
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kasper Eersels | + 4173 word(s) | 4173 | 2020-10-01 13:51:53 | | | |
| 2 | Rita Xu | -840 word(s) | 3333 | 2020-10-09 04:55:06 | | |
Video Upload Options
Point of care (PoC) diagnostics have been a hot topic for many decades now. In high-income countries they have the potential to streamline diagnostics, making healthcare more personal, cheaper, faster and more efficient in general. However, in resource-limited settings such as low-income countries, the need for PoC implementation is much more pronounced as professional laboratories are often hours away from patients which makes it hard to accurately diagnose them. Therefore, research on and implementation of PoC diagnostics in local health care systems in LICs will be essential in order to alleviate suffering due to infectious disease.
This entry discusses the entire value chain of the development of PoC diagnostic tool. Bottlenecks that hinder the implementation of PoC in LICs are highlighted at every step of the value chain. We will provide examples of PoC success stories to illustrate how potential bottlenecks can be overcome. Finally, the entry provides a recommendation on how we can avoid these typical ‘leaks in the pipeline’ in the future and prevent a promising technology being abandoned before it can even have an impact on healthcare.
1. Introduction
Low- and middle-income countries (LIC/MICs) face severe challenges due to limited economic opportunities. In addition to the economic struggles, LICs also bear a large burden of transmittable diseases, posing severe risks to the population’s wellbeing [1]. Healthcare systems and healthcare providers in LICs are often ill-equipped to treat the patients in the best possible way, especially in rural areas. Given the economical and infrastructural challenges in LIC, PoC diagnostics, which are often characterized by being independent of laboratory or medical infrastructure, as well as being highly affordable and holding considerable promise to improve the situation. Yet the actual commercialization of PoC diagnostic-tests lags well behind the innovative research and developments done in laboratories.
Due to this strange dichotomy between promising, innovative research and very limited valorization into real products, several review articles on the topic have been written in past years. However, most are specialized on one specific aspect—for example some authors looked in depth at logistical shortcomings [2]; others investigated funding and collaboration considerations [3]. Reviews also summarized the topic in consideration of different viewing angles, such as the technological aspects and their implications, as seen in Figure 1 [4]. Another approach is to distinguish between different usage profiles, from the use at home up to the use in a laboratory, and argued that what point-of-care means depends tremendously on how and where it is used, which is shown in Figure 2 [5].
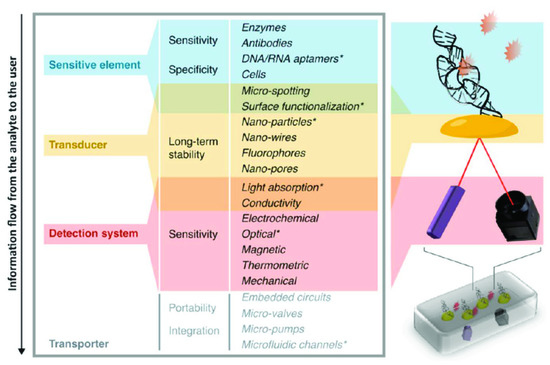
Figure 1. Migliozzi and Guibentif et al., looked at PoC bottlenecks from a technological standpoint. Figure re-used from [4] with permission under open-access creative commons copyright agreement.
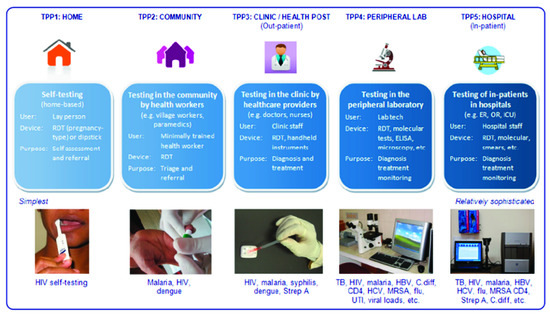
Figure 2. Pai et al., investigated PoC use within different usage scenarios. Figure adapted from [5] with permission under open-access copyright agreement.
