Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | gül kozalak | -- | 2450 | 2022-05-18 05:26:59 | | | |
| 2 | Vivi Li | Meta information modification | 2450 | 2022-05-19 03:29:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kozalak, G.; Yong, K.W.; Choi, J.R.; Di Bari, I.; Babar, Q.; Niknam, Z.; Rasmi, Y. In Vitro Human Cancer Models for Biomedical Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/23034 (accessed on 07 February 2026).
Kozalak G, Yong KW, Choi JR, Di Bari I, Babar Q, Niknam Z, et al. In Vitro Human Cancer Models for Biomedical Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/23034. Accessed February 07, 2026.
Kozalak, Gül, Kar Wey Yong, Jane Ru Choi, Ighli Di Bari, Quratulain Babar, Zahra Niknam, Yousef Rasmi. "In Vitro Human Cancer Models for Biomedical Applications" Encyclopedia, https://encyclopedia.pub/entry/23034 (accessed February 07, 2026).
Kozalak, G., Yong, K.W., Choi, J.R., Di Bari, I., Babar, Q., Niknam, Z., & Rasmi, Y. (2022, May 18). In Vitro Human Cancer Models for Biomedical Applications. In Encyclopedia. https://encyclopedia.pub/entry/23034
Kozalak, Gül, et al. "In Vitro Human Cancer Models for Biomedical Applications." Encyclopedia. Web. 18 May, 2022.
Copy Citation
Cancer is one of the leading causes of death worldwide, and its incidence is steadily increasing. Although years of research have been conducted on cancer treatment, clinical treatment options for cancers are still limited. Animal cancer models have been widely used for studies of cancer therapeutics, but these models have been associated with many concerns, including inaccuracy in the representation of human cancers, high cost and ethical issues. Therefore, in vitro human cancer models are being developed quickly to fulfill the increasing demand for more relevant models in order to get a better knowledge of human cancers and to find novel treatments.
in vitro model
human cancers
biomedical applications
therapeutic development
tumor biology
cancer markers
1. Introduction
Cancer is known as one of the most life-threatening diseases worldwide [1]. In-depth studies of cancer mechanisms are essential, given that this disease is complex and progressive. Traditionally, animal models have been widely used for studies of cancer therapeutics as they provide a relevant tumor microenvironment (TME) to evaluate drug safety and efficacy [2]. However, problems associated with the accurate representation of human cancers, as well as challenges involving ethical controversies, high cost and difficult handling of animal models, have limited their usefulness [3]. Differences in tumor biology in animal models compared to human models explain why some drugs tested in animals are ineffective in humans [4]. To address these challenges, in vitro human cancer models, including two-dimensional (2D) (e.g., transwell-based model) and three-dimensional (3D) models (e.g., spheroid, microfluidic tumor-microvascular and scaffold-based) have been developed for cancer studies [5]. These models offer simple model design, easy operation and result interpretation, leading to a better understanding of different aspects of cancer, such as tumor growth and proliferation, tumor invasion and drug delivery [6]. Indeed, in vitro cancer models are being developed quickly to fulfill the increasing demand for more sophisticated models in order to get a better view of both cancer biology and cancer therapies.
The existing 2D cancer models have revealed tumor progression, which includes genetic alterations of tumorigenic phenotypes, tumor migration and angiogenesis. For example, 2D transwell-based cancer models have been used to study the effects of angiogenesis on tumor cells [7]. However, studies have shown the absence of key receptors and signaling molecules in these models. They were not able to represent the key characteristics of the TME, rendering them less suitable for drug screening [8]. In contrast, 3D in vitro cancer models resemble tumor behavior and complex multicellular TME, providing a more accurate and reliable platform for studies of disease processes and analysis of drug efficacy [9]. For instance, spheroids form clusters, which mimic the morphology and activities of human solid tumors to better understand tumor activities in the human body [10]. Various types of biomaterials (e.g., natural and synthetic hydrogels) have also been investigated to create scaffold-based cancer models for cancer studies [11]. In addition, previous studies have also developed microfluidic tumor-microvascular models for anticancer drug screening [12]. The 3D models discussed above are able to elucidate the role of different types of cells, extracellular matrix (ECM) components and different stimuli in TME. They are particularly suitable for in-depth studies of oncogenesis-related cellular pathways and transcriptomic profiles to develop better anticancer therapeutic agents.
