Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pierandrea Mirino | -- | 2861 | 2022-05-17 16:13:34 | | | |
| 2 | Conner Chen | Meta information modification | 2861 | 2022-05-18 04:21:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mirino, P.; Pecchinenda, A.; Boccia, M.; , .; Guariglia, C. The Role of the Cerebellum in Spatial Navigation. Encyclopedia. Available online: https://encyclopedia.pub/entry/23018 (accessed on 01 March 2026).
Mirino P, Pecchinenda A, Boccia M, , Guariglia C. The Role of the Cerebellum in Spatial Navigation. Encyclopedia. Available at: https://encyclopedia.pub/entry/23018. Accessed March 01, 2026.
Mirino, Pierandrea, Anna Pecchinenda, Maddalena Boccia, , Cecilia Guariglia. "The Role of the Cerebellum in Spatial Navigation" Encyclopedia, https://encyclopedia.pub/entry/23018 (accessed March 01, 2026).
Mirino, P., Pecchinenda, A., Boccia, M., , ., & Guariglia, C. (2022, May 17). The Role of the Cerebellum in Spatial Navigation. In Encyclopedia. https://encyclopedia.pub/entry/23018
Mirino, Pierandrea, et al. "The Role of the Cerebellum in Spatial Navigation." Encyclopedia. Web. 17 May, 2022.
Copy Citation
Although the function of the cerebellum has typically been associated with motor functions, several recent studies point to the cerebellum being involved in various cognitive functions, including spatial navigation. More specifically, clinical and neuroimaging evidence suggests a functional and anatomical distinction between sensorimotor and cognitive cerebellum. The latter includes lobule VI, Crus I and II and lobule VIIB of the posterior lobe, which have been linked to different aspects of executive functions.
spatial navigation
cerebellum
inferior olivary nucleus
1. Visuospatial Skills and Spatial Navigation
Spatial navigation represents the individuals’ ability to acquire new environmental knowledge, develop cognitive maps, and determine the correct path to reach a goal using multiple cues, landmarks and beacons [1]. To perform accurate spatial navigation, individuals must identify their current position and destination in the environment and trace the best path between the starting position and the destination. These activities involve higher cognitive processes, including planning and problem solving [2], which are based on perceiving, integrating and interpreting the multisensory environment signals (the so-called allothetic information, including optic flow, visual and auditory inputs) as well as signals from individual movements (the so-called idiothetic information, including motor efferent copy, proprioceptive and vestibular inputs) to generate, update and transform cognitive maps of the environment [3]. Namely, successful navigation relies on the integration of allothethic and idiotehetic cues. Therefore, visuo-spatial skills represent a set of abilities involved in visual and non-verbal reasoning, allowing to identify and estimate the observer’s spatial relations with environmental objects or between different objects located in the surrounding environment. Processing spatial relationships and mental imagery, especially mental rotation, are also essential to correctly navigate the environment [4]. Moreover, spatial navigation strongly depends on the ability to perceive, act and operate on environmental representations as a function of spatial coordinates [5]. In fact, environments can be represented in two different formats: egocentric and allocentric coordinate frameworks (Figure 1). In the egocentric coordinate framework, the position of each environmental object is coded based on the observer’s position (i.e., on the right, in front, etc.). The egocentric representation requires that idiothetic and allothetic information is dynamically updated during spatial navigation. In contrast, in the allocentric coordinate framework, each environmental object is related to the position of the spatial cues (i.e., cardinal points), static objects or landmarks located in the environment (for example with respect to the sea) [6][7] to get a spatial representation of the environment independent from the observer’s point of view.
