Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natália Cruz-Martins | -- | 3049 | 2022-04-29 16:11:32 | | | |
| 2 | Catherine Yang | -1 word(s) | 3048 | 2022-05-05 04:41:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cruz-Martins, N.; Sharifi-Rad, J.; Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; , .; Özçelik, B.; et al. Avocado–Soybean Unsaponifiables (ASU). Encyclopedia. Available online: https://encyclopedia.pub/entry/22512 (accessed on 07 February 2026).
Cruz-Martins N, Sharifi-Rad J, Salehi B, Rescigno A, Dettori T, Calina D, et al. Avocado–Soybean Unsaponifiables (ASU). Encyclopedia. Available at: https://encyclopedia.pub/entry/22512. Accessed February 07, 2026.
Cruz-Martins, Natália, Javad Sharifi-Rad, Bahare Salehi, Antonio Rescigno, Tenuccia Dettori, Daniela Calina, Anca Oana Docea, Laxman Singh, , Beraat Özçelik, et al. "Avocado–Soybean Unsaponifiables (ASU)" Encyclopedia, https://encyclopedia.pub/entry/22512 (accessed February 07, 2026).
Cruz-Martins, N., Sharifi-Rad, J., Salehi, B., Rescigno, A., Dettori, T., Calina, D., Docea, A.O., Singh, L., , ., Özçelik, B., Bhia, M., Sharopov, F., & Cho, W.C. (2022, April 29). Avocado–Soybean Unsaponifiables (ASU). In Encyclopedia. https://encyclopedia.pub/entry/22512
Cruz-Martins, Natália, et al. "Avocado–Soybean Unsaponifiables (ASU)." Encyclopedia. Web. 29 April, 2022.
Copy Citation
Avocado and soybean unsaponifiables (ASU) constitute vegetable extracts made from fruits and seeds of avocado and soybean oil. Characterized by its potent anti-inflammatory effects, this ASU mixture is recommended to act as an adjuvant treatment for osteoarthritic pain and slow-acting symptomatic treatment of hip and knee osteoarthritis; autoimmune diseases; diffuse scleroderma and scleroderma-like states (e.g., morphea, sclerodactyly, scleroderma in bands). Besides, it can improve the mood and quality of life of postmenopausal women in reducing menopause-related symptoms.
avocado–soybean unsaponifiables
tocopherol
1. Avocado–Soybean Unsaponifiables: Extraction, Analysis and Chemical Compounds
1.1. Extraction and Analysis of ASU
Edible oils and fats have been used for a long time. From the lipid soap production, it was clear that some lipids escaped from the chemical saponification process. According to this observation, natural oils and fats are characterized by the unsaponifiable fraction [1]. In fact, lipids present in animal or vegetable have a remarkable variety of physical forms, and can be present as aggregates, or they can be associated with proteins and carbohydrates, e.g., the biological membranes.
1.1.1. Extraction Methods of ASU
ASU is prepared in two steps: (i) obtain the avocado and soybean crude oils by cold pressing through mechanical procedures at temperatures below 50 °C; (ii) extraction of unsaponifiable lipids: molecular distillation of crude avocado and soybean oil, saponification, extraction, purification.The extraction methods normally used when lipids subjected to further characterization of the fat which called cold methods.Since the extraction conditions of these methods limit the oxidative processes of the lipidic fractionas much as possible, allowing one to preserve the original composition [2]. These methods are generally based on the use of a binary mixture of solvents, such as chloroform/methanol [3][4], dichloromethane/methanol, or hexane/isopropanol [5].
Molecular distillation is a method of separating the unsaponifiable substances from the crude avocado oilat low pressures. It is based on the intensification of the four elementary processes—the diffusion of components through liquid, the vaporization ofthe surface of the liquid, vapor transport to the condensation surface, and condensation on the surface of the capacitor. Separation by molecular distillation is useful for the purification and concentration of unsaponifiable, low-vapor pressure thermoset substances. This method is applied when the conventional distillation methods lead to the thermal degradation of the products or if the vapor pressure of the components separately is verylow that separation at atmospheric pressure or at medium vacuum would require extremely high temperatures.
Consequently, at the end of the saponification process of vegetable oils by extraction with an organic solvent, an unsaponifiable fraction could beobtained.
