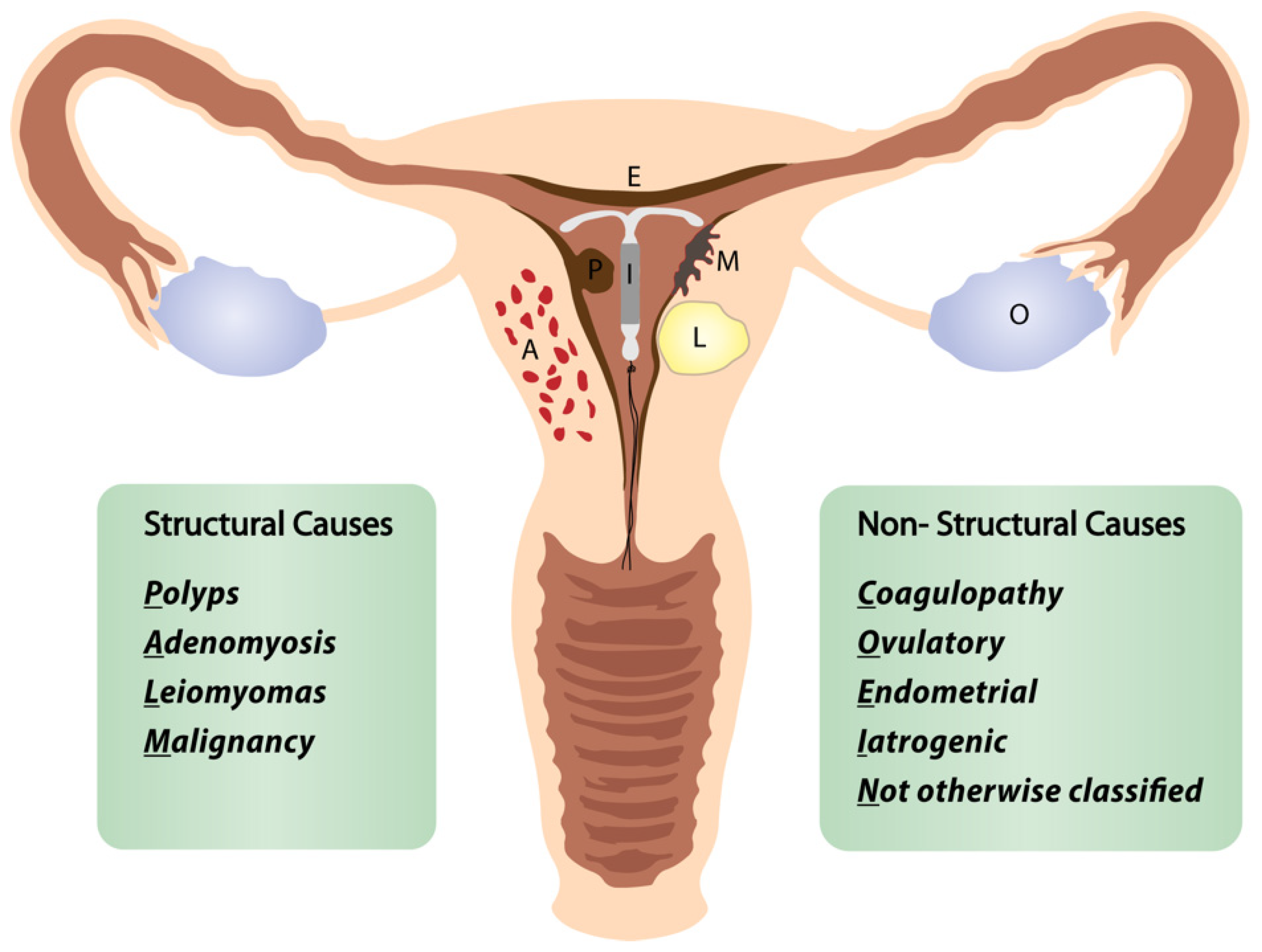
Figure 1. FIGO classification of abnormal uterine bleeding. Adapted from [8].