2. The Usage
Limited testing capabilities are often a bottleneck for adequate therapy. This is especially observable in HIV treatment, where CD4+ counts and viral load are used to monitor antiretroviral therapy (ART). In Sub-Saharan Africa, the median of patients that were retained between HIV diagnosis and CD4+ count was 59% [6]. HIV is a good example of a disease that is difficult to get a conclusive diagnosis on in the field as it needs a nucleic acid test that usually has to be performed in a laboratory with trained personnel [7][8][9]. While there are several HCTs PoC platforms available that can conduct HIV monitoring, such as the GeneXpert (Cepheid), the PIMA CD4+ (Abbot) or the Alere q (Abbot) [10][11][12], their high initial cost are still a problem. This makes ART therapy challenging to start and monitor in rural areas. Therefore, not only is supplementation with tests an important factor, but also the whole infrastructure of usage in combination with treatment, especially in rural healthcare settings where the different parts need to act together to create sensible plans for PoC testing and treatment delivery. Due to this interconnectedness of different factors surrounding the end-users, several bottlenecks can appear. HIV is, therefore, a good example of how healthcare, as well as patient management, are integral factors and can negate any positive effects that PoC can bring, if they are mishandled.
2.1. Healthcare Management
Political will towards PoC might be reduced when PoC tests lead to more demand for treatment, while at the same time treatment capabilities are scarce [5]. On the other hand, PoC diagnosis might not be feasible (for a test and treat scenario) when adequate treatment capabilities are not in place [13]. When treatment is available, patients might also just opt-out of tests in favor of direct use of medication, such as over-the-counter-antibiotics. This has been reported in Thailand, where missing information about disease origin among the public leads to a preference of medication instead of proper diagnosis, as medication is connected to symptoms instead of disease origin. For example, patients associate antibiotics with the symptoms of a bacterial infection instead of the infection itself and thus demand antibiotics even when the reason for the illness is not bacterial in nature but has similar symptoms [14]. PoC testing might offer proper diagnoses, preventing people from self-diagnoses and taking inadequate medicine. This also shows the importance of looking at PoC applications not independently but in the whole context of the healthcare system, that acts and is acted upon by various factors. Some researchers assess that the introduction of a PoC device into a system that is already in place changes the role from a technical to a social device. However, this also has its upsides—a more elusive benefit of why healthcare workers in rural areas might want to use PoC testing is the psychological effect it can have on patients, giving healthcare workers more certainty which, in turn, transfers to the patient and improves compliance. Patients also might overestimate the capabilities of the tests, which encourages compliance [14]. PoC can provide evidence without the need of laboratory infrastructure and highly trained lab technicians [15].
2.2. Patient Management
In addition to the infrastructural bottlenecks related to the availability of electricity and water, which were discussed in the research section, one of the most immediate problems is patient management. In rural areas, traditional laboratory diagnostics are limited by distance to central laboratories. For laboratory diagnostics, samples, for example, in the form of dried blood spots, have to be transported via motorcycle, creating problems of long turnaround times for test results of up to two weeks and the danger of sample damage or loss. Another problem is the loss-to-follow-up when patients have to either wait for their results or have to go to another facility. This may be a problem for two step diagnostic processes in which the second step is not PoC and the patient has to return or travel to a second facility [16]. Especially in rural LICs areas, there are large barriers to get to healthcare facilities, due to poor transport infrastructure and time constraints that might prevent a second visit [13].
For example, a third of the women in Ghana live further than two hours away from facilities with the capabilities of emergency obstetric and neonatal care [17][18]. Barriers such as these create gaps in the diagnostic and treatment pipeline and can lead to high levels of LTFU if patients do not return to collect their results and start treatment [9][19]. However, this also shows that highly effective laboratory diagnostics might not be suitable in LICs, even if their sensitivity and selectivity is far superior to PoC devices. If they pose the risk of LTFU, a one-step PoC device might be the more pragmatic and better solution [46]. In HIV diagnostic and treatment, the decentralization of diagnostics from large hospitals to rural healthcare centers (RHC) proved essential to give people outside of urban areas access to therapies, such as ART. Before the implementation of testing in RHCs, the rate of LTFU was unacceptably high [20] as over half of patients did not return to get their results [21].
The large potential of PoC diagnostics in this context is, in part, due to their ability to ensure the start of treatment in the same encounter, which is essential as the rapid initiation of treatment is immensely important in diseases such as aids and tuberculosis [22]. In India, just one round for combined screening and treatment of HPV reduced the cervical cancer rate and mortality for over 30-year-old women by 50% [23]. For this reason, the WHO recommends a screen and treat strategy for 30–39 year old women [13]. A PoC test-time of under one hour from test to result would be ideal, as treatment can follow in the same encounter [24]. In a healthcare worker survey, the participants argued that a sample-to-answer time of less than an hour is indeed optimal [25]. This forms a barrier for most nucleic acid based tests, which take several hours [25].