1.1.2. Analysis Methods of ASU
The extracted lipids then can be further fractionated by means of chromatographic techniques, such as thin layer chromatography (TLC), gas–liquid chromatography (GLC), and high-performance liquid chromatography (HPLC). In reality, chromatographic techniques can be combined with sophisticated analytical techniques, such as mass spectrometry (MS). In addition, nuclear magnetic resonance (NMR) is becoming routine for the study of the lipid fraction in biological matrices.
1.2. Chemical Composition of Avocado–Soybean Unsaponifiables
Typically, from this extraction process, a complex mixture of compounds is obtained, the main classes are tocopherols and tocotrienols, phytosterols, carotenes, chlorophylls and a mixture of other unsaponifiable compounds [2].
1.2.1. Tocopherols and Tocotrienols
Tocopherols and tocotrienols have α-, β-, γ-, and δ-isomers that differ in number and position of the methyl groups in the chromane ring and constitute a series of benzopyranols that present in plants and photosynthetic organisms. The synthesis starts from homogentisic acid with a complex series of reactions [6]. Taken together, these two groups of molecules are called tocochromanols. Tocotrienols have an unsaturated farnesyl isoprenoid tail with three trans double bonds, whereas tocopherols have a saturated phytyl tail (Table 1). Tocochromanols are often found in chloroplasts and, collectively, they have been termed vitamin E (the individual tocopherols are properly called ‘vitamers’) though only α-tocopherol has this designation, because of its biological activity and presence in the human body [7].
Table 1. Chemical structure of tocopherols and tocotrienols. Tocopherols have a saturated phytyl tail.
| Form | R1 | R2 | R3 |
| α-Tocopherol | CH3 | CH3 | CH3 |
| β-Tocopherol | CH3 | CH3 | H |
| γ-Tocopherol | H | CH3 | CH3 |
| δ-Tocopherol | H | CH3 | H |
| Basic structure of tocotrienols | 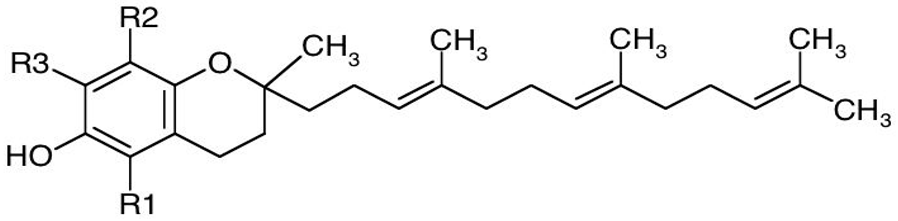 |
||
| Form | R1 | R2 | R3 |
| α-Tocotrienol | CH3 | CH3 | CH3 |
| β-Tocotrienol | CH3 | H | CH3 |
| γ-Tocotrienol | H | CH3 | CH3 |
| δ-Tocotrienol | H | H | CH3 |
The properties of vitamin E have been known for many decades. Many studies have analyzing the effects of vitamin E in cardiovascular diseases [8], immunity [9], treating and preventing osteoarthritis [10]. However, interest in tocotrienols has awakened due to their biological effects and the therapeutic properties [11][12]. Many studies have shown that tocotrienols are useful in the treatment of high cholesterol levels due to their ability to inhibit the key enzyme of cholesterol biosynthesis, HMG-CoA reductase [13]. The tocotrienols also show excellent antioxidant properties thanks to their lateral unsaturated tail, which allows easier access to the lipid bilayer of biological membranes [14]. Moreover, the anti-cancer [15] and neuroprotective [12] properties of tocotrienols have also been documented.
1.2.2. Phytosterols
Phytosterols are bioactive sterols present in vegetables, especially in natural oils, nuts and cereals, and are structurally similar to sterols from animal sources. Compared to cholesterol, they have an additional methyl or ethyl group in their side chain. The absorption of dietary plant sterols in humans is low compared to cholesterol [16]. All phytosterols, some of which are depicted in Figure 1, have a hydroxyl group at the 3-position. In oils, the sterol hydroxyl group is not linked to any other moiety, but phytosterols are usually present as conjugates with the hydroxyl group covalently bound via an ester bond to a fatty acid. When the double bond in the sterols is saturated, the resulting compounds are termed stanols [17].