A detailed medical history (including hereditary disposition for uterine malignancies), the vaginal speculum exam, and transvaginal ultrasound (TVS) are essential parts of evaluating patients complaining of AUB [7][14][21]. However, standard diagnostic criteria and reproducibility of TVS are not consistent [14], and the accuracy of TVS in diagnosing benign uterine conditions is suboptimal [22][23]. TVS is helpful to exclude endometrial cancer (EC) in postmenopausal women if the endometrial echo is less than or equal to 4 mm, providing a negative predictive value of >99% [24]. The advantage of TVS is the holistic assessment of the uterus and its surrounding structures [7][24]. Saline infusion sonohysterography (SIS) offers a superior detection rate of benign lesions compared to TVS, but costs, convenience, and tolerability are the limiting factors [25][26]. Laboratory tests, e.g., hemoglobin and human chorionic gonadotropin determination, supplement the physical examination and sonography. Further laboratory tests may be indicated to uncover hereditary bleeding disorders or hormonal alterations depending on the history and the developed clinical suspicion.
3. Role of Hysteroscopy in the Management of Women with AUB
Hysteroscopy is considered the gold standard technique for diagnosing and managing pathological conditions affecting the uterine cavity [27][28]. In turn, AUB is the most common indication to perform hysteroscopy in perimenopausal women [29]. The hysteroscopic “see-and-treat” approach allows exploration of the uterine cavity, targeted endometrial and endocervical biopsies, and—if indicated—immediate treatment of endocervical, endometrial, or submucosal pathologies (polyps, myomas) [24][30][31][32][33][34]. It is essential to highlight that hysteroscopy is unsuitable for evaluating and treating deep myometrial pathologies (such as adenomyosis or myomas FIGO-Grade ≥ 3). Most hysteroscopic procedures can be performed in an office setting depending on the patient’s preferences, available infrastructure (staffing, equipment), surgeon’s experience, and comfort level [24][28][31][32][33][34]. More complex and prolonged procedures, such as hysteroscopic myomectomy and extensive lysis of intrauterine adhesions, are typically performed in the operating room, thus providing the patient with general anesthesia and the ability of the surgeon to perform more extensive surgery. Moreover, hysteroscopy can be complemented with laparoscopy if necessary [24][28][35][36]. Regardless of the setting in which the hysteroscopic procedure is completed, it is helpful to distinguish diagnostic and operative hysteroscopy.
3.1. Diagnostic Hysteroscopy
Diagnostic hysteroscopy aims to diagnose lesions within the endometrial cavity and, if necessary, to obtain targeted biopsies [24][28][37]. Diagnostic hysteroscopy can be a single intervention or may immediately precede hysteroscopic surgery. The feasibility of diagnostic hysteroscopy decreases in patients with previous surgeries, pelvic infections, IUD use, and postmenopausal status. Cobellis et al. [38] developed a predictive score for office hysteroscopy failure using different predictors, the most significant of which were history of procedures on the cervix, cesarean section, recurrent vaginitis, retroflexed uterus, and menopause. Depending on the previously indicated factors, office hysteroscopy could not be completed in 6 to 76% of the interventions [38].
AUB is the presenting sign in >90% of postmenopausal women with EC [39]. In turn, the prevalence of EC or atypical hyperplasia in postmenopausal women with AUB is 21%, rising to 29% when AUB is accompanied by an endometrium thickness of ≥4 mm on TVS [39]. The systematic review by Clark et al. [40] confirmed high diagnostic accuracy of hysteroscopy with regard to EC, but only moderate for other types of endometrial disease. When comparing studies on the diagnostic accuracy of hysteroscopy, it is helpful to distinguish between studies reporting results based only on the hysteroscopic view and those obtained after a hysteroscopically-guided biopsy. For example, Elfayomy et al. [41] found “hysteroscopy” (meaning hysteroscopic image of the pathology) insufficient to exclude endometrial hyperplasia and cancer in women with AUB, based on 0.57/0.50 sensitivity and 0.92/0.94 specificity for endometrial hyperplasia and cancer, respectively (Figure 2).