Some researchers therefore argue for more holistic thinking in terms of health services. There needs to be better linkage that connects testing, diagnosis and treatment [26]. Given the large impact LTFU has, resources could also be used in preventing LTFU instead of perfecting diagnostic devices [13].
For example, getting infants on ART could be achieved with combinations of tests of different sensitivity, but proper linkage. The initiation rate of 71% could be achieved with PoC devices with a limited sensitivity of only 72%, but a successful linkage rate of 99%, or with a test of 100% sensitivity and 70% successful linkage [7][9]. Some researchers suggest that PoC tests should be evaluated just as much on their ability to facilitate linkage as they are on their performance [9]. As demonstrated before, the cost-benefit also favors a two-step system. The absence of functional referral systems is seen as a huge roadblock by other authors as well [21].
Therefore, information technology plays a key role in the context of PoC, maybe even more so than new and better devices themselves. The rapid reporting of results and counseling via mobile phones are essential for a decentralized use of PoC. Mobile phone-linked PoC devices can also assist in data capture and quality control, medication distribution, PoC tracking and data storage [5][25][27]. The usage of mobile phones in this way is generally categorized as mHealth, an expanding subfield of eHealth, and concerns itself with the use of wireless technology instead of connection through ordinary landline infrastructure, such as in eHealth. This is especially interesting for LMICs, as mobile phone usage outperforms other communication infrastructure usage [28]. In total, 70% of the 7.4 billion users of cellular phones reside in LMICs, and especially in sub-Saharan Africa, mHealth had a rapid expansion, making this approach hugely promising [17][29][30]. The response to Ebola PoC in the last epidemic might serve as an example, as it was very fast as a result of the effective surveillance systems in place. However, healthcare systems are slow to use this connectivity to their full potential [26]. This is starting to change as mHealth is more utilized. In a review of 255 studies of mHealth applications, 93 studies fell into the realm of health monitoring and surveillance, the second largest group with 88 publications concerned themselves with raising health awareness [28]; another study found the most used context to be increased patient follow-up, as well as patient compliance [30].
2.3. Training
While large hospitals in central areas might also have an appropriate workforce, the staff in rural healthcare clinics, which are the main access points to healthcare for the rural population, consist mainly of untrained individuals or inadequately small workforces; often just one doctor, nurse or pharmacist, with the possible addition of lay healthcare workers (LHWs) [25][31][21][32].
Human resources are in surprisingly short supply when it comes to healthcare workers and may stretch out the system, especially in rural sites [26][33]. Additional onsite testing could put even more strain on the already overworked staff; as already mentioned, this might be a major problem if there are no additional staff or incentives available, and might discourage PoC use [17][25][34]. There is also a problem in the lack of educated healthcare personnel, especially in Africa, which has over 24% of the worldwide disease burden while only having 2% of physicians of the world [31][35][36][37]. The shortage of skilled healthcare workers was seen as a problem by several authors [17][38].
Surveyed healthcare workers assessed PoC diagnostics as easy to use; however, they still expressed fear of knowledge gaps among their users and concern of incorrect use. For example, the use of a wrong buffer solution or no buffer solution at all was observed [38]. Other researchers also reported reluctance for PoC in LICs due to the need for training, and the costs associated with implementation, as well as diagnosis [22]. It is especially feared that lay healthcare workers will not have the adequate training or knowledge to conduct even simple PoC tests, which could lead to inaccurate results that could damage the perception of PoC in these settings [17]. Despite those fears, the world health organization recommends task shifting to LHWs to meet human resources needs [6][39] and it is a tool that is increasingly used to combat the estimate estimated shortage of 7.2 million healthcare workers, which is the most severe in Sub-Saharan Africa [40].
The question is, can task shifting from healthcare professionals to LHWs be achieved without a loss in reliability of test results. Lay health workers provide an important opportunity to give more people access to healthcare; this is especially the case for rural areas. However, medical devices are usually not designed to account for task shifting [31].