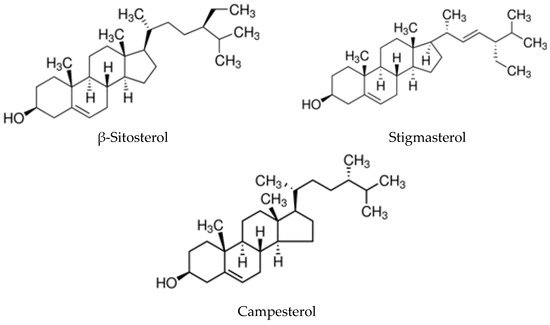
Figure 1. Chemical structure of the three most representative phytosterols in vegetable oils.
Phytosterols are amphiphilic and important constituents of all membranes, especially the plasma membrane, mitochondrial outer membrane and endoplasmic reticulum. They can regulate membrane fluidity and permeability in plasma membranes by restricting the mobility of fatty acyl chains in a similar manner to cholesterol in mammalian cells [18]. Phytosterols present in diet are well-known for their inhibitory effects on intestinal cholesterol absorption and in decreased LDL-cholesterol levels [19]. Furthermore, evidence is accumulating that these compounds have effects beyond cholesterol-lowering effects. Recently, Plat et al. (2019) reviewed the possible side effects in the field of immunology, hepatology, gastroenterology and rheumatology arising from the increasing consumption of foods rich in plant sterols and stanols [20]. The emerging scenario is that, along with multiple positive health effects, an excessive intake of phytosterols and phytostanols requires further investigation to understand the complete health effects of plant sterols and stanols in both healthy individuals as well as in individuals suffering from specific diseases.
1.2.3. Carotenes, Chlorophylls, and Other Unsaponifiable Compounds
The main pigments found in vegetable oils are carotenoids and chlorophylls [21]. Carotenoids cover a wide range of functions in human health [22]. They primarily exert antioxidant effects, but individual carotenoids (α- and β-carotene, lutein, zeaxanthin, lycopene) may also act through other mechanisms as in eye function [23]. There is evidence that carotenoids could improve cognitive function, skin-UV protection, and even may prevent some types of cancer [24][25].
Chlorophyll is an ester of chlorophyllic acid and phytol alcohol [26]. It occurs in several distinct forms; chlorophylls a and b are the major types typical of higher plants and green algae; chlorophylls c and d are found in different algae. Bacterio-chlorophyll occurs in certain bacteria [27]. The chlorophyll molecule consists of a central magnesium ion surrounded by a nitrogen-containing structure, a porphyrin ring; attached to the ring is the phytol chain. The variations are due to minor modifications of certain side groups [28].
It has long been debated whether chlorophylls, and their natural or synthetic derivatives, could be absorbable by humans, despite the fact that their consumption has been documented for a long time in traditional medicine [29][30][31]. However, recent research has confirmed that chlorophyll derivatives are absorbable by the human intestine [32]. The biological activities attributed to chlorophylls are various. Particularly interesting are those consistent with cancer prevention, such as antioxidant and antimutagenic effects, mutagen trapping, modulation of xenobiotic metabolism, and induction of apoptosis [33].
A miscellany of other compounds, extractable in organic solvents, can be found in the unsaponifiable fraction of numerous plants and their composition varies according to the plant being considered. It is necessary to consider that among these compounds, present in vegetable oils, hydrocarbons may also be present since they do not saponify. For example, squalene is present in some vegetable oils, where it can also represent 50% of the unsaponifiable fraction, such as in olive oil.
1.2.4. Main Components of ASU
An ASU fraction is obtained by the purification and fractionation of the respective oils [34]. The quantity and composition of extractable lipids depend on various factors, including the botanic cultivar, harvesting time, and the growth condition of the plant.
At 20 °C, ASU looks like an oily and thick paste, insoluble in water. Studies performed on these vegetable oils showed that unsaponifiables differ in the content of total unsaponifiables and, also, in their relative abundance [35]. Avocado oils contain more unsaponifiables than soybean oils (4.8%–12.2 % from the fruit flesh oils), whereas unsaponifiables account for over 50% of the oils from the avocado kernel. Total sterols were also more represented in avocados (3770–10720 μg/g oil) than in soybeans (on average 3600 μg/g oil). Concerning the sterol composition of avocado oils, β-sitosterol was the dominant one (ca. 90 %), with a limited amount of campesterol and stigmasterol. The sterol fraction of soybean oil had about 50% β-sitosterol and the remaining 50% was almost equally divided between campesterol and stigmasterol [36]. The tocopherol content was at least ten times higher in soybean oils (1130–1450 μg/g oil) than the content of both avocado flesh and kernel oil. Soybean tocopherols were rich in γ-tocopherol (>66 %), whereas δ-tocopherol was >21% and α-tocopherol was 11%. Interestingly, α-tocopherol accounted for 100% of the total tocopherols in avocado oils [37][38].