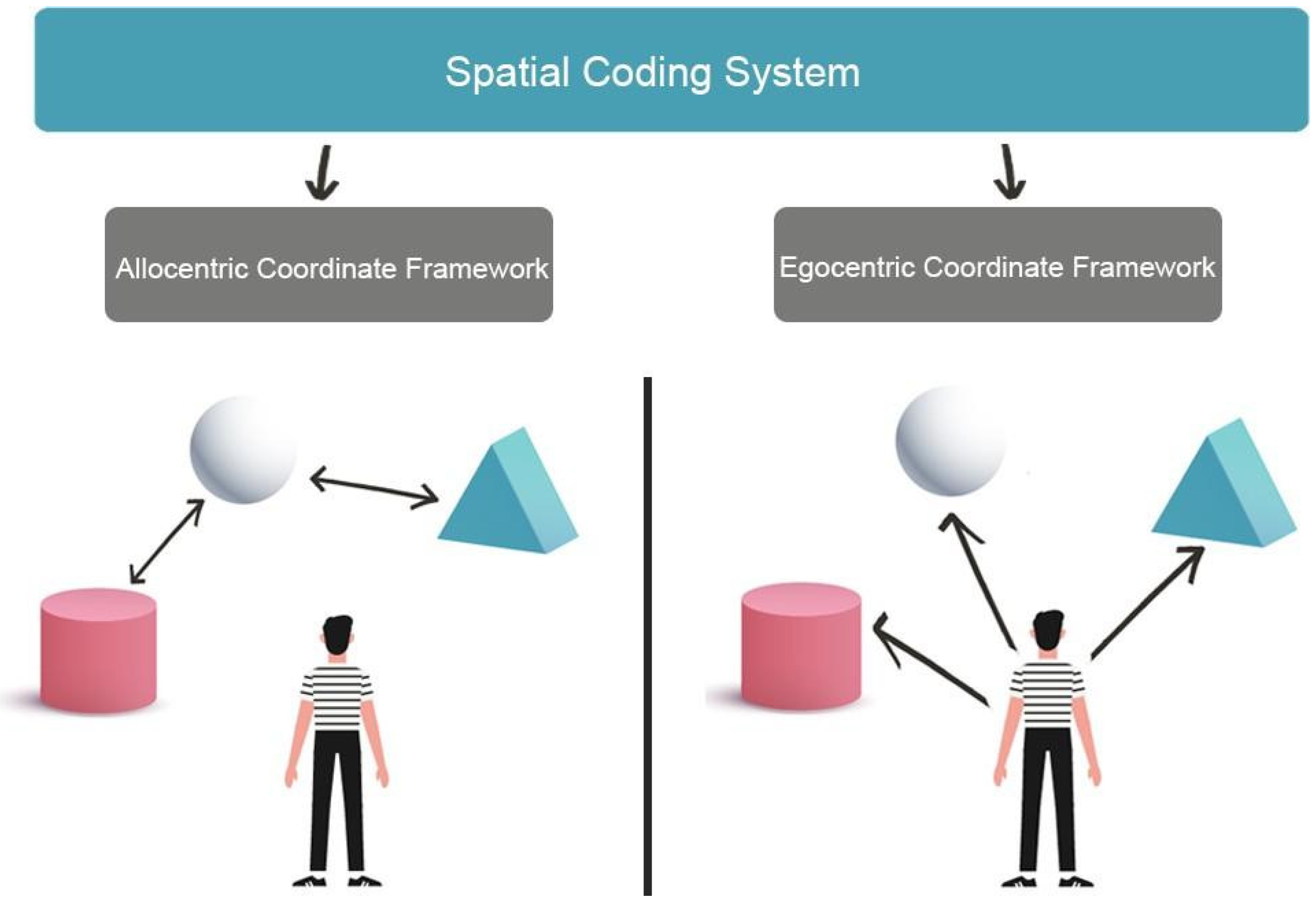
Figure 1. The image shows two different formats of environments representation. Allocentric Coordinate Framework encoding spatial information based on the navigator’s perception of relative landmark positions (left). The Egocentric Coordinate Framework bases spatial representations from the point of view of the navigator (right).
There are several models of spatial navigation that acknowledge the role of visuo-spatial skills and specify the neural circuits involved. For instance, Byrne et al. (2007) [8], in the BBB model–so called after the three authors initials–propose that in everyday life almost all navigation tasks require a translation from the egocentric to the allocentric representation and vice versa. This suggests that most navigational tasks likely involve a mix of the two forms of representations. According to the BBB model, the neural bases for egocentric representations lay in the parietal cortex, where information of the individual’s current viewpoint based on current position and bearing is represented [8]. In contrast, allocentric representations rely on the medial temporal lobe (MTL) and involve hippocampal place cells (neurons that code specific spatial locations), providing some degree of metric knowledge about locations in the environment [9]. Finally, the retrosplenial cortex performs translation between egocentric and allocentric coordinate frames and allows aligning the current “map” in the medial temporal lobe with the current viewpoint in the parietal cortex [8].
