
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | muhammad alauddin | -- | 2521 | 2022-05-11 12:28:13 | | | |
| 2 | Peter Tang | Meta information modification | 2521 | 2022-05-11 12:33:08 | | |
Video Upload Options
Guided bone and tissue regeneration remains an integral treatment modality to regenerate bone surrounding teeth and dental implants. Barrier membranes have been developed and produced commercially to allow space for bone regeneration and prevent the migration of unwanted cells. Ideal membrane properties, including biocompatibility, sufficient structural integrity and suitable shelf life with easy clinical application, are important to ensure good clinical regenerative outcomes. Membranes have various types, and their clinical application depends on the origin, material, structure and properties.
1. Introduction
2. Resorbable Membranes
2.1. Collagen Membranes
2.2. Clinical Evidence
2.3. Fibrin
2.4. Placenta
2.5. Chitosan
2.6. Current Development of Resorbable Membranes
3. Non-Resorbable Membranes
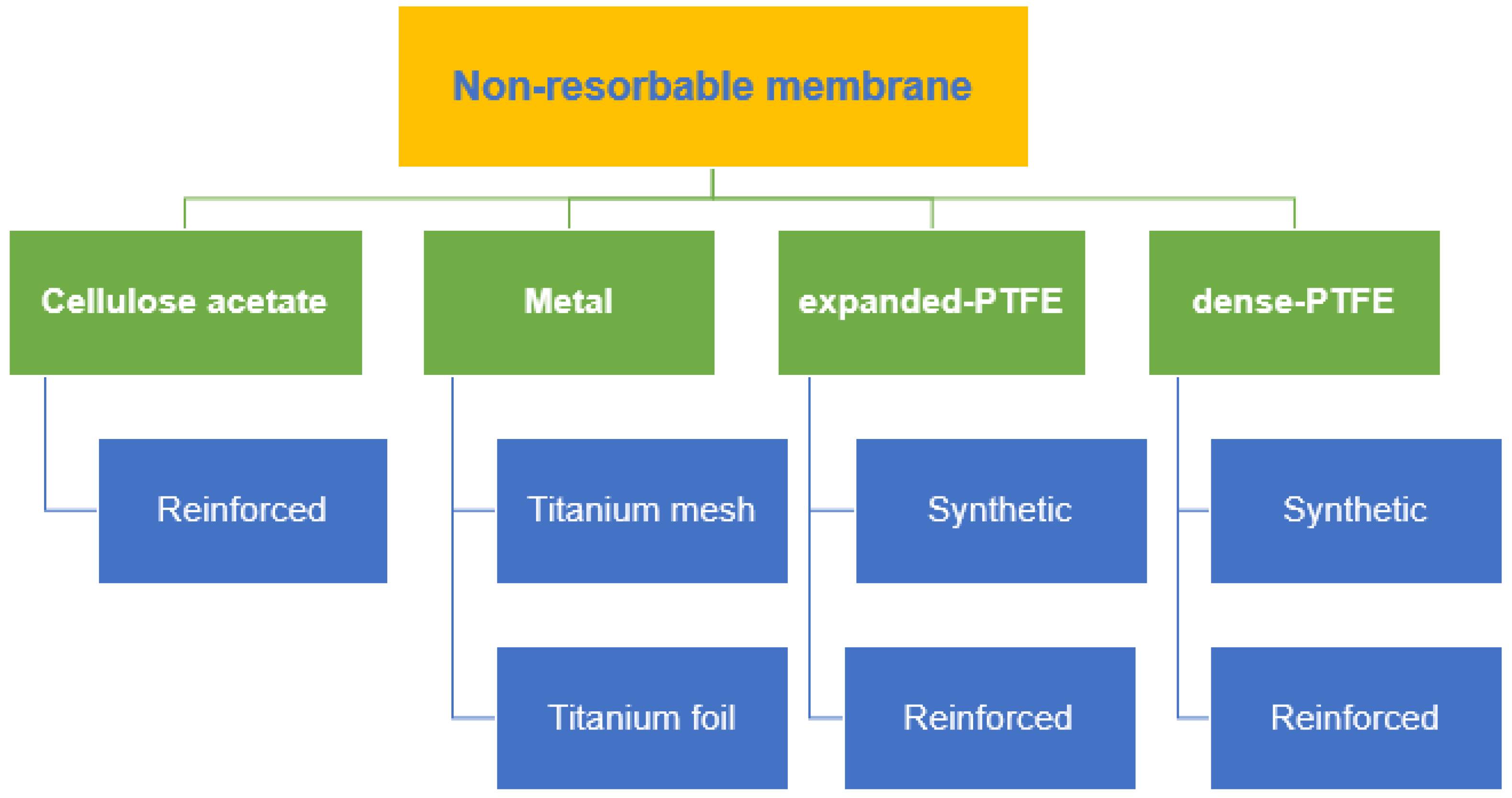
|
Product (Company) |
Material |
|---|---|
|
Ti- Micromesh (ACE) |
Titanium mesh |
|
Tocksystem (MeshTM) |
Titanium mesh |
|
Millipore |
Cellulose acetate |
|
Gore-Tex® |
ePTFE |
|
Cytoplast™ |
dPTFE |
|
Ti-Reinforced Gore-Tex® |
Titanium-reinforced ePTFE |
|
Cytoplast™ Ti-Reinforced 250 |
Titanium-reinforced dPTFE |
4. Synthetic Membranes
5. Autologous Platelet Concentrate (APC)
5.1. Types of Autologous Platelet Concentrate
6. High-Performance Polymer
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337.