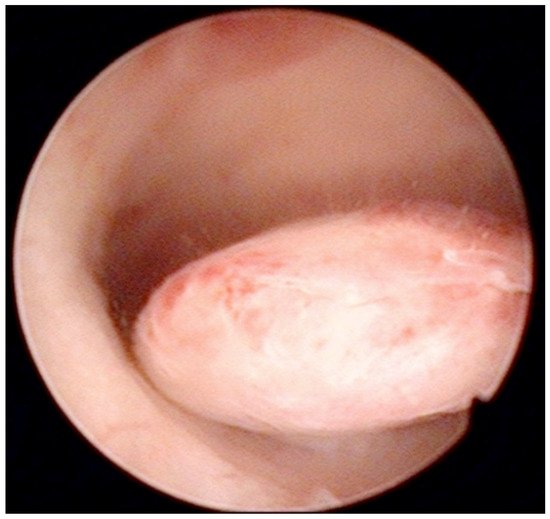
Figure 2. Pedunculate low-risk endometrial polyp in the posterior uterine wall during diagnostic hysteroscopy (intraoperative picture by F.B. and S.F.).
Similarly, Garuti et al. [42] used the terms “hysteroscopy” and “hysteroscopic view” interchangeably, reporting a low sensitivity (0.64 and 0.61) and specificity (0.92 and 0.95) for the diagnosis of endometrial hyperplasia. In the study of De Franciscis et al. [43], the concordance between the final histopathological result and the hysteroscopic impression was 86% for benign disease or normal endometrium, but only 58% for endometrial hyperplasia. In addition, the lowest agreement (52%) was noted for postmenopausal endometrial hyperplasia. When restricted to women with postmenopausal AUB, the sensitivity and specificity of in-office hysteroscopy for endometrial hyperplasia (with histopathology as reference) was 87% and 43%, respectively [43]. In contrast, Tinelli et al. [44] not only confirmed the diagnostic superiority of hysteroscopy with an eye-directed biopsy for detecting endometrial pathologies compared to TVS (and thus recommended hysteroscopy for all postmenopausal women with AUB and endometrial thickness > 4 mm) but also demonstrated the unique efficiency of hysteroscopy for diagnosing focal abnormalities (including EC) in the atrophic endometrium that would otherwise likely be missed by TVS. The authors advocate hysteroscopic evaluation even in patients with AUB with endometrium on TVS of <4 mm, aiming to decrease the chance of failing to diagnose carcinomas that develop focally in the atrophic endometrium (Figure 3) [44].
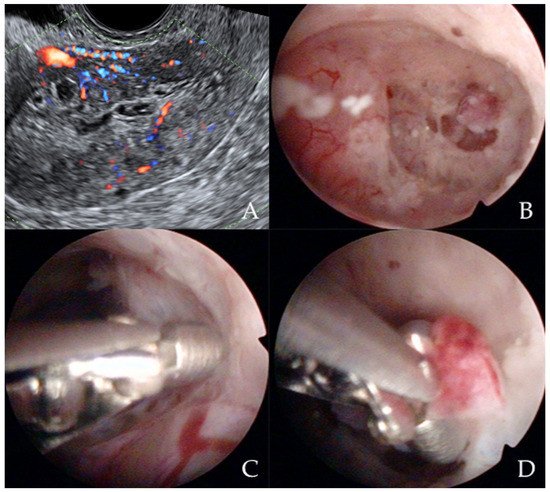
Figure 3. Ultrasonographic appearance of uterine lesion suspected for early-stage endometrial cancer (A). The diagnostic hysteroscopic allows for visualizing the suspected uterine area (B) and for obtaining the histologic biopsy (C,D). Intraoperative photographs by F.B. and S.F.