Cognitive models also acknowledge the role of visuo-spatial skills in navigation. For instance, Siegel and White (1975) [10] propose a hierarchical developmental model of human navigation, based on three different navigational processes: (a) Landmark navigation, which refers to a process that allows individuals to reach an environmental goal by navigating toward a visible landmark in the environment; (b) Route navigation, which allows individuals to reach their goal based on the egocentric representations of the sequence of landmarks in the environment and the directions that link a landmark to the following one; (c) Survey navigation relies on the development of an allocentric cognitive map and it is characterised by configurational-gestalt elements. It gives something more than a minimal map and provides an advantage in way-finding and in organising the navigational experience [10]. A similar model was proposed by Montello [11] who suggest that route and survey representations are alternatively used according to the navigational task request. Similarly, Wang and Sperke [12], argued that three cognitive systems underlie spatial navigation in mammals: (1) the path integration system that, based on the processing of idiothetic information on movements in the environment, operates a dynamic update of the individual position, maintaining a vector between the starting position and the actual position; (2) the view-dependent recognition system involved in recognizing places, by comparing current perceptions of the surrounding environment with the images of the same place in the same perspective stored in the long-term memory; (3) the reorientation system that, allows the individual to reorient in the environment by means of a cognitive map [12][13]. In humans, other processes are also involved such as the use of language and/or of paper maps [12]. Finally, Wolbers and Hegarty [7], focus on three interdependent domains related to navigation skills: cognitive and perceptual factors, neural information processing, and variability in brain microstructure [7]. Their model predicts that even if these levels may be independent, when navigating in most natural environments, the relevant features are not detected from a single vantage point and it is essential to keep track of the individual’s position and orientation to integrate all relevant features in a complete environment representation. To sum up, existing models converge in proposing that spatial navigation relies on basic perceptual and memory processes as well as on complex multisensory processes, hence it involves wide neural networks, in which information must be integrated and manipulated in space and time [14][15]. However, none of these models points to the contribution of the cerebellum.
2. The Role of the Cerebellum in Spatial Navigation
Although the function of the cerebellum has typically been associated with motor functions, several recent studies point to the cerebellum being involved in various cognitive functions [16], including spatial navigation. More specifically, clinical and neuroimaging evidence [17][18] suggests a functional and anatomical distinction between sensorimotor and cognitive cerebellum. The latter includes lobule VI, Crus I and II and lobule VIIB of the posterior lobe, which have been linked to different aspects of executive functions [19].
Already in 2013, Rochefort [20] reviewing the available evidence, underlined how many studies point to the role of the cerebellum in environmental navigation, especially when considering the existing connections to the hippocampal formation. They posit that the cerebellum contributes to spatial navigation at two levels: Firstly, in processing information on autonomous movements, to build a spatial representation in the hippocampus at the level of the place cells. Secondly, in the use of this spatial representation to perform an optimal trajectory towards a goal destination. Hence they propose that the cerebellum takes part in the navigation system by shaping the firing of the place cells in the hippocampus [21]. This contribution could occur either through direct projections to the hippocampus or via multi-synaptic connections involving a thalamic relay to the posterior parietal cortex or to the retrosplenial cortex.