2. Biomedical Applications of In Vitro Human Cancer Model
2.1. Therapeutic Development for Cancer Therapy
2.1.1. Anticancer Drug
In vitro disease models are utilized for medication development. This approach usually begins with basic research, which aids in the identification of pharmacological targets implicated in illness development. Following that, in vitro disease models may be utilized to screen drug libraries for medicines that affect the pharmacological target of interest. In vitro disease models will be utilized in parallel to analyze, investigate and optimize particular factors such as medication dose in order to predict drug efficacy and toxicity in people. The principle is the same: scientists test a variety of medicines on cells in vitro in order to swiftly exclude those that would not function in mice or people. These models were employed in the development and testing of current anticancer medicines, as well as the creation of novel treatments to substitute animal cancer models in chemotherapeutic testing [13]. In fact, in vitro cancer models are critical in cancer research for investigating genetic, epigenetic and cellular pathways, studying proliferative deregulation, apoptosis and cancer development, defining possible molecular markers and cancer therapies [14][15]. One of the first phases in medication development is generally the evaluation and testing of drugs in tumor cell lines. It permits a huge number of candidate medicines to be tested prior to subscribing to large-scale, costly in vivo clinical studies. Characterizing tumor cell lines in terms of their anchorage independence (soft agarose assay) is also imperative since it can be utilized to figure out which genes and pathways are involved in metastasis as well as their metastatic migration potential and invasiveness capacity [16][17]. Molecular profiling of cell lines that reveals changes in cell cycle regulators and other molecules is essential, enabling anticancer medicines to target cell cycle abnormalities [17]. For example, Hakozaki et al. reported upregulation of the epidermal growth factor receptor and cyclooxygenase-2 genes in a tumor cell line (FPS-1) acquired from an undifferentiated pleomorphic sarcoma (UPS), indicating that this cell line could be used to develop drugs that target these genes or cellular pathways [18]. When Fang et al. characterized cell lines acquired from patients with metastatic and recurrent malignant peripheral nerve sheath tumors, they discovered genes linked to metastatic potential, indicating that these genes could be targeted by therapeutic approaches [19]. Finally, DNA, RNA, proteins, chromosomal and functional profiling were performed on a panel of 60 distinct kinds of human tumor cell lines (NCI60) designed for the generation of anticancer medicines, allowing for an improved clinical translation of the findings of anticancer drug testing [20]. The molecular profiling of tumor cell lines also allows for a more accurate evaluation of cancer types and subtypes, as well as determining which cell lines are most suited for certain studies, improving the screening and research of anticancer medicines [21].
Tumor spheroids are commonly employed to evaluate tumor sensitivity and response to chemotherapeutics, including combination treatments (e.g., chemotherapeutics and small-molecule inhibitors), targeted chemotherapy and drug delivery vehicles [22]. Spheroids are frequently utilized as a high-throughput technique for both negative and positive drug candidate screening in novel drug development [23]. According to studies, gene expression patterns and responses to treatments in tumor spheroid models are more comparable to those in the native tumors [24]. For instance, liver tumor spheroids exhibited drug resistance, which was equivalent to that in native tumors [25]. In comparison to tumor cells grown in a monolayer culture, BT-549, BT-474 and T-47D breast tumor cell lines cultivated as spheroids demonstrated higher resistance to paclitaxel and doxorubicin [26]. Resistance to 5-fluorouracil, regorafenib and erlotinib was found to increase when HCT-116, SW-620 and DLD-1 colorectal carcinoma cell lines were cultured as spheroids with or without co-culturing with fibroblasts and endothelial cells [27]. Altogether, tumor spheroid models are better than monolayer cultured cells as tumor spheroids exhibit drug resistance seen in native tumors [28].