Some argue that task-shifting has to be accommodated when designing the PoC device. The interface has to be straightforward and user-friendly, even for laymen, which is often not considered in the design phase [15]. Ideally, the device should be a fully autonomous, robust, “black box” [4], which is fully automated with a simple interface and everything integrated into a simple “sample to answer” process [25]. Others suggest that the technological complexity must be as simple as a home pregnancy test [24], or assess modern PoC diagnostic platforms used for CD4+ testing as too sophisticated for usage in LICs [19]. Laboratory professionals also doubt that diagnostics can be performed by lay-healthcare-workers with appropriate quality assurance [5][22]. This viewpoint might be understandable, given the findings of doctors observing the wrong use of lateral flow devices [38]. The question, therefore, remains whether PoC devices demand usage by healthcare professionals.
Research is suggesting otherwise. A study about task shifting for the use of the HCTs Pima CD4+ Analyzer (Allere) in Namibia showed that lay-health-workers can produc valid tests as nurses. In a large study of 1429 CD4+ tests, in which 500 were performed by nurses and 929 by LHWs, the reception of test results by the patients was in favor of LHWs, with 98.1% contrary to 95.6%. LHCs were only slightly slower, with a median turnaround time of 21 minutes compared to 20 minutes for nurses. However, both were a tremendous improvement from the turnaround time of a laboratory test, which had a median of 4 days (IRq 2–8). Therefore, task shifting to LHWs may be an appropriate choice, even for more complex tests [6]. Other studies agree that LHWs can perform rapid testing just as well as trained laboratory staff, if trained properly. However, when implementing a training program, it has to be considered that the training package is adapted to the local environment [39]. LHWs in Malawi have named a lack of disease- and job-specific training as a key problem hindering their role as TB care providers [40][41]. Lateral flow tests for HIV testing were so successful in their ease of use, that task shifting could be greatly implemented and the tests in LMIC are now often done by expert-patients or trained lay healthcare workers [21].
2.3.1. Use by Trained Doctors
In cases where trained medical professionals are available, the bottlenecks present themselves differently. One issue is time constraints. In India, doctors prefer clinical diagnoses coupled with empiric treatments over a higher diagnostic security. Broadband antibiotic prescription after only a short symptomatic observation is a common example; this is faster than doing an additional PoC test that might not even be necessary, just in order to make the diagnosis more secure [5]. The general overburden of doctors in India is one factor for this. Generally, visits only last a few minutes, which is generally not enough time for PoC testing. On the doctor’s side, it is better for his reputation to treat several other waiting patients in this time and keep waiting-lines short. Many doctors only have a single room with one nurse as an assistant, even simple lateral flow tests are difficult to conduct under such conditions [5].
Awareness is another issue which is suggested by the literature. Healthcare providers might not be aware of PoC tests on the market [5]. Lack of knowledge about PoC testing among people living in rural areas could be addressed by, for example, advertising and explaining the use of PoC tests for rapid diagnosis of specific diseases. This could raise awareness and prevent last-minute visits to the doctor. However, general awareness of PoC devices in Kenya was surveyed and is high throughout high-, mid- and low-tier healthcare providers and seems to not be a critical barrier. In total, 95% of healthcare providers in his survey could name a disease that can be diagnosed with PoC tests (71% could name two and 24% could name three); only 5% were not able to name any disease which has PoC tests available. However, only 10% of healthcare workers which named more than three PoC devices had actually applied them in their practice [42]. This indicates that the bottleneck is systemic rather than knowledge-based. Higher knowledge and usage about HIV tests were shown by doctors in richer hospitals compared to rural doctors. For example, in malaria diagnostics there is a wide gap between the knowledge about (57%) and actual use of PoC diagnostics (36%), independent of socioeconomic factors. It is suggested that this is because PoC devices for malaria are seen as of limited usefulness as the disease has strong symptoms and the prevalence is foreseeable due to the seasons. Another possible reason might be the lack of availability, the devices not complementing other diagnostic methods or greater success with other diagnostic methods, such as a symptomatic approach. For other identified diagnostics, the knowledge about the device was 1- to 3-times higher than the actual use [42]. Healthcare workers in other studies could identify various PoC tests. The most known ones were for Malaria, HIV, Syphilis, Blood Glucose and Pregnancy. These findings concurred with other surveys [38][43].