An important contribution also comes from a recent study [22] showing that the cerebellum contributes to visuospatial working memory and decision-making, by optimizing the task-related modulation of mPFC-hippocampus (dCA1) gamma coherence. Findings about neural recording during both cognitive and sensorimotor processing strongly suggest that the cerebellum has a pivotal role in coordinating communication within network modulating the frequency coherence of cerebral cortical areas. Such a role is consistent with the long recognized role of the cerebellum in timing and temporal coordination [22]. Taken together, this evidence suggests that the cerebellum assumes a modulatory role in each process in which it is involved, being it motor or cognitive processes. Indeed, this proposal together with the cortico-nuclear micro-complex architecture of the cerebellum [23] inspired the universal cerebellar transform (UTC), a model describing the cerebellum activity as an unique, universal type of computation [24]. In other words, according to these models, all the cerebellar areas work in similar ways, by providing the same type of input and playing the same role in all the networks in which the cerebellum is involved. In the UTC model, the cerebellum integrates internal representations with external stimuli, modulating the different information flows that underlie a wide range of functional domains, optimizing individual’s performance based on context [25].
Importantly, the model predicts that lesions in different cerebellum areas should have similar effect on different domains depending on the cerebrellum-brain network affected by the lesion (Universal Cerebellar Impairment-UCI) [25][26]. Indeed, there is some evidence that this is the case as in humans, cerebellar lesions can result in different cognitive deficits [27]. This led some authors to suggest that alterations in sequential processing is the common denominator of the cerebellum contribution to cognitive, affective and behavioral deficits [28]. However, animal studies show that cerebellectomized rats exhibit different exploration patterns of a novel environment, suggesting that cerebellum is involved in processing spatial information and elaborating motor strategies during exploration [29]. Specifically, the growing evidence on the involvement of the cerebellum in navigation has raised the question of the potential roles of the two major cerebellar inputs, the olivo-cerebellar input (climbing fiber; CF) and the pontine nuclei-granule cells-parallel fibers input (mossy fiber; MF). Indeed, lesion studies provide some evidence that cerebellar dysfunctions may depend, at least in part, on alterations of the olivo-cerebellar inputs. For instance, Rondi-Reig [30] tested rats with lesion of CF and/or MF of the cerebellum in either the cued or the place protocol of the water maze. Rats with CF lesions and partial or total MF lesions presented a deficit in the latency to find a hidden platform in the water maze but were still able to find it when visual cues were available.
Cerebellum as Part of a Complex Network
The neural system underlying visuospatial abilities and spatial navigation includes the posterior parietal cortex (PPC) as a central area that is part of a complex, wide network of different brain areas (Figure 2). The posterior parietal cortex receives visual, auditory, and vestibular information and integrates different sources of information in a complex representation of the environment. Three different neural circuits originate from different posterior parietal cortex areas [31].
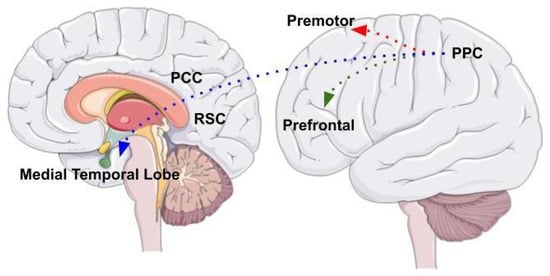
Figure 2. The image shows the three different neural circuits originating from PPC areas. The parieto-prefrontal pathway, the parieto-premotor pathway, and the parieto-medial temporal pathway. The figure was drawn based from Kravitz et al., (2021).
One is the parieto-prefrontal pathway, which involves lateral intraparietal area (LIP), ventral intraparietal area (VIP), medial temporal area (MT) and medial superior temporal area (MST) that project to the prefrontal cortex. This pathway contributes to the visual processing of information and spatial working memory [32]. The other is the parieto-premotor pathway, which includes two different parallel projections that connect the PPC to the premotor areas. The first projection connects the parietal reach region (PRR) to the dorsal premotor cortex whereas the second projection connects VIP with the ventral motor cortex. Overall, the parieto-premotor pathway mediates eye moments, reaching, grasping and other forms of visually guided actions [31]. Finally, the parieto-medial temporal pathway connects the caudal region of the inferior parietal lobe (cIPL) to several areas located in the medial temporal lobe (MTL). This pathway is involved in different spatial functions related to spatial orientation [31]. The cIPL processes the egocentric information and sends efferences to different MTL areas: the posterior cingulate cortex (PCC), that participates in directing and shifting attention; the retrosplenial cortex that is involved in memory, imagery and planning processes and translating representations from the egocentric to the allocentric format and vice versa; the parahippocampal gyrus, that contributes to the allocentric representation of the environment; the hippocampus that provides a crucial contribution in spatial navigation but whose role has not yet been fully clarified [32].