Three-dimensional bioprinting technology also allows for the construction of in vitro cancer models for anticancer drug screening. For instance, a 3D bioprinted breast cancer model that demonstrated doxorubicin resistance was used to evaluate the anticancer effect of lysyl oxidase inhibitor. Lysyl oxidate inhibitor was able to enhance doxorubicin sensitivity of the breast cancer model [29]. In another study, a 3D bioprinted vascularized human glioblastoma model was used to assess the therapeutic effects of the anticancer drug temozolomide and angiogenic inhibitor sunitinib. It was found that the combined treatment was better than temozolomide alone and sunitinib alone in reducing the tumor size [30]. Further research in the field of 3D bioprinting will allow the creation of high-efficiency 3D in vitro cancer models to gain an innovative basic understanding about carcinogenesis mechanisms, as well as to more accurately screen potential anticancer drugs and assist individual drug selection [31]. On the other hand, a microfluidic tumor-microvascular model of human liver cancer was used to assess the anticancer effect of Metuzumab. Metuzumab was able to induce antibody-dependent cell-mediated cytotoxic effects on the liver cancer model in the presence of peripheral blood mononuclear cells [32]. In another study, a microfluidic tumor-microvascular model of human glioblastoma was used to evaluate the effect of antioxidants on glioblastoma [33]. It was found that antioxidant catechins were able to reduce reactive oxygen species in the tumor cells, which could decrease vascular endothelial growth factor secretion from the tumor cells to TME for inducing tumor angiogenesis.
2.1.2. Therapeutic Cells
In vitro tissue models are becoming increasingly important in regenerative medicine, and attempt to replace, restore or regenerate tissue. In the realm of cancer, this has lately taken the form of altering immune cells in vitro so that when they are re-implanted into the patient, they can better fight cancer. For example, chimeric antigen receptor (CAR) T cells were tested on a 3D hydrogel-based in vitro model of human ovarian cancer to assess the anticancer effect of the immune cells before introducing them into patients. CAR-T cells were better than unmodified T cells in mediating the cytotoxic effects on ovarian cancer [13]. Besides CAR-T cells, tumor-reactive T cells can be generated by the co-culture of peripheral blood lymphocytes and tumor spheroids. Peripheral blood lymphocytes co-cultured with human colorectal tumor spheroid and lung tumor spheroid were able to produce CD8+ T cells that kill the colorectal tumor cells and lung tumor cells, respectively (Figure 1) [34]. Immune cells co-cultured with tumor spheroids are a reliable model for assessing the effects of CAR-T and tumor-reactive T cell infusion on cancers, training T cells to identify tumor antigens and predicting patient response to immunotherapy. In general, 3D in vitro cancer models are an important preclinical tool for developing novel immunotherapy tactics for cancer treatment [35].
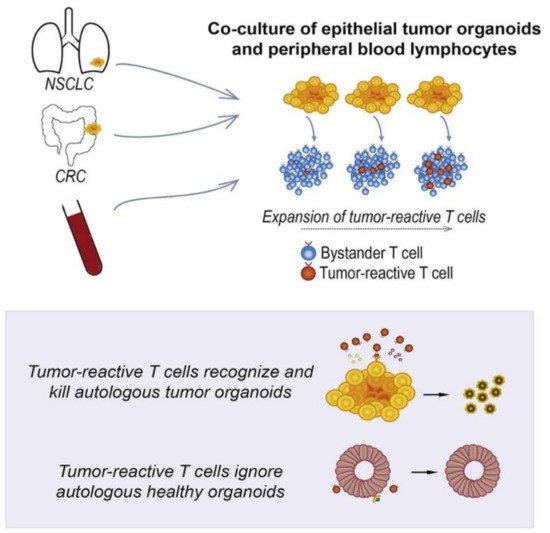
Figure 1. In vitro human cancer models for T cell therapy development. Adapted with permission from [34] © Elsevier (2018).