Dijkstra (2016) reported the absence of tocotrienols in soybean oil [33]. Tocopherols were found in soybean oil, by means of a simultaneous analytical method HPLC-DAD-FLD based, and also confirmed the absence of tocotrienols [39]. The unsaponifiable fraction of avocado contains tocotrienols, but in very low amounts. A study aimed at determining the tocochromanol content in raw and processed fruits and vegetables revealed the presence of tocotrienols in quantities of about 1% of the total tocochromanols, only in some avocado cultivars [40]. Tocotrienols have been shown to possess higher antioxidant and anti-inflammatory effects than α-tocopherol [11].
The presence of total sterols can also vary depending on the different growth conditions and the variety considered. De Souza et al. (2015) extracted by centrifugation the most cultivated avocado varieties in Brazilian territories and compared them to a commercial product. The Margarida and Hass varieties showed a phytosterol content that reached almost 100 mg/100 mL of oil [41]. Such a high amount of phytosterols makes ASU an important vehicle for bioactive natural compounds for human health.
The content of the various lipid classes of the unsaponifiable fraction mainly depends on plant variety, and on the purification and fractionation process adopted. However, although the ASU is chemically well characterized, it is not possible to exclude the fact that a part of its therapeutic action may be mediated by unidentified factors. Therefore, it is preferable to use the unsaponifiable fraction “in toto”, which, in this way, can preserve the glycosylated fraction [42].
2. Avocado–Soybean Unsaponifiables for Medical Purposes
2.1. Autoimmune Disorders
The human immune system is known to protect the body against the foreign invaders that basically exert a wide variety of deleterious effects [43]. Sometimes the optimal functioning mechanism of the immune system is said to act as a ‘double-edged sword’, either by healing the physiological state or by damaging it. The act of immune dysfunction acting against its own normal components of the body results in autoimmune disorders [44]. Many pieces of conclusive experimental evidence have suggested that they result from the interaction between various genetic and environmental factors, and even the distinct functioning of the endocrine system [45].
The known etiology of these autoimmune dysfunctions is associated with the overproduction and up-regulation of several pro-inflammatory substrates, such as interleukin 1β (IL-1β), a stimulant cytokine which further stimulates the synthesis of other pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-8 (IL-8), macrophage inflammatory protein (MIP), and reactive oxygen species (ROS), such as NO., O2−, H2O2 [46]. Other components that lead to this immune disorder include tumor necrosis factor-α (TNF-α) and -β (TNF-β), prostaglandin E-2 (PGE-2), inducible nitric oxide synthase (iNOS), metalloproteinases, including collagenases (MMP-1, 8, 13), aggrecanases (ADAM-TS4), stromelysin-1 (MMP-3), and gelatinases (MMP-2) [47][48].
Scleroderma is a rare autoimmune disorder, in which skin and connective tissue gets thickened due to too much collagen production. In scleroderma, the collagen content of the skin increases while the number of adipocytes decreases.
ASU was suggested by Jablonska et al. as being an effective agent in the treatment of scleroderma by increasing the collagen solubility and reducing cutaneous fibrosis. A larger cohort study is needed to investigate the effect of ASU in the treatment of scleroderma [49].
The ASU effect on autoimmune disorders seems to be an unexplored subject. However, considering the soybean content of ASU, it is worth mentioning the effects of soy on many immune disorders. A crossover randomized clinical trial was performed with 14 diabetic patients and reported that soy protein inclusion in the diet was beneficiary to the serum lipid profile and renal function. This effect was attributed to the isoflavones in soy protein [50]. Another study conducted on eight people reported that consuming soy protein as half of the daily protein intake did not show any significant effects on renal function or proteinuria. However, the study reported a significant association between soy protein intake and reduction in serum cholesterol and triacylglycerol concentrations [51]. It should be noted that there is some conflict regarding the effects of phytoestrogens of soy on immune disorders. A mouse model study on lupus disease reported that a soy diet compared to a casein diet worsened the clinical course of lupus [52].