Less threatening but a more common finding in patients with AUB (20–31% of cases) are endometrial polyps [9][10][11][25][33]. The sensitivity of TVS for the diagnosis of polyps is particularly low (0.51) [25]. In contrast, in women with postmenopausal AUB, the sensitivity and specificity of hysteroscopy for the diagnosis of polyps are reported at 0.81–0.92 and 0.85–0.98, respectively [11][26]. The study by de Godoy Borges et al. [23] confirmed the superiority of diagnostic hysteroscopy (96.4% sensitivity, 74.6% specificity) in comparison to TVS (88.7% sensitivity, 25.4% specificity) for detecting endometrial polyps in women aged 41 to 82 presenting with AUB. Saline Infusion Sonohysterogram (SIS) provides a similar sensitivity compared with hysteroscopy but lower specificity (0.93 and 0.83 compared to 0.95 and 0.90, respectively). Finally, a recent meta-analysis reported sensitivity and specificity of 0.87 and 0.86, 0.62 and 0.73, and 0.92 and 0.85 for SIS, TVS, and hysteroscopy, respectively, for detecting endometrial polyps in women with AUB [11]. Similarly, hysteroscopy offers the highest diagnostic accuracy (>90%) for detecting submucous myomas as compared to TVS or SIS [45]. Further developments in the field of sonography, based on the three-dimensional virtual image synthesis (so called “virtual sonographic hysteroscopy”), will show to what extent diagnostic hysteroscopy could be replaced by next-generation, three-dimensional imaging modalities for detecting intracavitary uterine pathologies [46].
3.2. Operative Hysteroscopy
Operative hysteroscopy uses mechanical, electrosurgical, and laser instruments to treat intracavitary pathologies. The introduction of the small-diameter coaxial bipolar electrode (Versapoint, Gynecare, Ethicon, NJ, USA) in 1999 was a milestone for outpatient operative hysteroscopy (Figure 4) [47]. The development of miniaturized mechanical instruments with small diameter scopes and working channels with continuous flow systems enabled the “see-and-treat” approach without general anesthesia [32][34][48][49][50]. Wortman et al. [51] confirmed that major operative hysteroscopic surgery could be performed in an office-based setting resulting in a 98.8% rate of “satisfied” or “very satisfied” patients. Both outpatient (73%) and inpatient (80%) hysteroscopic polypectomy offer comparable success rates, as determined by the patients’ subjective bleeding and QoL assessment after six months [52]. However, procedure failure is higher (19% vs. 7%), and acceptability is lower (83% vs. 92%) with outpatient compared to inpatient polypectomy [52]. An essential aspect of hysteroscopic surgery is a very high level of physician satisfaction (e.g., 95% reported in [53]) associated with this approach. Factors such as incomplete resection and recurrence of the pathology decrease the acceptability of hysteroscopic surgery [38][52].
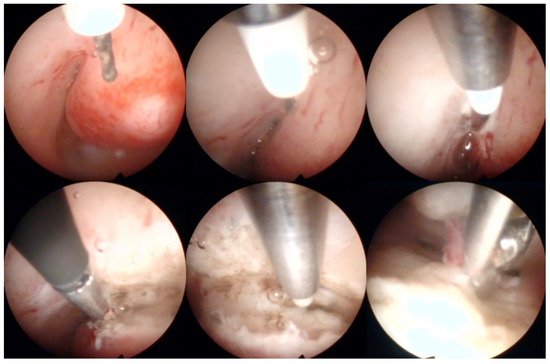
Figure 4. Operative hysteroscopic removal of an endometrial polyp (mean diameter 13 mm) by Versapoint system (Gynecare, Ethicon Inc., Raritan, NJ, USA). Intraoperative photographs by F.B. and S.F.
As with any other surgical procedure, hysteroscopic operations can be associated with complications. Every second complication of operative hysteroscopy is mechanical (52%), including cervical lacerations, uterine perforations, and injuries to the adjacent organs such as the bowel or bladder, sometimes associated with internal bleeding and conversion to laparoscopy or laparotomy [54][55]. Further short-term complications, such as excess fluid absorption, pulmonary edema, critical electrolyte disbalance, or genital tract burns, and long-term consequences (intrauterine adhesions) should be mentioned [54][55][56]. Venous air embolism during endometrial resection/endometrial ablation (ER/EA) or hysteroscopic myomectomy, although exceedingly rare (1:1140 surgeries), is a potentially fatal complication [57][58]. Myomectomies are hysteroscopic procedures with the highest complication rate (up to 14%) and the highest risk of distension medium-related complications (more than seven times more common compared to polypectomy) [54]. The application of monopolar energy is associated with more frequent local and systemic adverse events (perforations, burns, hyponatremia) as compared to bipolar energy [59][60]. Many of these complications can be prevented by strict adherence to basic surgical principles, e.g., limited use of monopolar devices [59][60]. Furthermore, some complications of intracavitary resections, e.g., intrauterine adhesions, can be successfully reduced by the use of antiadhesive barriers [61].