2.1.3. Phototherapy
PDT (photodynamic therapy) is a new theranostic treatment option for a variety of malignancies and illnesses. In the 4T1 cell line (a breast tumor cell line) that is extensively metastatic, Xiaobing et al. investigated the implications of Sinoporphyrin sodium-mediated PDT (DVDMS-PDT) on tumor cell proliferation and metastasis. DVDMS-PDT was cytotoxic to 4T1 cells and able to inhibit the migration of 4T1 cells [36]. Drug resistance is a significant obstacle to cancer therapy. The synergistic impact of drug and phototherapy on the bladder tumor cell line 5637 was examined, and the results showed that blue light irradiation enhances the cytotoxic effect of cisplatin on bladder tumor cells [37]. Analyzing the photosensitizer absorption and penetration through several cell layers has been aided by tumor spheroid culture. For example, the Beckman Laser Institute has employed a human glioma spheroid model to investigate the uptake and localization of 5-aminolevulinic acid/protoporphyrin IX-based regimens for PDT [38]. Xiao et al. investigated the uptake patterns of various porphyrin photosensitizers in a human bladder tumor spheroid model [39]. Hypocrellins and benzoporphyrin derivative monoacid ring A (BPD-MA) were found to penetrate the spheroid deeper than other photosensitizers, including aluminum phthalocyanine, photofrin and protoporphyrin IX. These results suggested that hypocrellins and BPD-MA could be used for bladder cancer phototherapy. In another study, a 3D ovarian tumor spheroid model was utilized to evaluate the phototoxicity of benzophenothiazinium dye EtNBS and its hydroxyl-terminated derivative (EtNBS-OH) [40]. EtNBS was effective in killing the tumor cells in the tumor core, while EtNBS-OH was able to mediate widespread structural degradation of tumors upon irradiation. These two photosensitizers could be used simultaneously for synergistic PDT. Moreover, photosensitizers can be conjugated with aptamers to achieve targeted PDT. For instance, pyropheophorbide, a conjugate with aptamer sgc8 selectively bound to cervical tumor spheroids that overexpressed protein tyrosine kinase 7 and generated singlet oxygen upon red laser irradiation to kill the tumor cells (Figure 2) [41].
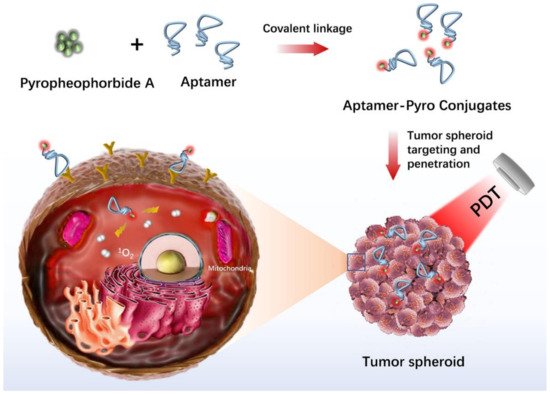
Figure 2. In vitro human cancer models for photodynamic therapy development. Adapted with permission from [41] © ACS Publications (2020).
2.2. Assessment of Tumor Cell Migration, Metastasis and Invasion
One of the most important factors for the progression of metastasis is the vascular system because metastasis of tumor cells mostly occurs within the vascular system. The vasculature of tumors is characterized as unorganized and leaky due to the new formation of vessels that supply nutrients to the tumor. Extravasation and metastasis of tumor cells can easily occur due to the leaky architecture of tumor vessels and secretions of endothelial cells (ECs). Studies in which ECs and tumor cells were co-cultured in both the microfluidic tumor-microvascular system and 3D bioprinted scaffold showed increased tumor cell migration [42][43].
In addition, studies with perivascular cells have shown that they can positively or negatively affect tumor growth and metastasis due to their capacity to stabilize blood vessel structure and permeability [44][45]. Cancer-associated fibroblasts (CAFs) are the main perivascular cells in TME, and their roles in TME are stimulating tumor cell proliferation and facilitating cancer progression by modifying ECM components and metastasis by modulating immune components [46]. In addition, CAFs initiate angiogenesis by supporting ECs that provide nutritional support for tumor growth and development [47]. Furthermore, CAFs positively affect the proliferation and metabolism of tumor cells via autophagy [48]. In a 3D hydrogel-based scaffold, human squamous cell carcinoma cells were unable to migrate independently, but they were shown to invade along CAFs within the matrix [48]. In another study, the requirement for direct cell contact from CAFs to induce tumor invasion was demonstrated in a 3D colon tumor spheroid model [49]. It has been revealed that different CAF signatures are associated with different survival rates of patients with ovarian cancer [50]. Overall, new insights into tumor biology and drug discovery can be gained by examining CAFs and tumor cell interactions in 3D in vitro cancer models.