ASU could also be useful to treat inflammatory bowel disease. The supplementation of soy isoflavones to neonates and piglets was also suggested to reduce the intestinal barrier damages of lipopolysaccharide [53]. A study using a pig model of intestinal inflammation tested the effect of soy-derived di- and tripeptides and reported the anti-inflammatory effects of these peptides in vivo [54].
2.2. Menopause
Menopause is described as the end of menstruating and is a normal condition that all women experience when they age [55]. In the initial days/years of menopause, the associated symptoms include hot flashes, vaginal dryness, and rapid bone loss as a result of osteoporosis and sleep disturbances [56]. Hormone replacement therapy (HRT) has been used as the most common therapy to get relief from menopausal dysfunction. But again, it comes with possible fallouts, such as breast or endometrial cancer, irregular bleeding, thromboembolic events, mastalgia, nausea, weight gain, migraine, among other issues. Presently, HRT is forbidden for women currently undergoing or who have a history of breast cancer, coronary heart disease (CHD), venous thromboembolic events or stroke, liver disease, mysterious vaginal bleeding, high-risk endometrial cancer, or transient ischemic attack [57].
In the light of these aspects, the use of other alternative therapies, possibly through the use of herbal formulations, is recommended. Recently, herbal remedies, particularly the ones inheriting phytoestrogens values, are in great demand for the treatment of such climacteric symptoms. Soy isoflavones and extracts are the preferred phytoestrogen sources, with estrogen-like properties. Phytoestrogens are the chief constituents of polyphenols, structurally similar to endogenous estrogen, but having weak estrogenic properties as compared to endogenous ones [56].Keeping this in mind, soybean rich in unique dietary phytoestrogens (i.e., isoflavones daidzein, genistin, and glycetin) has gained considerable importance; apart from this, gabapentin, clonidine, selective serotonin reuptake inhibitors (SSRI’s), black cohosh, and vitamin E are other alternatives used for conventional HRT [58]. These phytoestrogens are reported to have selective estrogen receptor modulators preferential for estrogen receptor-beta (ER-β) rather than for estrogen receptor-alpha (ER-α). As a result, when these phytoestrogens bound to ER-β trigger an effective transcriptional activity, either the response can be an agonist response or antagonist, depending on the compound (stimulus) and the site of action (target tissue) [59].
ASU is an herbal medicine derived from avocado and soy which is used to relieve hot flashes in menopausal women [60]. It is known that ASU has a potent phytoestrogenic value and exerts significant positive effects in reducing menopausal-related symptoms, such as hot flashes, besides being able to improve mood and quality of life in postmenopausal women [61].
However, there are conflicts regarding the effect of soybean on climacteric symptoms of menopause. Some authors have suggested positive effects [62], while others did not report any significant effect [63][64]. A study investigating the association between dietary fiber intake and serum estrogen levels also studied ASU intake. The study suggested that ASU is related to higher serum estrogen levels, but the source of the effect needs to be explored [65].
An open label randomized study included 49 women and tested the ability of ASU to relieve menopausal symptoms. The women were divided into two groups; one group received 1 mg ASU daily and another group were treated with HRT (0.625 mg conjugated estrogen and 2.5 mg medroxyprogesterone acetate tablets). The visual analog scale (VAS) was used to determine the intensity of hot flashes, and the climacteric symptom was determined by the Greene Climacteric Scale (GCS) and Blatt–Kupperman Menopausal Index (BKMI). No significant differences were stated in the hot flash severity decrease for the ASU and HRT groups (GCS; p = 0.571 and BMKI; p = 0.891) [60]. Thus, studies investigating the effect of ASU vs HRT are limited, but they report similar symptom relief effects. Concerning the HRT side effects, ASU seems a feasible alternative to HRT.
2.3. Other Pharmacotherapeutic Uses of ASU
There are studies that highlighted other beneficial effects of ASU on different medical situations, such as chronic dorsalgia [66], gingival inflammation, periodontitis [67] or back pain [68]. Other health benefits include its ability to decrease the risk of osteoporosis, heart disease, and breast cancer [69].