2.3.2. View on PoC
With regard to the view of patients on PoC, it was found that, according to healthcare personnel, patients were satisfied with PoC results (97%) and would recommend them (96%). However, only half of clinicians thought they would give reliably accurate results; 46% were unsure and 4% considered them not accurate. In total, 65% of healthcare workers used medication even on a negative test, showing that trust in the test is limited. This concurs with only 20% of healthcare workers stating that they rely on the test alone. The majority sees the test as a complement to other means of diagnosis, such as symptoms [42], as 54% of surveyed encountered barriers preventing PoC use. The likelihood of encountering barriers correlated with hospital tier (45% in high-end hospitals and 53% in mid-tier). In total, 50% of personnel who encountered barriers named reliability issues as a large problem; the second largest obstacle named was availability, with 46%. Only a smaller percentage saw cost (14%) and awareness or training deficiencies (12%) as major obstacles. When asked about improvements to increase PoC use, the respondents replied with improved tests (44%), improved reliability (22%) and standardization (20%) which was specifically mentioned, even though it was not in the survey as an answer. Oddly, increased availability was named by just 22%, despite it being the second highest identified barrier. In total, 85% agreed that PoC is an opportunity for more affordable healthcare in Kenya [42].
From the first start of research in the beginning, to the view of end-users at the patient-side, the bottlenecks of PoC diagnostics along the value chain seem to be as diverse and as different from each other as the actors and circumstances that present themselves on the way, as is summarized in Figure 4. However, as Figure 5 shows, there are many possible solutions at each step as well, which are, next to technological advancements, often based upon the connection of different stakeholders.
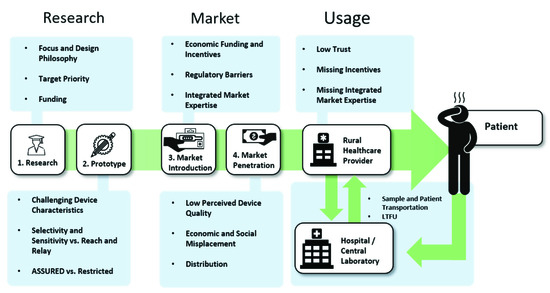
Figure 4. Identified Problems along the value chain.
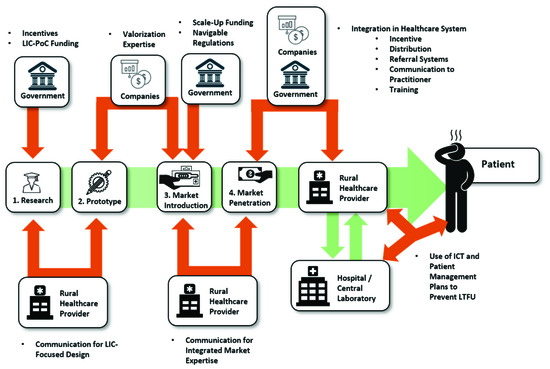
Figure 5. Identified Pivotal Points and Influencing Forces.
References
- Boutayeb, A. The impact of infectious diseases on the development of Africa. In The Handbook of Disease Burdens and Quality of Life Measures; Preedy, V.R., Watson, R.R., Eds.; Springer: New York, NY, USA, 2010; pp. 1171–1188. ISBN 978-0-387-78664-3.
- Desmond Kuupiel; Vitalis Bawontuo; Paul K. Drain; Nonjabulo Gwala; Tivani Mashamba-Thompson; Supply chain management and accessibility to point-of-care testing in resource-limited settings: a systematic scoping review. BMC Health Services Research 2019, 19, 519, 10.1186/s12913-019-4351-3.
- Amy Starr; Katy M Graef; Jennifer Dent; Fostering innovative product development for neglected tropical diseases through partnerships. Pharmaceutical Patent Analyst 2016, 5, 391-400, 10.4155/ppa-2016-0038.
- D. Migliozzi; Thomas Guibentif; Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects. Biosensors 2018, 8, 119, 10.3390/bios8040119.
- Nitika Pant Pai; Caroline Vadnais; Claudia Denkinger; Nora Engel; Madhukar Pai; Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries. PLOS Medicine 2012, 9, e1001306, 10.1371/journal.pmed.1001306.
