Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jayalakshmi Subramanian | -- | 1365 | 2022-04-28 16:53:49 | | | |
| 2 | Catherine Yang | Meta information modification | 1365 | 2022-04-29 04:01:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Subramanian, J.; Farooq, S.A.; , .; Singh, A.; Irfan Ul Haq, M. Ceramic Nanostructured Coatings. Encyclopedia. Available online: https://encyclopedia.pub/entry/22458 (accessed on 08 February 2026).
Subramanian J, Farooq SA, , Singh A, Irfan Ul Haq M. Ceramic Nanostructured Coatings. Encyclopedia. Available at: https://encyclopedia.pub/entry/22458. Accessed February 08, 2026.
Subramanian, Jayalakshmi, Sheikh Aamir Farooq, , Arvind Singh, Mir Irfan Ul Haq. "Ceramic Nanostructured Coatings" Encyclopedia, https://encyclopedia.pub/entry/22458 (accessed February 08, 2026).
Subramanian, J., Farooq, S.A., , ., Singh, A., & Irfan Ul Haq, M. (2022, April 28). Ceramic Nanostructured Coatings. In Encyclopedia. https://encyclopedia.pub/entry/22458
Subramanian, Jayalakshmi, et al. "Ceramic Nanostructured Coatings." Encyclopedia. Web. 28 April, 2022.
Copy Citation
Ceramic nanocoatings are widely used in many applications such as engine valves, boiler parts, automotive body parts, orthopaedic implants, etc., due to their excellent resistance to corrosion, oxidation and wear, as compared to metals, especially in high-temperature applications. They also have excellent thermal and electrical insulation properties.
nanostructured coatings
nanomaterials
ceramics
corrosion
1. Alumina (Al2O3) Nanostructured Coatings
Alumina (Al2O3) is popular as ceramic coating material due to its excellent inherent resistance to corrosion and mechanical abrasion, and low electrical/thermal conductivity [1][2][3].
The synthesising route of Al2O3 nanocoatings has an influence on their anticorrosion performance. This is demonstrated in Ref. [4], where a comparison of the performance of nanocoatings produced by plasma-enhanced atomic layer deposition (ALD) with those produced via thermal-enhanced atomic layer deposition was made [4]. Al2O3 nanocoatings with thickness ranging from 10 to 50 nm were deposited on 100Cr6 steel and Al2024-T3 aluminium substrates. Nanocoatings produced by plasma-enhanced ALD were less porous due to their better film nucleation compared to thermal-enhanced ALD nanocoatings. It was observed that 10 nm thick nanocoatings produced by plasma-enhanced ALD remained intact over the substrates, whereas 10 nm thick nanocoatings produced by thermal-enhanced ALD showed poor adhesion and were detached from the substrates. Furthermore, it was identified that thickness of the nanocoatings affected their quality. Among the Al2O3 nanocoatings produced by both ALD techniques, the 50 nm thick nanocoatings were found to be the least porous on both the substrates [4]. Thicker Al2O3 nanocoatings (50 nm thickness) produced by both ALD techniques showed better resistance to corrosion due to their low porosity and strong adherence to substrates [4]. The presence of porosity and weak adhesion of nanocoatings to substrates are detrimental to corrosion resistance. Overall, it was concluded that nanocoatings deposited by plasma-enhanced ALD provide higher corrosion resistance, and that 50 nm thick nanocoatings produced by both ALD techniques provide the best corrosion resistance. A preliminary step that can improve the corrosion resistance of nanocoatings is their pre-treatment. Hydrogen–argon plasma pre-treatment of Al2O3 nanocoatings deposited on steel via both plasma-enhanced ALD and thermal-enhanced ALD and its effect on corrosion performance of the deposited nanocoatings demonstrated this fact [5]. Plasma pre-treatment and increased pre-treatment time improve corrosion resistance in the nanocoatings produced by both methods. The improvement was observed to be more pronounced in thermal-enhanced ALD coatings due to their enhanced adhesion to substrates and reduced porosity, imparted by the pre-treatment. Surface treatments such as pre-annealing of substrates before the deposition of nanocoatings results in the removal of heterogeneities, resulting in better formation of nanocoatings. Pre-annealing of copper substrates and subsequent deposition of 10–50 nm Al2O3 coatings by ALD [6] has shown enhancement in the corrosion resistance of the nanocoatings. In the ALD process, it has been observed that the deposition temperature influences the corrosion behaviour of nanoceramic coatings. The corrosion resistance of Al2O3 nanocoating deposited on 316 L stainless steel with ALD at the temperature of 250 °C was found to be superior to that of the coating deposited at 160 °C. Deposition at higher temperature improves the coating’s sealing effectiveness, i.e., reduces porosity and thereby improves corrosion resistance [7]. Certain carbon steels, on the other hand, require lower deposition temperatures to avoid adverse effects on their microstructure. Al2O3 nanocoating deposited on 100Cr6 carbon steel at 160 °C using the ALD process requires a nanocoating thickness of >10 nm to achieve effective sealing and avoid corrosion [8]. In view of the above discussion, it can be concluded that the synthesis parameters have a vital role in determining the properties of the nanocoating.