Macrophages are the most abundant immune cells in TME that are derived from monocytes. In general, M1 phenotypes show anticancer properties, while the M2 secrete cytokines and growth factors for promoting inflammation [51]. Results of a study with macrophage/breast tumor spheroid models showed increased cytokines associated with the M2 phenotype, faster oxygen consumption and resistance to cytotoxic drugs [52]. Studying macrophages in 3D in vitro cancer models is essential to better understand the clinical implications of immunotherapeutics. Cancer stem cells (CSC) are a special subpopulation that maintains tumor growth with their self-renewal and differentiation capacity. It provides activation of signaling pathways involved in the cell cycle, growth factor secretion and stemness properties. CAFs were shown to enrich CSCs in lung tumor cells through the de-differentiation process, such as epithelial to mesenchymal transition (EMT) [53]. The EMT process has an important place in the acquisition and maintenance of stem cell-like properties and the invasive phenotype in tumor cells [54]. A study with a colorectal tumor/macrophage transwell model revealed that macrophages produce IL-6, inducing the EMT program to promote tumor invasion, migration and metastasis (Figure 3) [55]. In a 3D bioprinted cervical cancer model, TGF-β was found to induce EMT in HeLa cells [56].
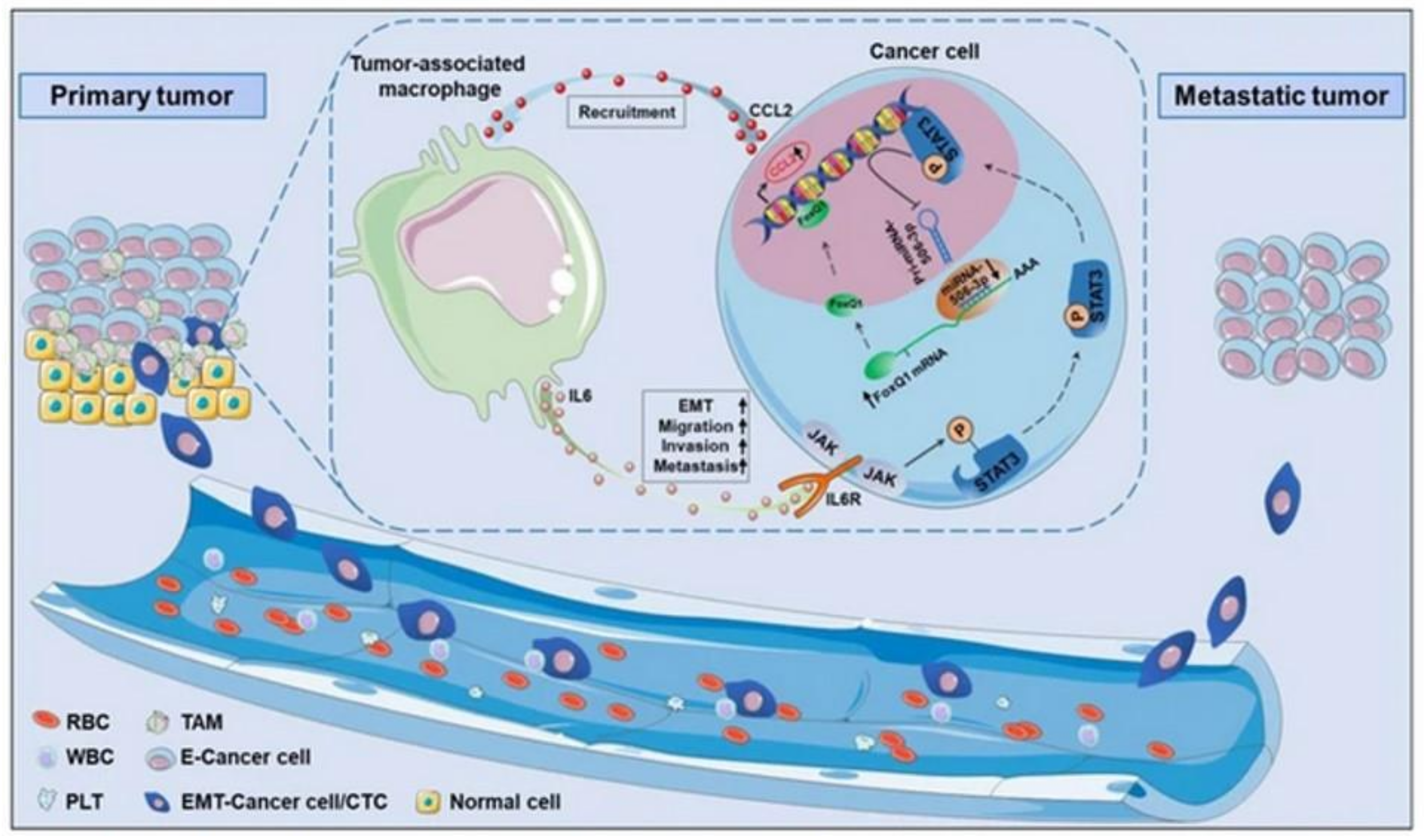
Figure 3. In vitro human cancer models for assessment of tumor biology. Adapted with permission from [55] © Creative Commons Attribution License (2019).