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Khoshkam, V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann. Maxillofac. Surg. 2017, 7, 263–277.
- Masquelet, A.C.; Begue, T. The Concept of Induced Membrane for Reconstruction of Long Bone Defects. Orthop. Clin. N. Am. 2010, 41, 27–37.
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65.
- Hoornaert, A.; D’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012.
- Caballé-Serrano, J.; Sawada, K.; Miron, R.J.; Bosshardt, D.D.; Buser, D.; Gruber, R. Collagen barrier membranes adsorb growth factors liberated from autogenous bone chips. Clin. Oral Implants Res. 2016, 28, 236–241.
- Huang, H.-L.; Ma, Y.-H.; Tu, C.-C.; Chang, P.-C. Radiographic Evaluation of Regeneration Strategies for the Treatment of Advanced Mandibular Furcation Defects: A Retrospective Study. Membranes 2022, 12, 219.
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63.
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regenera-tion: A literature review. Materials 2020, 13, 786.
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17.
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503.
- Cortellini, P.; Carnevale, G.; Sanz, M.; Tonetti, M.S. Treatment of deep and shallow intrabony defects A multicenter randomized controlled clinical trial. J. Clin. Periodontol. 1998, 25, 981–987.
- Gulameabasse, S.; Gindraux, F.; Catros, S.; Fricain, J.C.; Fenelon, M. Chorion and amnion/chorion membranes in oral and per-iodontal surgery: A systematic review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1216–1229.
- Qasim, S.S.B.; Baig, M.R.; Matinlinna, J.P.; Daood, U.; Al-Asfour, A. Highly Segregated Biocomposite Membrane as a Functionally Graded Template for Periodontal Tissue Regeneration. Membranes 2021, 11, 667.
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C 2020, 109, 110618.
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, I.U. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83.
- Hassan, M.; Prakasam, S.; Bain, C.; Ghoneima, A.; Liu, S.S.-Y. A randomized split-mouth clinical trial on effectiveness of am-nion-chorion membranes in alveolar ridge preservation: A clinical, radiologic, and morphometric study. Int. J. Oral Maxillofac. Implants 2017, 32, 1389–1398.
- Chan, E.C.; Kuo, S.-M.; Kong, A.M.; Morrison, W.A.; Dusting, G.J.; Mitchell, G.M.; Lim, S.Y.; Liu, G.-S. Three Dimensional Collagen Scaffold Promotes Intrinsic Vascularisation for Tissue Engineering Applications. PLoS ONE 2016, 11, e0149799.
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials 2016, 84, 167–183.
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158.
- Allan, B.; Ruan, R.; Landao-Bassonga, E.; Gillman, N.; Wang, T.; Gao, J.; Ruan, Y.; Xu, Y.; Lee, C.; Goonewardene, M.; et al. Collagen Membrane for Guided Bone Regeneration in Dental and Orthopedic Applications. Tissue Eng. Part A 2021, 27, 372–381.
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation via coating nanometer-sized bioactive glass to improve guided bone re-generation. Biomater. Sci. 2018, 6, 1007–1019.
- Annen, B.M.; Ramel, C.F.; Hämmerle, C.H.; Jung, R.E. Use of a new cross-linked collagen membrane for the treatment of pe-ri-implant dehiscence defects: A randomised controlled double-blinded clinical trial. Eur. J. Oral Implantol. 2011, 4, 87–100.
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. dense polytetrafluoroethylene membranes in vertical ridge augmentation around dental implants: A prospective randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 859–866.
- Ruggiero, R.; de Almeida Carvalho, V.; da Silva, L.G.; de Magalhães, D.; Ferreira, J.A.; de Menezes, H.H.M.; de Melo, G.P.; Naves, M.M. Study of in vitro degradation of cellulose acetate membranes modified and incorporated with tetracycline for use as an adjuvant in periodontal reconstitution. Ind. Crop. Prod. 2015, 72, 2–6.