The neural architecture involving connections to the posterior parietal cortex is acknowledged by the BBB model [8], according to which the posterior parietal cortex underlies processing the spatial visual information and represents the environment in an egocentric coordinate framework. Subsequently, the MTL areas receive the egocentric representation through the parieto-medial temporal pathway and integrate it with the allocentric representation elaborated by the parahippocampal gyrus. Finally, the retrosplenial cortex converts egocentric in allocentric representations and vice versa, in a bidirectional way, while the hippocampus allows for the long-term storage of these representations [8]. However, this architecture does not account for evidence indicating that deficits in navigation may result from cerebellar alterations, which affect brain areas to which the cerebellum is connected, therefore mimicking the impairments generated by direct alterations of frontal and parietal areas.
More specifically, studies using viral tracers in nonhuman primates show a di-synaptic projection from the dentate nucleus (DN) towards the prefrontal cortex, passing through the median-dorsal and ventrolateral nuclei of the thalamus [20]. This suggests the presence of a circuit connecting the lateral part of the cerebellar hemispheres and the dentate nucleus with the dorsal region of the prefrontal cortex [33]. Animal studies also suggest that in mice this thalamic projection supports the prefrontal activity during the maintenance of spatial working memory [34]. These prefrontal areas are involved in executive functions, such as strategic behavior, planning, abstraction, cognitive flexibility, and working memory, all involved in spatial navigation. On the other hand, the prefrontal cortex areas send rich projections to the pontine nuclei, from which the mossy fibers (MF) originate. Importantly, these projections constitute one of the two main inputs of the lateral cerebellar hemispheres [33][35][36][37]. Bidirectional connections between the cerebral cortex and the cerebellum form the cerebro-cerebellar circuit, which represents the anatomical substrate for the cerebellar influence on higher cognitive functions [38]. In addition, MRI studies show robust functional connectivity between the dentate nucleus–the major cerebellar output nucleus–and several cortical and subcortical cerebral areas involved in spatial navigation, including the parahippocampal gyrus and the hippocampus, but also parietal, frontal areas, thalamus and insular cortex [39].
Clinical studies also provide some converging evidence. For example, Tedesco et al. [40] tested navigational working memory in 12 cerebellar patients and 12 healthy age-matched participants using 2 comparable navigational tests (Walking Corsi Test and the Magic Carpet) [41][42]. The Walking Corsi Test (i.e., WalCT) [3][43] includes nine black tiles (30 cm × 30 cm) that are placed on a light gray carpet (2.50 m × 3 m). At the edge of the carpet, a 10th tile shows the participant’s position. The examiner presents sequences of increasing length by walking on the carpet and stopping on each tile for 2 s. After the presentation of each sequence, the participant is required to walk on the carpet and repeat the sequence. The Magic Carpet (i.e., E-WalCT) [42][44], has the same dimensions as the WalCT (2.50 m × 3 m), with nine white tiles (30 cm × 30 cm) placed on the carpet in the same array. Each tile is 10-mm thick with a luminous white surface of 75 mm × 95 mm on top and 6 pressure sensors that are regularly spaced under the surface. As in the WalCT, at the edge of the carpet, a tenth tile shows the participant’s position. The tiles are connected to an electronic device that turns the luminous surface on or off and detects the sensors activated by walking on the tiles. Sequences of increasing length of lighting tiles are presented. At the end of each sequence, all the lights go out and an acoustic warning is the participant’s signal to reproduce the observed sequence by walking on the E-WalCT. Patients with cerebellar lesions performed significantly worse than control participants only at the E-WaLCT. This finding has been attributed to the fact that the E-WalCT involves a higher cognitive load to detect and order single, independent stimuli as a sequence and that this type of processing is highly dependent on the contribution of cerebellum. In fact, Perrochon [42] suggest that spatial mapping requires a translation from stimulus space onto response space and this translation entails higher processing costs in the E-WalCT than in the WalCT. Accordingly, impaired cerebellar function affects stimulus-response associations in working memory, preventing sequence elaboration. It should be noted that this account is in keep with the theory proposed by Spencer [45], according to which the cerebellum works with cortical regions “to sustain representations of the stimulus-response mappings, a form of action-based working memory” (pp. 1302).