2. Titanium Oxide (TiO2) Nanostructured Coatings
Titanium oxide (TiO2) is a popular ceramic material known for its resistance to corrosion and mechanical abrasion [9], photocatalysis [10], protection against UV [11] and self-cleaning property [12].
A factor that controls the corrosion resistance of ceramic nanocoatings is the size of nanoceramic particles. Corrosion studies of TiO2 nanocoating on carbon steel substrate showed improved corrosion resistance with the reduction in the size of nano-TiO2 particles [13]. Corrosion rates of nanocoatings with 10 nm, 50 nm, 100 nm and 150 nm TiO2 particle sizes in 1 M H2SO4 solution (determined using polarisation and electrochemical impedance spectroscopy), deposited on carbon steel surfaces, revealed that these nanocoatings prevent corrosion. However, it was found that the physical adhesion of the nanocoatings to the substrate surface depends on the nanoparticle size—the smaller the particle size, the better the coating–substrate interface bonding. Corrosion resistance of nanocoatings improved with the reduction in the size of nano-TiO2 particles primarily due to decreased O2 and H2O permeability into nanocoatings [13]. The addition of graphene oxide (GO) to TiO2 nanocoatings has shown a significant improvement in the anticorrosion performance of the nanocoatings. Nanocomposite TiO2/GO (graphene oxide) ceramic coating produced for a cast iron pipeline showed a remarkable 94% reduction in corrosion rates compared to bare substrate in seawater. This is mainly due to the reduced porosity and capacitance of the coating [14]. Another factor that controls corrosion behaviour of TiO2 nanocoatings is their thickness. Figure 1 shows scanning electron microscope (SEM) images of an uncoated AA2024 aluminium alloy substrate, and a substrate with TiO2 nanocoatings deposited at time durations of 40 s and 80 s [15]. The TiO2 nanocoating with greater thickness achieved by longer deposition time had the best corrosion resistance, with current density lower by one order of magnitude.
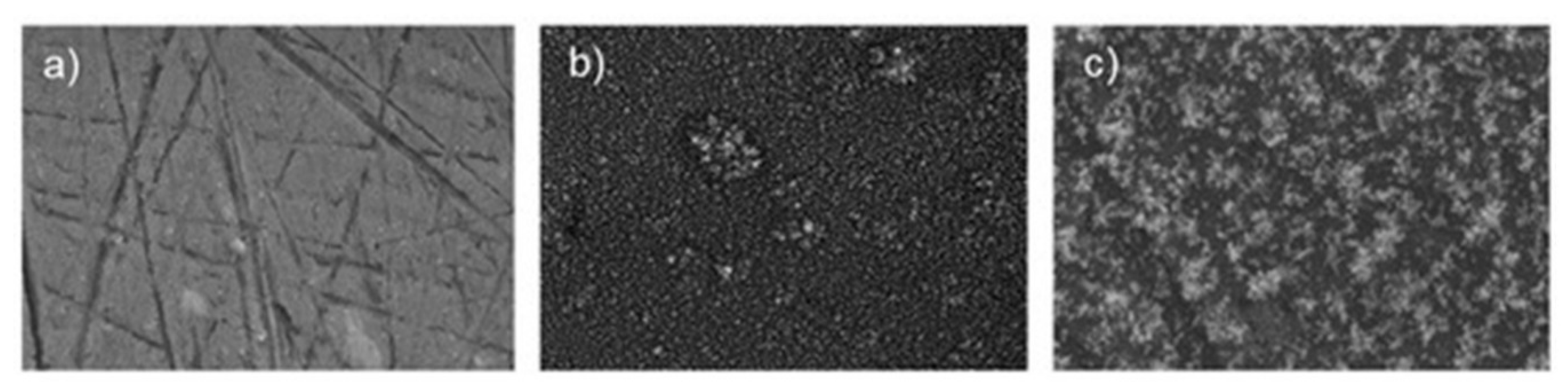
Figure 1. SEM images of (a) uncoated AA2024 sample, (b) TiO2-coated AA2024 with deposition time of 40 s and (c) TiO2-coated AA2024 with deposition time of 80 s [15].