References
- Zhou, Y.; Abel, G.A.; Hamilton, W.; Pritchard-Jones, K.; Gross, C.P.; Walter, F.M.; Renzi, C.; Johnson, S.; McPhail, S.; Elliss-Brookes, L. Diagnosis of cancer as an emergency: A critical review of current evidence. Nat. Rev. Clin. Oncol. 2017, 14, 45–56.
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug--delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805.
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114.
- Ayuso, J.M.; Park, K.-Y.; Virumbrales-Muñoz, M.; Beebe, D.J. Toward improved in vitro models of human cancer. APL Bioeng. 2021, 5, 010902.
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998.
- Langer, E.M.; Allen-Petersen, B.L.; King, S.M.; Kendsersky, N.D.; Turnidge, M.A.; Kuziel, G.M.; Riggers, R.; Samatham, R.; Amery, T.S.; Jacques, S.L. Modeling tumor phenotypes in vitro with three-dimensional bioprinting. Cell Rep. 2019, 26, 608–623.
- Roudsari, L.C.; West, J.L. Studying the influence of angiogenesis in in vitro cancer model systems. Adv. Drug Deliv. Rev. 2016, 97, 250–259.
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13.
- Han, Y.L.; Pegoraro, A.F.; Li, H.; Li, K.; Yuan, Y.; Xu, G.; Gu, Z.; Sun, J.; Hao, Y.; Gupta, S.K. Cell swelling, softening and invasion in a three-dimensional breast cancer model. Nat. Phys. 2020, 16, 101–108.
- Gopal, S.; Kwon, S.-J.; Ku, B.; Lee, D.W.; Kim, J.; Dordick, J.S. 3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity. Commun. Biol. 2021, 4, 1–14.
- Dondajewska, E.; Juzwa, W.; Mackiewicz, A.; Dams-Kozlowska, H. Heterotypic breast cancer model based on a silk fibroin scaffold to study the tumor microenvironment. Oncotarget 2018, 9, 4935.
- Shi, Y.; Cai, Y.; Cao, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic technology and applications for anti-cancer drug screening. TrAC Trends Anal. Chem. 2021, 134, 116118.
- Ando, Y.; Siegler, E.L.; Ta, H.P.; Cinay, G.E.; Zhou, H.; Gorrell, K.A.; Au, H.; Jarvis, B.M.; Wang, P.; Shen, K. Evaluating CAR--T Cell Therapy in a Hypoxic 3D Tumor Model. Adv. Healthc. Mater. 2019, 8, 1900001.
- Louzada, S.; Adega, F.; Chaves, R. Defining the sister rat mammary tumor cell lines HH-16 cl. 2/1 and HH-16. cl. 4 as an in vitro cell model for Erbb2. PLoS ONE 2012, 7, e29923.
- Nakatsu, N.; Yoshida, Y.; Yamazaki, K.; Nakamura, T.; Dan, S.; Fukui, Y.; Yamori, T. Chemosensitivity profile of cancer cell lines and identification of genes determining chemosensitivity by an integrated bioinformatical approach using cDNA arrays. Mol. Cancer Ther. 2005, 4, 399–412.
- Hughes, L.; Malone, C.; Chumsri, S.; Burger, A.M.; McDonnell, S. Characterisation of breast cancer cell lines and establishment of a novel isogenic subclone to study migration, invasion and tumourigenicity. Clin. Exp. Metastasis 2008, 25, 549–557.
- Albini, A.; Benelli, R.; Noonan, D.M.; Brigati, C. The “chemoinvasion assay”: A tool to study tumor and endothelial cell invasion of basement membranes. Int. J. Dev. Biol. 2004, 48, 563–571.
- Hakozaki, M.; Hojo, H.; Sato, M.; Tajino, T.; Yamada, H.; Kikuchi, S.; Abe, M. Establishment and characterization of a new cell line, FPS-1, derived from human undifferentiated pleomorphic sarcoma, overexpressing epidermal growth factor receptor and cyclooxygenase-2. Anticancer Res. 2006, 26, 3393–3401.