- Francina Kaindjee-Tjituka; Souleymane Sawadogo; Graham Mutandi; Andrew D. Maher; Natanael Salomo; Claudia Mbapaha; Marytha Neo; Anita Beukes; Justice Gweshe; Alexinah Muadinohamba; et al.David W. Lowrance Task-shifting point-of-care CD4+ testing to lay health workers in HIV care and treatment services in Namibia. African Journal of Laboratory Medicine 2017, 6, 643, 10.4102/ajlm.v6i1.643.
- Marvin Hsiao; Kathryn Stinson; Landon Myer; Linkage of HIV-Infected Infants from Diagnosis to Antiretroviral Therapy Services across the Western Cape, South Africa. PLoS ONE 2013, 8, e55308, 10.1371/journal.pone.0055308.
- Andrea Ciaranello; Ji-Eun Park; Lynn Ramirez-Avila; Kenneth A. Freedberg; Rochelle P. Walensky; Valériane Leroy; Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Medicine 2011, 9, 59, 10.1186/1741-7015-9-59.
- Lorna Dunning; Marvin Hsiao; Landon Myer; Point-of-care HIV early infant diagnosis: is test sensitivity everything?. Journal of the International AIDS Society 2015, 18, 20235.
- Zibusiso Ndlovu; Emmanuel Fajardo; Elton Mbofana; Tatenda Maparo; Daniela Garone; Carol Metcalf; Helen Bygrave; Kekeletso Kao; Sekesai Zinyowera; Multidisease testing for HIV and TB using the GeneXpert platform: A feasibility study in rural Zimbabwe. PLoS ONE 2018, 13, e0193577, 10.1371/journal.pone.0193577.
- Clement Zeh; Charles E. Rose; Seth Inzaule; Mitesh A. Desai; Fredrick Otieno; Felix Humwa; Benta Akoth; Paul Omolo; Robert T. Chen; Yenew Kebede; et al.Taraz Samandari Laboratory-based performance evaluation of PIMA CD4+ T-lymphocyte count point-of-care by lay-counselors in Kenya.. Journal of Immunological Methods 2017, 448, 44-50, 10.1016/j.jim.2017.05.006.
- Nei-Yuan Hsiao; Lorna Dunning; Max Kroon; Landon Myer; Laboratory Evaluation of the Alere q Point-of-Care System for Early Infant HIV Diagnosis. PLoS ONE 2016, 11, e0152672, 10.1371/journal.pone.0152672.
- Nicole G. Campos; Vivien Davis Tsu; Jose Jeronimo; Mercy Mvundura; Jane J. Kim; Estimating the value of point-of-care HPV testing in three low- and middle-income countries: a modeling study. BMC Cancer 2017, 17, 791, 10.1186/s12885-017-3786-3.
- Marco J. Haenssgen; Nutcha Charoenboon; Thomas Althaus; Rachel C Greer; Daranee Intralawan; Yoel Lubell; The social role of C-reactive protein point-of-care testing to guide antibiotic prescription in Northern Thailand. Social Science & Medicine 2018, 202, 1-12, 10.1016/j.socscimed.2018.02.018.
- Shuqi Wang; Mark A. Lifson; Fatih Inci; Li-Guo Liang; Ye-Feng Sheng; Utkan Demirci; Advances in addressing technical challenges of point-of-care diagnostics in resource-limited settings. Expert Review of Molecular Diagnostics 2016, 16, 449-459, 10.1586/14737159.2016.1142877.
- Léa Duchesne; Gilles Hejblum; Ndèye Coumba Toure Kane; Richard Njouom; Thomas-D'aquin Toni; Raoul Moh; Babacar Sylla; Nicolas Rouveau; Alain Attia; Karine Lacombe; et al. Model-based cost-effectiveness estimates of testing strategies for diagnosing hepatitis C virus infection in people who use injecting drugs in Senegal. International Journal of Drug Policy 2020, 75, 102613, 10.1016/j.drugpo.2019.102613.
- Desmond Kuupiel; Vitalis Bawontuo; Tivani P. Mashamba-Thompson; Improving the Accessibility and Efficiency of Point-of-Care Diagnostics Services in Low- and Middle-Income Countries: Lean and Agile Supply Chain Management. Diagnostics 2017, 7, 58, 10.3390/diagnostics7040058.
- Peter W. Gething; Fiifi Amoako Johnson; Faustina Frempong-Ainguah; Philomena Nyarko; Angela Baschieri; Patrick Aboagye; Jane Falkingham; Zoë Matthews; P.M. Atkinson; Geographical access to care at birth in Ghana: a barrier to safe motherhood. BMC Public Health 2012, 12, 991, 10.1186/1471-2458-12-991.