3. Tantalum Pentoxide (Ta2O5) Nanostructured Coatings
Tantalum pentoxide (Ta2O5) ceramic is an excellent material for making corrosion-resistant nanocoatings [16]. Ta2O5 has high hardness [17] and high resistance to chemical attack under extreme environments [18]. Due to its high dielectric constant (~25), it is used in capacitors for automobile electronics, high-speed tools and cell phones [19][20][21].
β-Ta2O5 nanoceramic nanocoating on a Ti-6Al-4V alloy substrate enhanced corrosion resistance in 3.5 wt % NaCl due to the formation of a stable passive oxide layer [22]. A comparative corrosion performance of filtered cathodic arc deposited (FCAD) tantalum oxide (Ta2O5) and chromium oxide (Cr2O3) nanocoatings on 100Cr6 steel substrate showed that the substrate coated with Ta2O5 nanocoating exhibited improved corrosion resistance than that coated with Cr2O3 nanocoating [18]. The deposition method also influenced corrosion performance, such that FCAD Ta2O5 nanocoatings exhibited nearly four times higher corrosion resistance than that of the nanocoatings deposited by ALD. The spurious interfacial oxide layer generated in ALD coating increases voids at the interface, coating degradation and dissolution of the coating. In the FCAD process, however, the native oxide layer is removed by pre-etching substrate surfaces by ion bombardment prior to actual oxide growth (passive oxide coating) [23].
4. Tantalum Nitride (Ta2N) Nanostructured Coatings
Tantalum nitride (Ta2N) deposited over Ti-6Al-4V bipolar plates using the reactive sputter deposition method [24] was studied for its corrosion properties in the simulated polymer electrolyte membrane fuel cell environment, with varying pH values and temperatures. The Ta2N nanocoated substrate had significantly higher corrosion resistance when compared to the uncoated Ti-6Al-4V at any particular pH or temperature value. An increase in acidity showed a reduction in corrosion resistance [24]. Investigation of nanocoatings on Ti-6Al-4V for biomaterial applications has shown that Ta2N nanocoating exhibited better corrosion resistance in Ringer’s physiological solution at 37 °C with lower Icorr values when compared to both pure Ta and bare Ti-6Al-4V, as can be seen from Figure 2 [25].