ASU was also studied for its effect in wound healing. A rat model study investigated the effect of ASU on rat wound healing by randomly dividing rats into three subgroups (20 rats each). Each rat in the control (saline), vehicle (cream) and treatment (cream plus ASU) groups were wounded on the dorsum (2×2 cm2 wound) and the wounds were screened daily. The study found that treatment (cream plus ASU) produced a significantly higher level of tissue glycosaminoglycan and collagen contents compared to controls. It was also reported that the treatment had a modulating effect on inflammation, improved fibroplasia and provided higher amounts of scar tissue; so, it was concluded that ASU is a promising agent in wound healing [70].
References
- Prieto Vidal, N.; Adeseun Adigun, O.; Pham, T.; Mumtaz, A.; Manful, C.; Callahan, G.; Stewart, P.; Keough, D.; Thomas, R. The Effects of Cold Saponification on the Unsaponified Fatty Acid Composition and Sensory Perception of Commercial Natural Herbal Soaps. Molecules 2018, 23, 2356.
- Uquiche, E.; Romero, V.; Ortiz, J.; Del Valle, J. Extraction of oil and minor lipids from cold-press rapeseed cake with supercritical CO2. Braz. J. Chem. Eng. 2012, 29, 585–598.
- EG, B. A rapid method of total lipid extraction and purification. Canj. Biochem. Physiol. 1959, 37, 911–917.
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509.
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978, 90, 420–426.
- Dewick, P.M.; Fattorusso, E. Chimica, biosintesi e bioattività delle sostanze naturali; Piccin: Padova, Italy, 2012.
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15.
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. Iubmb Life 2019, 71, 507–515.
- Lee, G.; Han, S. The role of vitamin E in immunity. Nutrients 2018, 10, 1614.
- Chin, K.Y.; Ima-Nirwana, S. The role of vitamin E in preventing and treating osteoarthritis-a review of the current evidence. Front. Pharmacol. 2018, 9, 946.
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169.
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular Basis of Vitamin E Action Tocotrienol potently inhibits glutamate-induced pp60c-Src Kinase activation and death of ht4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055.
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.K. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992, 35, 3595–3606.
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275.
- Bagchi, D.; Preuss, H.G. Phytopharmaceuticals in Cancer Chemoprevention; CRC Press: Boca Raton, FL, USA, 2004.
- Jones, P.J.; MacDougall, D.E.; Ntanios, F.; Vanstone, C.A. Dietary phytosterols as cholesterol-lowering agents in humans. Can. J. Physiol. Pharmacol. 1997, 75, 217–227.
- Moreau, R.A.; Whitaker, B.D.; Hicks, K.B. Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res. 2002, 41, 457–500.
- Xu, F.; Rychnovsky, S.D.; Belani, J.D.; Hobbs, H.H.; Cohen, J.C.; Rawson, R.B. Dual roles for cholesterol in mammalian cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14551–14556.
- Kritchevsky, D.; Chen, S.C. Phytosterols—health benefits and potential concerns: A review. Nutr. Res. 2005, 25, 413–428.
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.; Burrin, D.; Guthrie, G.; Thijs, C.; Te Velde, A.; Vreugdenhil, A. Plant-based sterols and stanols in health & disease:“Consequences of human development in a plant-based environment?”. Prog. Lipid Res. 2019.
- Pohndorf, R.; Cadaval Jr, T.; Pinto, L. Kinetics and thermodynamics adsorption of carotenoids and chlorophylls in rice bran oil bleaching. J. Food Eng. 2016, 185, 9–16.
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65.
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26.
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738.
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S.
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Products Olive Tree, 1st ed.; Intech: London, UK, 2016; pp. 67–98.
- Canniffe, D.P.; Thweatt, J.L.; Chew, A.G.M.; Hunter, C.N.; Bryant, D.A. A paralog of a bacteriochlorophyll biosynthesis enzyme catalyzes the formation of 1, 2-dihydrocarotenoids in green sulfur bacteria. J. Biol. Chem. 2018, 293, 15233–15242.
- Hoober, J.K.; Eggink, L.L.; Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 2007, 94, 387–400.
- Bowers, W.F. Chlorophyll in wound healing and suppurative disease. Am. J. Surg. 1947, 73, 37–50.
- Edwards, B. Treatment of chronic leg ulcers with ointment containing soluble chlorophyll. Physiotherapy 1954, 40, 177–179.
- Mishra, V.K.; Bacheti, R.; Husen, A. Medicinal uses of chlorophyll: A critical overview. Chlorophyll Struct. Funct. Med. Uses. Hauppauge Nova Sci. Publ. 2012, 177–196.