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296.
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308.
- Soldatos, N.K.; Stylianou, P.; Angelov, N.; Koidou, P.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147.
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implants Res. 2010, 21, 567–576.
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier Membranes Used for Ridge Augmentation: Is There an Optimal Pore Size? J. Oral Maxillofac. Surg. 2009, 67, 1218–1225.
- Lee, J.-Y.; Kim, Y.-K.; Yun, P.-Y.; Oh, J.-S.; Kim, S.-G. Guided bone regeneration using two types of non-resorbable barrier mem-branes. J. Korean Assoc. Oral Maxillofac. Surg. 2010, 36, 275–279.
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14.
- Vroom, M.; Gründemann, L. Nietresorbeerbare membranen. Tandartspraktijk 2014, 35, 8–13.
- Barber, H.D.; Lignelli, J.; Smith, B.M.; Bartee, B.K. Using a Dense PTFE Membrane Without Primary Closure to Achieve Bone and Tissue Regeneration. J. Oral Maxillofac. Surg. 2007, 65, 748–752.
- Rodriguez, I.A.; Selders, G.S.; Fetz, A.E.; Gehrmann, C.J.; Stein, S.H.; Evensky, J.A.; Green, M.S.; Bowlin, G.L. Barrier membranes for dental applications: A review and sweet advancement in membrane developments. Mouth Teeth 2018, 2, 1–9.
- Castro, A.G.; Diba, M.; Kersten, M.; Jansen, J.A.; Beucken, J.J.V.D.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mater. Sci. Eng. C 2018, 85, 154–161.
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115.
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864.
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engi-neering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126.
- Alauddin, M.S.; Ramli, H. Management of Membrane Exposure Utilizing Concentrated Growth Factor (CFG) in Guided Bone Regeneration: A Clinical Report. Open Dent. J. 2020, 14, 763–768.
- Alauddin, M.S.; Yusof, N.M.; Adnan, A.S.; Said, Z. Preliminary Novel Analysis on Antimicrobial Properties of Concentrated Growth Factor against Bacteria-Induced Oral Diseases. Eur. J. Dent. 2022, 1–239.
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44.
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167.
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777.
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869.
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implant. 2013, 39, 743–749.
- Toth, J.M.; Wang, M.; Estes, B.T.; Scifert, J.L.; Seim, H.B., III; Turner, A.S. Polyetheretherketone as a biomaterial for spinal applica-tions. Biomaterials 2006, 27, 324–334.
- Santing, H.J.; Meijer, H.J.; Raghoebar, G.M.; Özcan, M. Fracture strength and failure mode of maxillary implant-supported provisional single crowns: A comparison of composite resin crowns fabricated directly over PEEK abutments and solid titanium abutments. Clin. Implant Dent. Relat. Res. 2012, 14, 882–889.
- Tannous, F.; Steiner, M.; Shahin, R.; Kern, M. Retentive forces and fatigue resistance of thermoplastic resin clasps. Dent. Mater. 2012, 28, 273–278.
- Costa-Palau, S.; Torrents-Nicolas, J.; Barberà, M.B.-D.; Cabratosa-Termes, J. Use of polyetheretherketone in the fabrication of a maxillary obturator prosthesis: A clinical report. J. Prosthet. Dent. 2014, 112, 680–682.
- Aldhuwayhi, S.; Alauddin, M.S.; Martin, N. The Structural Integrity and Fracture Behaviour of Teeth Restored with PEEK and Lithium-Disilicate Glass Ceramic Crowns. Polymers 2022, 14, 1001.
- Papia, E.; Brodde, S.A.; Becktor, J.P. Deformation of polyetheretherketone, PEEK, with different thicknesses. J. Mech. Behav. Biomed. Mater. 2022, 125, 104928.
- Alauddin, M.S. A Review of Polymer Crown Materials: Biomechanical and Material Science. J. Clin. Diagn. Res. 2019, 13, ZE01–ZE05.
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19.
- Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The modern and digital transformation of oral health care: A mini review. Healthcare 2021, 9, 118.
- El Morsy, O.A.; Barakat, A.; Mekhemer, S.; Mounir, M. Assessment of 3-dimensional bone augmentation of severely atrophied maxillary alveolar ridges using patient-specific poly ether-ether ketone (PEEK) sheets. Clin. Implant Dent. Relat. Res. 2020, 22, 148–155.
- Mounir, M.; Shalash, M.; Mounir, S.; Nassar, Y.; El Khatib, O. Assessment of three dimensional bone augmentation of severely atrophied maxillary alveolar ridges using prebent titanium mesh vs customized poly-ether-ether-ketone (PEEK) mesh: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 960–967.
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.; Habib, S.R.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging bio-material for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95.