- Fang, Y.; Elahi, A.; Denley, R.C.; Rao, P.H.; Brennan, M.F.; Jhanwar, S.C. Molecular characterization of permanent cell lines from primary, metastatic and recurrent malignant peripheral nerve sheath tumors (MPNST) with underlying neurofibromatosis-1. Anticancer Res. 2009, 29, 1255–1262.
- Ikediobi, O.N.; Davies, H.; Bignell, G.; Edkins, S.; Stevens, C.; O’Meara, S.; Santarius, T.; Avis, T.; Barthorpe, S.; Brackenbury, L. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol. Cancer Ther. 2006, 5, 2606–2612.
- Ferreira, D.; Adega, F.; Chaves, R. The Importance of Cancer Cell Lines as in Vitro Models in Cancer Methylome Analysis and Anticancer Drugs Testing. In Oncogenomics and Cancer Proteomics—Novel Approaches in Biomarkers Discovery and Therapeutic Targets in Cancer; Lopez-Camarillo, C., Ed.; InTech: London, UK, 2013; pp. 139–166.
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In vitro tumor models: Advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016, 4, 12.
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324.
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218.
- Uchida, Y.; Tanaka, S.; Aihara, A.; Adikrisna, R.; Yoshitake, K.; Matsumura, S.; Mitsunori, Y.; Murakata, A.; Noguchi, N.; Irie, T. Analogy between sphere forming ability and stemness of human hepatoma cells. Oncol. Rep. 2010, 24, 1147–1151.
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T. Comparison of 2D-and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843.
- Zoetemelk, M.; Rausch, M.; Colin, D.J.; Dormond, O.; Nowak-Sliwinska, P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci. Rep. 2019, 9, 1–14.
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204.
- Wang, Y.; Shi, W.; Kuss, M.; Mirza, S.; Qi, D.; Krasnoslobodtsev, A.; Zeng, J.; Band, H.; Band, V.; Duan, B. 3D bioprinting of breast cancer models for drug resistance study. ACS Biomater. Sci. Eng. 2018, 4, 4401–4411.
- Han, S.; Kim, S.; Chen, Z.; Shin, H.K.; Lee, S.-Y.; Moon, H.E.; Paek, S.H.; Park, S. 3D bioprinted vascularized tumour for drug testing. Int. J. Mol. Sci. 2020, 21, 2993.
- Knowlton, S.; Onal, S.; Yu, C.H.; Zhao, J.J.; Tasoglu, S. Bioprinting for cancer research. Trends Biotechnol. 2015, 33, 504–513.
- Li, Y.; Zhang, T.; Pang, Y.; Li, L.; Chen, Z.-N.; Sun, W. 3D bioprinting of hepatoma cells and application with microfluidics for pharmacodynamic test of Metuzumab. Biofabrication 2019, 11, 034102.
- Liu, H.; Jie, M.; He, Z.; Li, H.-F.; Lin, J.-M. Study of antioxidant effects on malignant glioma cells by constructing a tumor-microvascular structure on microchip. Anal. Chim. Acta 2017, 978, 1–9.
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018, 174, 1586–1598.
- Boucherit, N.; Gorvel, L.; Olive, D. 3D tumor models and their use for the testing of immunotherapies. Front. Immunol. 2020, 11, 603640.
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics 2015, 5, 772.
- Shakibaie, M.; Vaezjalali, M.; Rafii-Tabar, H.; Sasanpour, P. Synergistic effect of phototherapy and chemotherapy on bladder cancer cells. J. Photochem. Photobiol. B 2019, 193, 148–154.
- Madsen, S.J.; Sun, C.H.; Tromberg, B.J.; Wallace, V.P.; Hirschberg, H. Photodynamic Therapy of Human Glioma Spheroids Using 5--Aminolevulinic Acid. Photochem. Photobiol. 2000, 72, 128–134.
- Xiao, Z.; Hansen, C.B.; Allen, T.M.; Miller, G.G.; Moore, R.B. Distribution of photosensitizers in bladder cancer spheroids: Implications for intravesical instillation of photosensitizers for photodynamic therapy of bladder cancer. J. Pharm. Sci. 2005, 8, 536–543.