- Egner, P.A.; Stansbury, K.H.; Snyder, E.P.; Rogers, M.E.; Hintz, P.A.; Kensler, T.W. Identification and characterization of chlorin e4 ethyl ester in sera of individuals participating in the chlorophyllin chemoprevention trial. Chem. Res. Toxicol. 2000, 13, 900–906.
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12.
- Werman, M.; Neeman, I.; Mokady, S. Avocado oils and hepatic lipid metabolism in growing rats. Food Chem. Toxicol. 1991, 29, 93–99.
- Gutfinger, T.; Letan, A. Studies of unsaponifiables in several vegetable oils. Lipids 1974, 9, 658–663.
- Normén, L.; Dutta, P.; Lia, Å.; Andersson, H. Soy sterol esters and β-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 2000, 71, 908–913.
- Veronezi, C.M.; Jorge, N. Effect of Carica papaya and Cucumis melo seed oils on the soybean oil stability. Food Sci. Biotechnol. 2018, 27, 1031–1040.
- Manaf, Y.N.; Rahardjo, A.P.; Yusof, Y.A.; Desa, M.N.; Nusantoro, B.P. Lipid characteristics and tocopherol content of the oils of native avocado cultivars grown in Indonesia. Int. J. Food Prop. 2018, 21, 2758–2771.
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous determination of tocols, γ-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019, 271, 630–638.
- Li, X.-K.; Ji, W.-J.; Zhao, J.; Wang, S.-J.; Au, C.-T. Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J. Catal. 2005, 236, 181–189.
- Jorge, T.d.S.; Polachini, T.C.; Dias, L.S.; Jorge, N.; Telis-Romero, J. Physicochemical and rheological characterization of avocado oils. Ciência E Agrotecnologia 2015, 39, 390–400.
- Patel, N.K. Phytotherapeutic Investigation of Major Herbal Steroids to Explore their Potential as an Alternative to Synthetic Steroids. Ph.D. Thesis, Saurashtra University, Rajkot, Gujarat, India, 2011.
- Carrillo, J.L.M.; García, F.P.C.; Coronado, O.G.; García, M.A.M.; Cordero, J.F.C. Physiology and Pathology of Innate Immune Response Against Pathogens. In Physiology and Pathology of Immunology; IntechOpen: London, UK, 2017.
- Aribi, M. Introductory Chapter: Immune system dysfunction and autoimmune diseases. Immunopathog. Immune-Based Ther. Sel. Autoimmune Disord. 2017, 1.
- Maranduba, C.M.d.C.; De Castro, S.B.R.; Souza, G.T.d.; Rossato, C.; da Guia, F.C.; Valente, M.A.S.; Rettore, J.V.P.; Maranduba, C.P.; Souza, C.M.d.; Carmo, A.M.R.d. Intestinal microbiota as modulators of the immune system and neuroimmune system: Impact on the host health and homeostasis. J. Immunol. Res. 2015, 2015.
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552.
- Henrotin, Y.E.; Sanchez, C.; Deberg, M.A.; Piccardi, N.; Guillou, G.B.; Msika, P.; Reginster, J.-Y.L. Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J. Rheumatol. 2003, 30, 1825–1834.
- Kut-Lasserre, C.; Miller, C.C.; Ejeil, A.; Gogly, B.; Dridi, M.; Piccardi, N.; Guillou, B.; Pellat, B.; Godeau, G. Effect of avocado and soybean unsaponifiables on gelatinase A (MMP-2), stromelysin 1 (MMP-3), and tissue inhibitors of matrix metalloproteinase (TIMP-1 and TIMP-2) secretion by human fibroblasts in culture. J. Periodontol. 2001, 72, 1685–1694.
- Jablonska, S. Avocado/soybean unsaponifiables in the treatment of scleroderma: Comment on the article by Maheu et al. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1998, 41, 1705.
- Azadbakht, L.; Shakerhosseini, R.; Atabak, S.; Jamshidian, M.; Mehrabi, Y.; Esmaill-Zadeh, A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur. J. Clin. Nutr. 2003, 57, 1292.
- Anderson, G.H.; Li, E.; Anthony, S.P.; Ng, L.T.; Bialik, R. Dissociation between plasma and brain amino acid profiles and short-term food intake in the rat. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1994, 266, R1675–R1686.