- R. Zachariah; S. D. Reid; P. Chaillet; M. Massaquoi; E. J. Schouten; A. D. Harries; Viewpoint: Why do we need a point-of-care CD4 test for low-income countries?. Tropical Medicine and International Health 2010, 16, 37-41, 10.1111/j.1365-3156.2010.02669.x.
- Katie Tayler-Smith; R. Zachariah; Moses Massaquoi; Marcel Manzi; Olesi Pasulani; Thomas Van Den Akker; Marielle Bemelmans; Ariane Bauernfeind; Beatrice Mwagomba; Anthony D Harries; et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system?. Transactions of The Royal Society of Tropical Medicine and Hygiene 2010, 104, 313-319, 10.1016/j.trstmh.2010.01.007.
- Steven Reid; Sarah J Fidler; Graham Cooke; Tracking the progress of HIV: the impact of point-of-care tests on antiretroviral therapy. Clinical Epidemiology 2013, 5, 387-396, 10.2147/CLEP.S37069.
- Tivani Phosa Mashamba-Thompson; Ngcwalisa A. Jama; Benn Sartorius; Paul K Drain; Rowan M. Thompson; Implementation of Point-of-Care Diagnostics in Rural Primary Healthcare Clinics in South Africa: Perspectives of Key Stakeholders. Diagnostics 2017, 7, 3, 10.3390/diagnostics7010003.
- Rengaswamy Sankaranarayanan; Bhagwan M. Nene; Surendra S. Shastri; Kasturi Jayant; Richard Muwonge; Atul M. Budukh; Sanjay Hingmire; Sylla G. Malvi; Ranjit Thorat; Ashok Kothari; et al.Roshan ChinoyRohini KelkarShubhada KaneSangeetha DesaiVijay R. KeskarRaghevendra RajeshwarkarNandkumar PanseKetayun A. Dinshaw HPV Screening for Cervical Cancer in Rural India. New England Journal of Medicine 2009, 360, 1385-1394, 10.1056/nejmoa0808516.
- Mickey Urdea; Laura A. Penny; Stuart S. Olmsted; Maria Y. Giovanni; Peter Kaspar; Andrew Shepherd; Penny Wilson; Carol Dahl; Steven Buchsbaum; Gerry Moeller; et al.Deborah C. Hay Burgess Requirements for high impact diagnostics in the developing world. Nature 2006, 444, 73-79, 10.1038/nature05448.
- Ahmad N. Abou Tayoun; Paul R. Burchard; Imran Malik; Axel Scherer; Gregory J. Tsongalis; Democratizing Molecular Diagnostics for the Developing World. American Journal of Clinical Pathology 2014, 141, 17-24, 10.1309/ajcpa1l4kpxbjnpg.
- Rosanna Peeling; Bringing diagnostics to developing countries: an interview with Rosanna Peeling. Expert Review of Molecular Diagnostics 2015, 15, 1107-1110, 10.1586/14737159.2015.1081802.
- Steven R. Steinhubl; Evan D. Muse; Eric J. Topol; The emerging field of mobile health. Science Translational Medicine 2015, 7, 283rv3, 10.1126/scitranslmed.aaa3487.
- Abaza, H.; Marschollek, M. mHealth application areas and technology combinations. A comparison of literature from high and low/middle income countries. Methods Inf. Med. 2017, 56, e105–e122.
- Sandeep Kumar Vashist; Peter B. Luppa; Leslie Y. Yeo; Aydogan Ozcan; John H.T. Luong; Emerging Technologies for Next-Generation Point-of-Care Testing. Trends in Biotechnology 2015, 33, 692-705, 10.1016/j.tibtech.2015.09.001.
- Vincent Duclos; Maurice Ye; Moubassira Kagoné; Hamidou Sanou; N. Hélène Sawadogo; Gilles Bibeau; Ali Sie; Situating mobile health: a qualitative study of mHealth expectations in the rural health district of Nouna, Burkina Faso. Health Research Policy and Systems 2017, 15, 47, 10.1186/s12961-017-0211-y.