References
- World Health Organization. Research on the Menopause in the 1990s: Report of a WHO Scientific Group; WHO Scientific Group on Research on the Menopause in the 1990s, Ed.; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1996; ISBN 978-92-4-120866-6.
- Shapley, M.; Blagojevic-Bucknall, M.; Jordan, K.; Croft, P. The epidemiology of self-reported intermenstrual and postcoital bleeding in the perimenopausal years. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1348–1355.
- Paramsothy, P.; Harlow, S.D.; Greendale, G.A.; Gold, E.B.; Crawford, S.L.; Elliott, M.R.; Lisabeth, L.D.; Randolph, J.F. Bleeding patterns during the menopausal transition in the multi-ethnic Study of Women’s Health Across the Nation (SWAN): A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1564–1573.
- Carugno, J. Clinical management of vaginal bleeding in postmenopausal women. Climacteric 2020, 23, 343–349.
- Goldstein, S.R. Appropriate evaluation of postmenopausal bleeding. Menopause 2018, 25, 1476–1478.
- Munro, M.G.; Critchley, H.O.; Broder, M.S.; Fraser, I.S. FIGO Working Group on Menstrual Disorders FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynecol. Obstet. 2011, 113, 3–13.
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; The FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynecol. Obstet. 2018, 143, 393–408.
- Chodankar, R.; Critchley, H.O.D. Biomarkers in abnormal uterine bleeding. Biol. Reprod. 2018, 101, 1155–1166.
- Soja, M.; Masternak, M.; Piwowarczyk, I.; Janas, Ł.; Szyłło, K.; Nowak, M. Analysis of the results of invasive diagnostic procedures in patients referred to gynecologic department due to abnormal uterine bleeding. Menopausal Rev. 2020, 19, 155–159.
- Kim, M.K.; Jung, Y.S.; Chon, S.J.; Yun, B.H.; Cho, S.; Choi, Y.S.; Lee, B.S.; Seo, S.K. Common Causes of Postmenopausal Bleeding in Korean Women: 10-Year Outcomes from a Single Medical Center. J. Korean Med. Sci. 2017, 32, 830–834.
- Kaveh, M.; Sadegi, K.; Salarzaei, M.; Parooei, F. Comparison of diagnostic accuracy of saline infusion sonohysterography, transvaginal sonography, and hysteroscopy in evaluating the endometrial polyps in women with abnormal uterine bleeding: A systematic review and meta-analysis. Videosurgery Other Miniinvasive Tech. 2020, 15, 403–415.
- Sun, Y.; Wang, Y.; Mao, L.; Wen, J.; Bai, W. Prevalence of abnormal uterine bleeding according to new International Federation of Gynecology and Obstetrics classification in Chinese women of reproductive age. Medicine 2018, 97, e11457.
- Johnatty, S.E.; Stewart, C.J.R.; Smith, D.; Nguyen, A.; Dwyer, J.O.; O’Mara, T.A.; Webb, P.M.; Spurdle, A.B. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci. Rep. 2020, 10, 362.
- Bosch, T.V.D.; Verbakel, J.Y.; Valentin, L.; Wynants, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladkevicius, P.; Alcazar, J.L.; Votino, A.; et al. Typical ultrasound features of various endometrial pathologies described using International Endometrial Tumor Analysis (IETA) terminology in women with abnormal uterine bleeding. Ultrasound Obstet. Gynecol. 2021, 57, 164–172.
- Von Mackensen, S. Quality of life in women with bleeding disorders. Haemophilia 2011, 17, 33–37.
- Tower, C. Pregnancy in peri- and postmenopausal women: Challenges in management. Menopause Int. 2009, 15 (Suppl. 1), 165–168.
- Andersen, A.-M.N.; Wohlfahrt, J.; Christens, P.; Olsen, J.; Melbye, M. Maternal age and fetal loss: Population based register linkage study. BMJ 2000, 320, 1708–1712.
- Levy-Zauberman, Y.; Pourcelot, A.-G.; Capmas, P.; Fernandez, H. Update on the management of abnormal uterine bleeding. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 613–622.