- Zhao, J.-h.; Sun, S.-j.; Horiguchi, H.; Arao, Y.; Kanamori, N.; Kikuchi, A.; Oguma, E.; Kayama, F. A soy diet accelerates renal damage in autoimmune MRL/Mp-lpr/lpr mice. Int. Immunopharmacol. 2005, 5, 1601–1610.
- Zhu, C.; Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X.; Ma, X.; Gao, K.; Hu, Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015, 28, 288–294.
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-derived di-and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2011, 142, 363–368.
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal symptoms and their management. Endocrinol. Metab. Clin. 2015, 44, 497–515.
- Baker, F.C.; De Zambotti, M.; Colrain, I.M.; Bei, B. Sleep problems during the menopausal transition: Prevalence, impact, and management challenges. Nat. Sci. Sleep 2018, 10, 73.
- Kuh, D.; Muthuri, S.; Cooper, R.; Moore, A.; Mackinnon, K.; Cooper, C.; Adams, J.; Hardy, R.; Ward, K. Menopause, reproductive life, hormone replacement therapy, and bone phenotype at age 60–64 years: A British birth cohort. J. Clin. Endocrinol. Metab. 2016, 101, 3827–3837.
- Suthar, A.; Banavalikar, M.; Biyani, M. Pharmacological activities of Genistein, an isoflavone from soy (Glycine max): Part II—Anti-cholesterol activity, effects on osteoporosis & menopausal symptoms. IJEB 2001, 39.
- Levis, S.; Strickman-Stein, N.; Ganjei-Azar, P.; Xu, P.; Doerge, D.R.; Krischer, J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: A randomized, double-blind trial. Arch. Intern. Med. 2011, 171, 1363–1369.
- Panahi, Y.; Beiraghdar, F.; Kashani, N.; Javan, N.B. Comparison of piascledine (avocado and soybean oil) and hormone replacement therapy in menopausal-induced hot flashing. Iran. J. Pharm. Res. Ijpr 2011, 10, 941.
- Nair, P.A. Dermatosis associated with menopause. J. Mid-Life Health 2014, 5, 168.
- Akbari, T.N.; DAVOODABADI, F.M. COMPARATIVE EFFECTS OF FLAXSEED, SOY ON MENOPAUSAL HOT FLASHES. Complementary Med. J. Fac. Nurs. Midwifery Fall 2012, 2, 52–60.
- Lewis, J.E.; Nickell, L.A.; Thompson, L.U.; Szalai, J.P.; Kiss, A.; Hilditch, J.R. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause 2006, 13, 631–642.
- Fontvieille, A.; Dionne, I.; Riesco, E. Long-term exercise training and soy isoflavones to improve quality of life and climacteric symptoms. Climacteric 2017, 20, 233–239.
- Monroe, K.R.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Stanczyk, F.Z.; Adlercreutz, H.; Pike, M.C. Dietary fiber intake and endogenous serum hormone levels in naturally postmenopausal Mexican American women: The Multiethnic Cohort Study. HNUC 2007, 58, 127–135.
- Merkulova, D.; Onsin, A.; Merkulov, Y.A. Piascledin in the treatment of chronic dorsalgia. Zhurnal Nevrol. I Psikhiatrii Im. Ss Korsakova 2013, 113, 18–22.
- Kut, C.; Assoumou, A.; Dridi, M.; Bonnefoix, M.; Gogly, B.; Pellat, B.; Guillou, G.; Godeau, G. Morphometric analysis of human gingival elastic fibres degradation by human leukocyte elastase protective effect of avocado and soybean unsaponifiables (ASU). Pathol. -Biol. 1998, 46, 571–576.
- Alekseev, V.; Alekseev, A.; Gol’dzon, G. Nonspecific low-back pain: From symptomatic treatment to pathogenesis-based treatment. Zhurnal Nevrol. I Psikhiatrii Im. Ss Korsakova 2014, 114, 51–55.
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419.
- Oryan, A.; Mohammadalipour, A.; Moshiri, A.; Tabandeh, M.R. Avocado/soybean unsaponifiables: A novel regulator of cutaneous wound healing, modelling and remodelling. Int. Wound J. 2015, 12, 674–685.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.5K
Revisions:
2 times
(View History)
Update Date:
05 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