- Amir Sabet Sarvestani; K.H. Sienko; Medical device landscape for communicable and noncommunicable diseases in low-income countries.. Globalization and Health 2018, 14, 65, 10.1186/s12992-018-0355-8.
- Lora Perry; Robert Malkin; Effectiveness of medical equipment donations to improve health systems: how much medical equipment is broken in the developing world?. Medical & Biological Engineering & Computing 2011, 49, 719-722, 10.1007/s11517-011-0786-3.
- McNerney, R; Diagnostics for developing countries. Diagnostics 2015, 5, 200–209.
- Minh D. Pham; Paul A. Agius; Lorena Romero; Peter McGlynn; David Anderson; Suzanne M. Crowe; Stanley Luchters; Acceptability and feasibility of point-of-care CD4 testing on HIV continuum of care in low and middle income countries: a systematic review. BMC Health Services Research 2016, 16, 343, 10.1186/s12913-016-1588-y.
- Global Health Workforce Alliance; WHO. The Cost-Effectiveness of Close-to-Community Health Programmes: What do We Know and Where Are the Gaps? Available online: https://www.who.int/workforcealliance/knowledge/resources/cost_effectiveness_brief/en/ (accessed on 21 August 2020).
- WHO. Treat. Train. Retain-Task Shifting: Global Recommendations and Guidelines; WHO Document Production Services: Geneva, Switzerland, 2008; ISBN 978-92-4-159631-2.
- Richard M Scheffler; Jenny X Liu; Yohannes Kinfu; Mario Dal Poz; Forecasting the global shortage of physicians: an economic- and needs-based approach. Bulletin of the World Health Organization 2008, 86, 516-523, 10.2471/BLT.07.046474.
- Reza Rasti; Deborah Nanjebe; Jonas Karlström; Charles Muchunguzi; Juliet Mwanga-Amumpaire; Jesper Gantelius; Andreas Mårtensson; Lourdes Rivas; Francesc Galban; Philippa Reuterswärd; et al.Helene Andersson SvahnHelle M. AlvessonYap BoumTobias Alfvén Health care workers’ perceptions of point-of-care testing in a low-income country—A qualitative study in Southwestern Uganda. PLoS ONE 2017, 12, e0182005, 10.1371/journal.pone.0182005.
- Katy Yao; Winnie Wafula; Ebi Bile; Rachanee Cheignsong; Stacy Howard; Austin Demby; John Nkengasong; Ensuring the Quality of HIV Rapid Testing in Resource-Poor Countries Using a Systematic Approach to Training. American Journal of Clinical Pathology 2010, 134, 568-572, 10.1309/AJCPOPXR8MNTZ5PY.
- Lisa M Puchalski Ritchie; Monique Van Lettow; Austine Makwakwa; Adrienne K Chan; Jemila S Hamid; Harry Kawonga; Alexandra L.C. Martiniuk; Michael J Schull; Vanessa Van Schoor; Merrick Zwarenstein; et al.Jan BarnsleySharon E. Straus The impact of a knowledge translation intervention employing educational outreach and a point-of-care reminder tool vs standard lay health worker training on tuberculosis treatment completion rates: study protocol for a cluster randomized controlled trial. Trials 2016, 17, 439, 10.1186/s13063-016-1563-2.
- L M Puchalski Ritchie; M Van Lettow; J Barnsley; A K Chan; M Joshua; Alexandra L.C. Martiniuk; Michael J Schull; Merrick Zwarenstein; Evaluation of lay health workers' needs to effectively support anti-tuberculosis treatment adherence in Malawi.. The International Journal of Tuberculosis and Lung Disease 2012, 16, 1492–1497, 10.5588/ijtld.12.0206.
- Faith W. Kimani; Samuel M. Mwangi; Benjamin Kwasa; Abdi M. Kusow; Benjamin Ngugi; Jiahao Chen; Xinyu Liu; Rebecca Cademartiri; Martin M. Thuo; Rethinking the Design of Low-Cost Point-of-Care Diagnostic Devices. Micromachines 2017, 8, 317, 10.3390/mi8110317.
- Lee F. Schroeder; Ali Elbireer; J. Brooks Jackson; Timothy K. Amukele; Laboratory Diagnostics Market in East Africa: A Survey of Test Types, Test Availability, and Test Prices in Kampala, Uganda. PLoS ONE 2015, 10, e0134578, 10.1371/journal.pone.0134578.