- Weisberg, E.; McGeehan, K.; Fraser, I.S. Effect of perceptions of menstrual blood loss and menstrual pain on women’s quality of life. Eur. J. Contracept. Reprod. Health Care 2016, 21, 431–435.
- Magnay, J.L.; O’Brien, S.; Gerlinger, C.; Seitz, C. Pictorial methods to assess heavy menstrual bleeding in research and clinical practice: A systematic literature review. BMC Women’s Health 2020, 20, 24.
- Kostov, S.; Watrowski, R.; Kornovski, Y.; Dzhenkov, D.; Slavchev, S.; Ivanova, Y.; Yordanov, A. Hereditary Gynecologic Cancer Syndromes—A Narrative Review. Onco Targets Ther. 2022, 15, 381–405.
- Vitale, S.G.; Riemma, G.; Pacheco, L.A.; Carugno, J.; Haimovich, S.; Tesarik, J.; De Angelis, M.C.; Sardo, A.D.S.; De Franciscis, P. Hysteroscopic endometrial biopsy: From indications to instrumentation and techniques. A call to action. Minim. Invasive Ther. Allied Technol. 2021, 30, 251–262.
- Borges, P.C.D.G.; Dias, R.; Machado, R.B.; Borges, J.B.R.; Spadoto-Dias, D. Transvaginal Ultrasonography and Hysteroscopy as Predictors of Endometrial Polyps in Postmenopause. Women’s Health 2015, 11, 29–33.
- Hill, M.J.; Levens, E.D.; Decherney, A.H. Committee on Practice Bulletins—Gynecology Practice Bulletin No. 128. Obstet. Gynecol. 2012, 120, 197–206.
- Maheux-Lacroix, S.; Li, F.; Laberge, P.Y.; Abbott, J. Imaging for Polyps and Leiomyomas in Women with Abnormal Uterine Bleeding. Obstet. Gynecol. 2016, 128, 1425–1436.
- Vroom, A.J.; Timmermans, A.; Bongers, M.Y.; Heuvel, E.R.V.D.; Geomini, P.M.A.J.; Van Hanegem, N. Diagnostic accuracy of saline contrast sonohysterography in detecting endometrial polyps in women with postmenopausal bleeding: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 54, 28–34.
- The Use of Hysteroscopy for the Diagnosis and Treatment of Intrauterine Pathology. Obstet. Gynecol. 2020, 135, 754–756.
- Carugno, J.; Grimbizis, G.; Franchini, M.; Alonso, L.; Bradley, L.; Campo, R.; Catena, U.; De Angelis, C.; Sardo, A.D.S.; Farrugia, M.; et al. International Consensus Statement for recommended terminology describing hysteroscopic procedures. Facts Views Vis. ObGyn 2021, 13, 287–294.
- Nagele, F.; O’Connor, H.; Davies, A.; Badawy, A.; Mohamed, H.; Magos, A. 2500 Outpatient diagnostic hysteroscopies. Obstet. Gynecol. 1996, 88, 87–92.
- Nappi, L.; Sorrentino, F.; Angioni, S.; Pontis, A.; Litta, P.; Greco, P. Feasibility of hysteroscopic endometrial polypectomy using a new dual wavelengths laser system (DWLS): Preliminary results of a pilot study. Arch. Gynecol. Obstet. 2017, 295, 3–7.
- Sardo, A.D.S.; Bettocchi, S.; Spinelli, M.; Guida, M.; Nappi, L.; Angioni, S.; Fernandez, L.M.S.; Nappi, C. Review of New Office-Based Hysteroscopic Procedures 2003–2009. J. Minim. Invasive Gynecol. 2010, 17, 436–448.
- Vitale, S.G.; Bruni, S.; Chiofalo, B.; Riemma, G.; Lasmar, R.B. Updates in office hysteroscopy: A practical decalogue to perform a correct procedure. Updat. Surg. 2020, 72, 967–976.
- Giampaolino, P.; Della Corte, L.; Di Filippo, C.; Mercorio, A.; Vitale, S.G.; Bifulco, G. Office hysteroscopy in the management of women with postmenopausal bleeding. Climacteric 2020, 23, 369–375.