To sum up, that the cerebellum plays an important role in many cognitive, sensorimotor, and behavioral functions is well accepted. In contrast, the role of the cerebellum in human navigation has been less explored despite evidence from animals’ studies [30][46] consistently showing its key role in developing, storing, and retrieving cognitive maps of the environment. For instance, data suggest that it plays an important role in allowing to put in the correct sequence a set of spatial position [40] but it is still unclear the type of inputs that allows the cerebellum to play its role in human navigation. Given the functional anatomy of cerebellum, the input from the inferior olivar complex seems a possible candidate as it plays an important role in modulating the cerebellar activity and it could be involved in coding temporal/sequential features of different types of stimuli [47].
References
- Brodbeck, D.R.; Tanninen, S.E. Place Learning and Spatial Navigation. In Encyclopedia of the Sciences of Learning; Springer: Boston, MA, USA, 2012; pp. 2639–2641.
- Bocchi, A.; Carrieri, M.; Lancia, S.; Quaresima, V.; Piccardi, L. The Key of the Maze: The Role of Mental Imagery and Cognitive Flexibility in Navigational Planning. Neurosci. Lett. 2017, 651, 146–150.
- Piccardi, L.; Iaria, G.; Ricci, M.; Bianchini, F.; Zompanti, L.; Guariglia, C. Walking in the Corsi Test: Which Type of Memory Do You Need? Neurosci. Lett. 2008, 432, 127–131.
- Guariglia, C.; Palermo, L.; Piccardi, L.; Iaria, G.; Incoccia, C. Neglecting the Left Side of a City Square but Not the Left Side of Its Clock: Prevalence and Characteristics of Representational Neglect. PLoS ONE 2013, 8, e67390.
- Dickerson, B.C.; Alireza, A. Dementia: Comprehensive Principles and Practice; Oxford University Press: Oxford, UK, 2014.
- Galati, G.; Lobel, E.; Vallar, G.; Berthoz, A.; Pizzamiglio, L.; Bihan, D. Le The Neural Basis of Egocentric and Allocentric Coding of Space in Humans: A Functional Magnetic Resonance Study. Exp. Brain Res. 2000, 133, 156–164.
- Wolbers, T.; Hegarty, M. What Determines Our Navigational Abilities? Trends Cogn. Sci. 2010, 14, 138–146.
- Byrne, P.; Becker, S.; Burgess, N. Remembering the Past and Imagining the Future: A Neural Model of Spatial Memory and Imagery. Psychol. Rev. 2007, 114, 340–375.
- O’Keefe, J.; Dostrovsky, J. The Hippocampus as a Spatial Map. Preliminary Evidence from Unit Activity in the Freely-Moving Rat. Brain Res. 1971, 34, 171–175.
- Siegel, A.W.; White, S.H. The Development of Spatial Representations of Large-Scale Environments. Adv. Child Dev. Behav. 1975, 10, 9–55.
- Montello, D.R. Navigation. In International Encyclopedia of the Social & Behavioral Sciences; Egenhofer, M.J., Golledge, R.G., Eds.; Pergamon: Oxford, UK, 1998; pp. 143–154.
- Wang, F.; Spelke, E. Human Spatial Representation: Insights from Animals. Trends Cogn. Sci. 2002, 6613, 376–382.
- Piccardi, L.; Bianchini, F.; Zompanti, L.; Guariglia, C. Pure Representational Neglect and Navigational Deficits in a Case with Preserved Visuo-Spatial Working Memory. Neurocase 2008, 14, 329–342.
- Teghil, A.; Bonavita, A.; Guariglia, C.; Boccia, M. Commonalities and Specificities between Environmental Navigation and Autobiographical Memory: A Synthesis and a Theoretical Perspective. Neurosci. Biobehav. Rev. 2021, 127, 928–945.