- Klein, O.J.; Bhayana, B.; Park, Y.J.; Evans, C.L. In vitro optimization of EtNBS-PDT against hypoxic tumor environments with a tiered, high-content, 3D model optical screening platform. Mol. Pharm. 2012, 9, 3171–3182.
- Xiong, H.; Yan, J.; Cai, S.; He, Q.; Wen, N.; Wang, Y.; Hu, Y.; Peng, D.; Liu, Y.; Liu, Z. Aptamer–pyropheophorbide a conjugates with tumor spheroid targeting and penetration abilities for photodynamic therapy. Mol. Pharm. 2020, 17, 2882–2890.
- Truong, D.; Fiorelli, R.; Barrientos, E.S.; Melendez, E.L.; Sanai, N.; Mehta, S.; Nikkhah, M. A three-dimensional (3D) organotypic microfluidic model for glioma stem cells–Vascular interactions. Biomaterials 2019, 198, 63–77.
- Meng, F.; Meyer, C.M.; Joung, D.; Vallera, D.A.; McAlpine, M.C.; Panoskaltsis--Mortari, A. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv. Mater. 2019, 31, 1806899.
- Mezheyeuski, A.; Lindh, M.B.; Guren, T.K.; Dragomir, A.; Pfeiffer, P.; Kure, E.H.; Ikdahl, T.; Skovlund, E.; Corvigno, S.; Strell, C. Survival-associated heterogeneity of marker-defined perivascular cells in colorectal cancer. Oncotarget 2016, 7, 41948.
- Cooke, V.G.; LeBleu, V.S.; Keskin, D.; Khan, Z.; O’Connell, J.T.; Teng, Y.; Duncan, M.B.; Xie, L.; Maeda, G.; Vong, S. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 2012, 21, 66–81.
- Kronemberger, G.S.; Miranda, G.A.; Tavares, R.S.; Montenegro, B.; Kopke, Ú.d.A.; Baptista, L.S. Recapitulating Tumorigenesis in vitro: Opportunities and Challenges of 3D Bioprinting. Front. Bioeng. Biotechnol. 2021, 9, 423.
- Zhou, W.; Xu, G.; Wang, Y.; Xu, Z.; Liu, X.; Xu, X.; Ren, G.; Tian, K. Oxidative stress induced autophagy in cancer associated fibroblast enhances proliferation and metabolism of colorectal cancer cells. Cell Cycle 2017, 16, 73–81.
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400.
- Attieh, Y.; Clark, A.G.; Grass, C.; Richon, S.; Pocard, M.; Mariani, P.; Elkhatib, N.; Betz, T.; Gurchenkov, B.; Vignjevic, D.M. Cancer-associated fibroblasts lead tumor invasion through integrin-β3–dependent fibronectin assembly. J. Cell Biol. 2017, 216, 3509–3520.
- Yeung, T.-L.; Sheng, J.; Leung, C.S.; Li, F.; Kim, J.; Ho, S.Y.; Matzuk, M.M.; Lu, K.H.; Wong, S.T.; Mok, S.C. Systematic identification of druggable epithelial–stromal crosstalk signaling networks in ovarian cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 272–282.
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 123–147.
- Tevis, K.M.; Cecchi, R.J.; Colson, Y.L.; Grinstaff, M.W. Mimicking the tumor microenvironment to regulate macrophage phenotype and assessing chemotherapeutic efficacy in embedded cancer cell/macrophage spheroid models. Acta Biomater. 2017, 50, 271–279.
- Chen, W.-J.; Ho, C.-C.; Chang, Y.-L.; Chen, H.-Y.; Lin, C.-A.; Ling, T.-Y.; Yu, S.-L.; Yuan, S.-S.; Louisa Chen, Y.-J.; Lin, C.-Y. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 2014, 5, 3472.
- Findlay, V.J.; Wang, C.; Watson, D.K.; Camp, E.R. Epithelial-to-mesenchymal transition and the cancer stem cell phenotype: Insights from cancer biology with therapeutic implications for colorectal cancer. Cancer Gene Ther. 2014, 21, 181–187.
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64.
- Pang, Y.; Mao, S.; Yao, R.; He, J.; Zhou, Z.; Feng, L.; Zhang, K.; Cheng, S.; Sun, W. TGF-β induced epithelial–mesenchymal transition in an advanced cervical tumor model by 3D printing. Biofabrication 2018, 10, 044102.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
984
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
19 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