- Vitale, S.G.; Haimovich, S.; Riemma, G.; Ludwin, A.; Zizolfi, B.; De Angelis, M.C.; Carugno, J. Innovations in hysteroscopic surgery: Expanding the meaning of “in-office”. Minim. Invasive Ther. Allied Technol. 2021, 30, 125–132.
- Fagioli, R.; Vitagliano, A.; Carugno, J.; Castellano, G.; De Angelis, M.C.; Sardo, A.D.S. Hysteroscopy in postmenopause: From diagnosis to the management of intrauterine pathologies. Climacteric 2020, 23, 360–368.
- Vitale, S.G.; Riemma, G.; Ciebiera, M.; Cianci, S. Hysteroscopic treatment of submucosal fibroids in perimenopausal women: When, why, and how? Climacteric 2020, 23, 355–359.
- Vitale, S.G. The Biopsy Snake Grasper Sec. VITALE: A New Tool for Office Hysteroscopy. J. Minim. Invasive Gynecol. 2020, 27, 1414–1416.
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; De Franciscis, P.; Signoriello, G.; Colacurci, N. Is it possible to predict office hysteroscopy failure? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 328–333.
- Saccardi, C.; Vitagliano, A.; Marchetti, M.; Turco, A.L.; Tosatto, S.; Palumbo, M.; De Lorenzo, L.S.; Vitale, S.G.; Scioscia, M.; Noventa, M. Endometrial Cancer Risk Prediction According to Indication of Diagnostic Hysteroscopy in Post-Menopausal Women. Diagnostics 2020, 10, 257.
- Clark, T.J.; Voit, D.; Gupta, J.K.; Hyde, C.; Song, F.; Khan, K.S. Accuracy of Hysteroscopy in the Diagnosis of Endometrial Cancer and Hyperplasia. JAMA 2002, 288, 1610–1621.
- Elfayomy, A.K.; Habib, F.A.; Alkabalawy, M.A. Role of hysteroscopy in the detection of endometrial pathologies in women presenting with postmenopausal bleeding and thickened endometrium. Arch. Gynecol. Obstet. 2012, 285, 839–843.
- Garuti, G.; Cellani, F.; Garzia, D.; Colonnelli, M.; Luerti, M. Accuracy of hysteroscopic diagnosis of endometrial hyperplasia: A retrospective study of 323 patients. J. Minim. Invasive Gynecol. 2005, 12, 247–253.
- De Franciscis, P.; Riemma, G.; Schiattarella, A.; Cobellis, L.; Guadagno, M.; Vitale, S.G.; Mosca, L.; Cianci, A.; Colacurci, N. Concordance between the Hysteroscopic Diagnosis of Endometrial Hyperplasia and Histopathological Examination. Diagnostics 2019, 9, 142.
- Tinelli, R.; Tinelli, F.G.; Cicinelli, E.; Malvasi, A.; Tinelli, A. The role of hysteroscopy with eye-directed biopsy in postmenopausal women with uterine bleeding and endometrial atrophy. Menopause 2008, 15, 737–742.
- Wanderley, M.D.S.; Álvares, M.M.; Vogt, M.D.F.B.; Sazaki, L.M.P. Accuracy of Transvaginal Ultrasonography, Hysteroscopy and Uterine Curettage in Evaluating Endometrial Pathologies. Rev. Bras. Ginecol. Obstet. 2016, 38, 506–511.
- Vitale, S.G.; Laganà, A.S.; Török, P.; Lasmar, R.B.; Carugno, J.; Palumbo, M.; Tesarik, J. Virtual sonographic hysteroscopy in assisted reproduction: A retrospective cost-effectiveness analysis. Int. J. Gynecol. Obstet. 2022, 156, 112–118.
- Vilos, G.A. Correspondence. Fertil. Steril. 1999, 72, 740–743.
- Sardo, A.D.S.; Mazzon, I.; Bramante, S.; Bettocchi, S.; Bifulco, G.; Guida, M.; Nappi, C. Hysteroscopic myomectomy: A comprehensive review of surgical techniques. Hum. Reprod. Updat. 2008, 14, 101–119.