- Eichenbaum, H.; Sauvage, M.; Fortin, N.; Komorowski, R.; Lipton, P. Towards a Functional Organization of Episodic Memory in the Medial Temporal Lobe. Neurosci. Biobehav. Rev. 2012, 36, 1597–1608.
- Molinari, M.; Leggio, M.G. Cerebellar Information Processing and Visuospatial Functions. Cerebellum 2007, 6, 214–220.
- Klein, A.P.; Ulmer, J.L.; Quinet, S.A.; Mathews, V.; Mark, L.P. Nonmotor Functions of the Cerebellum: An Introduction. Am. J. Neuroradiol. 2016, 37, 1005–1009.
- Boccia, M.; Nemmi, F.; Guariglia, C. Neuropsychology of Environmental Navigation in Humans: Review and Meta-Analysis of FMRI Studies in Healthy Participants. Neuropsychol. Rev. 2014, 24, 236–251.
- Stoodley, C.J.; Schmahmann, J.D. Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage 2009, 44, 489–501.
- Rochefort, C.; Lefort, J.; Rondi-Reig, L. The Cerebellum: A New Key Structure in the Navigation System. Front. Neural Circuits 2013, 7, 35.
- Rochefort, C.; Arabo, A.; André, M.; Poucet, B.; Save, E.; Rondi-Reig, L. Cerebellum Shapes Hippocampal Spatial Code. Science 2011, 334, 385–389.
- Liu, Y.; McAfee, S.S.; Van Der Heijden, M.E.; Dhamala, M.; Sillitoe, R.V.; Heck, D.H. Causal Evidence for a Role of Cerebellar Lobulus Simplex in Prefrontal-Hippocampal Interaction in Spatial Working Memory Decision-Making. Cerebellum, 2022; in press.
- Itō, M. The Cerebellum and Neural Control; Raven Press: New York, NY, USA, 1984; ISBN 978-0890041062.
- Schmahmann, J.D. The Role of the Cerebellum in Affect and Psychosis. J. Neurolinguistics 2000, 13, 189–214.
- Schmahmann, J.D. The Role of the Cerebellum in Cognition and Emotion: Personal Reflections since 1982 on the Dysmetria of Thought Hypothesis, and Its Historical Evolution from Theory to Therapy. Neuropsychol. Rev. 2010, 20, 236–260.
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum 2014, 13, 151–177.
- Baillieux, H.; De Smet, H.J.; Dobbeleir, A.; Paquier, P.F.; De Deyn, P.P.; Mariën, P. Cognitive and Affective Disturbances Following Focal Cerebellar Damage in Adults: A Neuropsychological and SPECT Study. Cortex 2010, 46, 869–879.
- Lupo, M.; Ferlazzo, F.; Aloise, F.; Di Nocera, F.; Tedesco, A.M.; Cardillo, C.; Leggio, M. New Protocol for Dissociating Visuospatial Working Memory Ability in Reaching Space and in Navigational Space. Behav. Res. Methods 2018, 50, 1602–1613.
- Dahhaoui, M.; Lannou, J.; Stelz, T.; Caston, J.; Guastavinot, J.M. Role of the Cerebellum in Spatial Orientation in the Rat. Behav. Neural Biol. 1992, 58, 180–189.
- Rondi-Reig, L.; Le Marec, N.; Caston, J.; Mariani, J. The Role of Climbing and Parallel Fibers Inputs to Cerebellar Cortex in Navigation. Behav. Brain Res. 2002, 132, 11–18.
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Mishkin, M. A New Neural Framework for Visuospatial Processing. Nat. Rev. Neurosci. 2011, 12, 217–230.
- Boccia, M.; Sulpizio, V.; Nemmi, F.; Guariglia, C.; Galati, G. Direct and Indirect Parieto-Medial Temporal Pathways for Spatial Navigation in Humans: Evidence from Resting-State Functional Connectivity. Brain Struct. Funct. 2017, 222, 1945–1957.
- Middleton, F.A.; Strick, P.L. Cerebellar Projections to the Prefrontal Cortex of the Primate. J. Neurosci. 2001, 21, 700–712.
- Bolkan, S.S.; Stujenske, J.M.; Parnaudeau, S.; Spellman, T.J.; Rauffenbart, C.; Abbas, A.I.; Harris, A.Z.; Gordon, J.A.; Kellendonk, C. Thalamic Projections Sustain Prefrontal Activity during Working Memory Maintenance. Nat. Neurosci. 2017, 20, 987–996.
- Schmahmann, J.D.; Pandya, D.N. Prefrontal Cortex Projections to the Basilar Pons in Rhesus Monkey: Implications for the Cerebellar Contribution to Higher Function. Neurosci. Lett. 1995, 199, 175–178.
- Schmahmann, J.D.; Pandya, D.N. Anatomic Organization of the Basilar Pontine Projections from Prefrontal Cortices in Rhesus Monkey. J. Neurosci. 1997, 17, 438.
- Palesi, F.; De Rinaldis, A.; Castellazzi, G.; Calamante, F.; Muhlert, N.; Chard, D.; Tournier, J.D.; Magenes, G.; D’Angelo, E.; Wheeler-Kingshott, C.A.M.G. Contralateral Cortico-Ponto-Cerebellar Pathways Reconstruction in Humans in Vivo: Implications for Reciprocal Cerebro-Cerebellar Structural Connectivity in Motor and Non-Motor Areas. Sci. Rep. 2017, 7, 12841.
- Schmahmann, J.D. From Movement to Thought: Anatomic Substrates of the Cerebellar Contribution to Cognitive Processing. Hum. Brain Mapp. 1996, 4, 174–198.
- Allen, G.; McColl, R.; Barnard, H.; Ringe, W.K.; Fleckenstein, J.; Cullum, C.M. Magnetic Resonance Imaging of Cerebellar-Prefrontal and Cerebellar-Parietal Functional Connectivity. Neuroimage 2005, 28, 39–48.
- Tedesco, A.M.; Bianchini, F.; Piccardi, L.; Clausi, S.; Berthoz, A.; Molinari, M.; Guariglia, C.; Leggio, M. Does the Cerebellum Contribute to Human Navigation by Processing Sequential Information? Neuropsychology 2017, 31, 564–574.
- Bianchini, F.; Di Vita, A.; Palermo, L.; Piccardi, L.; Blundo, C.; Guariglia, C. A Selective Egocentric Topographical Working Memory Deficit in the Early Stages of Alzheimer’s Disease: A Preliminary Study. Am. J. Alzheimers Dis. Other Demen. 2014, 29, 749–754.
- Perrochon, A.; Kemoun, G.; Dugué, B.; Berthoz, A. Cognitive Impairment Assessment through Visuospatial Memory Can Be Performed with a Modified Walking Corsi Test Using the “Magic Carpet”. Dement. Geriatr. Cogn. Dis. Extra 2014, 4, 1–13.
- Piccardi, L.; Bianchini, F.; Argento, O.; De Nigris, A.; Maialetti, A.; Palermo, L.; Guariglia, C. The Walking Corsi Test (WalCT): Standardization of the Topographical Memory Test in an Italian Population. Neurol. Sci. 2013, 34, 971–978.
- Meilinger, T.; Berthoz, A.; Wiener, J.M. The Integration of Spatial Information across Different Viewpoints. Mem. Cognit. 2011, 39, 1042–1054.
- Spencer, R.M.C.; Ivry, R.B. Sequence Learning Is Preserved in Individuals with Cerebellar Degeneration When the Movements Are Directly Cued. J. Cogn. Neurosci. 2009, 21, 1302.
- Mandolesi, L.; Leggio, M.G.; Spirito, F.; Petrosini, L. Cerebellar Contribution to Spatial Event Processing: Do Spatial Procedures Contribute to Formation of Spatial Declarative Knowledge? Eur. J. Neurosci. 2003, 18, 2618–2626.
- Xu, D.; Liu, T.; Ashe, J.; Bushara, K.O. Role of the Olivo-Cerebellar System in Timing. J. Neurosci. 2006, 26, 5990–5995.
More
Information
Subjects:
Psychology, Biological
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
18 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