- Vitale, S.G.; Laganà, A.S.; Caruso, S.; Garzon, S.; Vecchio, G.M.; La Rosa, V.L.; Casarin, J.; Ghezzi, F. Comparison of three biopsy forceps for hysteroscopic endometrial biopsy in postmenopausal patients (HYGREB-1): A multicenter, single-blind randomized clinical trial. Int. J. Gynecol. Obstet. 2021, 155, 425–432.
- Luerti, M.; Vitagliano, A.; Sardo, A.D.S.; Angioni, S.; Garuti, G.; De Angelis, C.; Del Zoppo, S.; Dealberti, D.; Nappi, L.; Perrini, G.; et al. Effectiveness of Hysteroscopic Techniques for Endometrial Polyp Removal: The Italian Multicenter Trial. J. Minim. Invasive Gynecol. 2019, 6, 1169–1176.
- Wortman, M.; Daggett, A.; Ball, C. Operative Hysteroscopy in an Office-Based Surgical Setting: Review of Patient Safety and Satisfaction in 414 Cases. J. Minim. Invasive Gynecol. 2013, 20, 56–63.
- Clark, T.J.; Middleton, L.J.; Cooper, N.A.; Diwakar, L.; Denny, E.; Smith, P.; Gennard, L.; Stobert, L.; E Roberts, T.; Cheed, V.; et al. A randomised controlled trial of Outpatient versus inpatient Polyp Treatment (OPT) for abnormal uterine bleeding. Health Technol. Assess. 2015, 19, 1–194.
- Scheiber, M.D.; Chen, S.H. A Prospective Multicenter Registry of Patients Undergoing Hysteroscopic Morcellation of Uterine Polyps and Myomas. J. Gynecol. Surg. 2016, 32, 318–323.
- Famada, A.V.; Plans, R.C.; Canals, L.C.; Torrijos, M.R.; Vicente, A.R.; Albadalejo, A.B. Outcomes of surgical hysteroscopy: 25 years of observational study. J. Obstet. Gynaecol. 2021, 1–5.
- MacLean-Fraser, E.; Penava, D.; Vilos, G.A. Perioperative Complication Rates of Primary and Repeat Hysteroscopic Endometrial Ablations. J. Am. Assoc. Gynecol. Laparosc. 2002, 9, 175–177.
- Jo, Y.Y.; Jeon, H.J.; Choi, E.; Choi, Y.-S. Extreme hyponatremia with moderate metabolic acidosis during hysteroscopic myomectomy -A case report-. Korean J. Anesthesiol. 2011, 60, 440–443.
- Van Dijck, C.; Timmerman, D.; Teunkens, A.; Rex, S.; Verguts, J.; Van De Velde, M. Venous Air Embolism during Hysteroscopic Myomectomy: An Analysis of 7 Cases. Gynecol. Obstet. Investig. 2017, 82, 569–574.
- Vilos, G.A.; Hutson, J.R.; Singh, I.S.; Giannakopoulos, F.; Abu Rafea, B.; Vilos, A.G. Venous Gas Embolism during Hysteroscopic Endometrial Ablation: Report of 5 Cases and Review of the Literature. J. Minim. Invasive Gynecol. 2020, 27, 748–754.
- Tammam, A.E. Comparative Study between Monopolar Electrodes and Bipolar Electrodes in Hysteroscopic Surgery. J. Clin. Diagn. Res. 2015, 9, QC11-1.
- De Franciscis, P.; Grauso, F.; Cobellis, L.; Messalli, E.M.; Cucinella, G.; Perino, A.; Colacurci, N.; Torella, M. Outcomes of monopolar versus bipolar endometrial ablation on uterine bleeding and psychophysical wellbeing. Minerva Ginecol. 2017, 69, 328–335.
- Vitale, S.G.; Riemma, G.; Carugno, J.; Perez-Medina, T.; Pacheco, L.A.; Haimovich, S.; Parry, J.P.; Sardo, A.D.S.; De Franciscis, P. Postsurgical barrier strategies to avoid the recurrence of intrauterine adhesion formation after hysteroscopic adhesiolysis: A network meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2022, 226, 487–498.e8.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
24 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