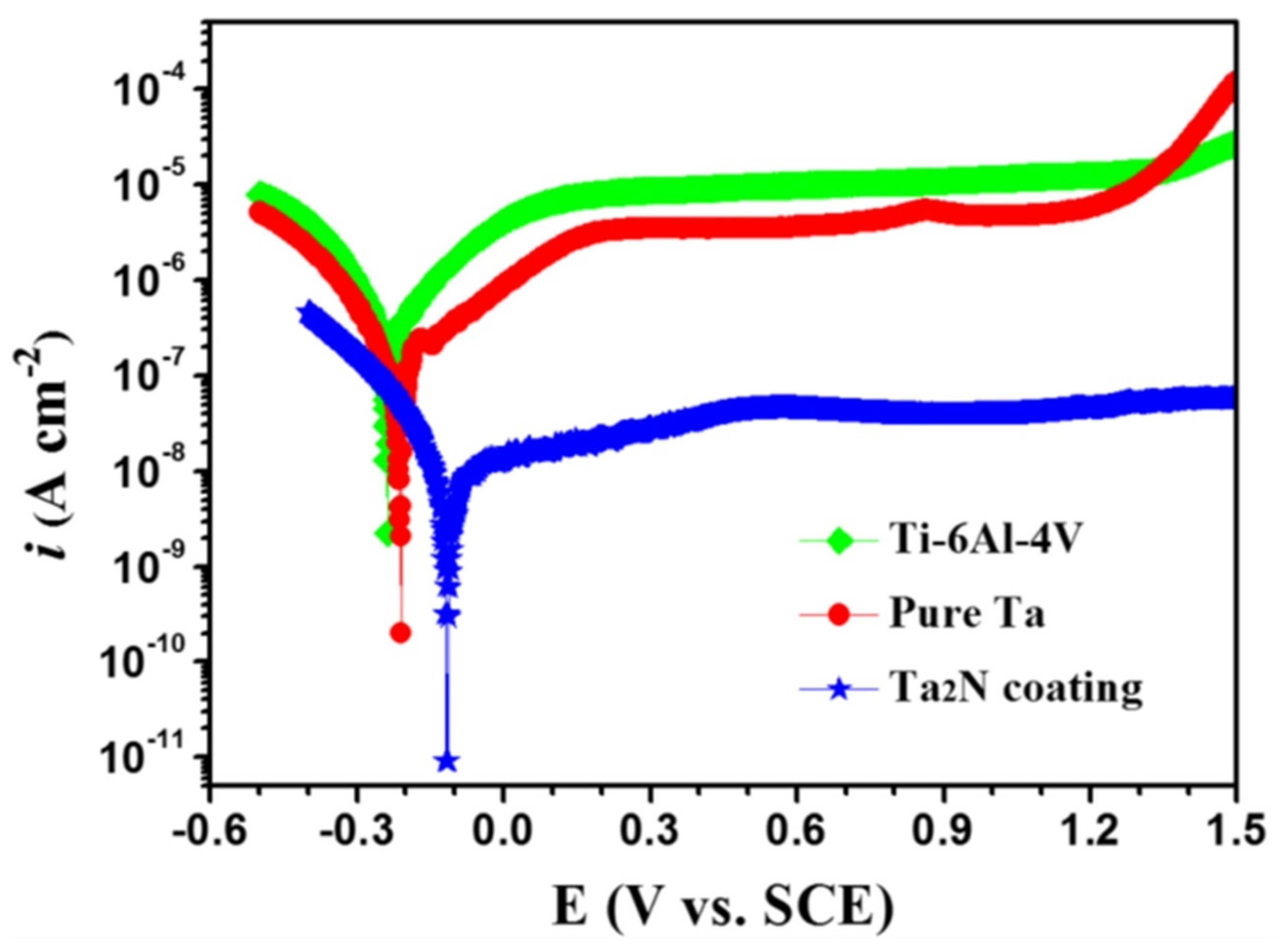
Figure 2. Potentiodynamic polarisation curves of bare Ti-6Al-4V, pure Ta and Ta2N coating [25].
Tantalum-based nanocoatings of Ta2O5, Ta3N5 and TaON (tantalum oxynitride) deposited on 306 stainless steel [26] showed enhancement in their corrosion performance by impeding the corrosion current density due to the formation of a passive film. Corrosion rates reduced by almost 50% when compared to the bare stainless steel and pure tantalum-coated stainless steel samples. TaON (tantalum oxynitride) demonstrated the best corrosion resistance among the Ta-based nanocoatings, followed by Ta2O5 (tantalum pentoxide) and Ta3O5 (tantalum nitride). The better corrosion resistance of TaON was attributed to its hydrophobic nature, aided by its texture. Furthermore, it was observed that the anticorrosive nature of the TaON nanocoating was influenced by the morphological, chemical and electrical properties of the deposited film. Thus, the TaON nanocoating significantly reduced the corrosion current density, resulting in enhanced anticorrosive behaviour [26].
References
- Piwoński, I.; Soliwoda, K. The effect of ceramic nanoparticles on tribological properties of alumina sol–gel thin coatings. Ceram. Int. 2010, 36, 47–54.
- Li, H.; Cui, Y.; Li, Z.; Zhu, Y.; Wang, H. Fabrication of microcapsules containing dual-functional tung oil and properties suitable for self-healing and self-lubricating coatings. Prog. Org. Coat. 2018, 115, 164–171.
- Mersagh Dezfuli, S.; Sabzi, M. A study on the effect of presence of CeO2 and benzotriazole on activation of self-healing mechanism in ZrO2 ceramic-based coating. Int. J. Appl. Ceram. Technol. 2018, 15, 1248–1260.
- Potts, S.E.; Schmalz, L.; Fenker, M.; Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Radnóczi, G.; Tóth, L. Ultra-thin aluminium oxide films deposited by plasma-enhanced atomic layer deposition for corrosion protection. J. Electrochem. Soc. 2011, 158, C132.
- Härkönen, E.; Potts, S.E.; Kessels, W.M.M.; Díaz, B.; Seyeux, A.; Światowska, J.; Maurice, V.; Marcus, P.; Radnóczi, G.; Tóth, L.; et al. Hydrogen–argon plasma pre-treatment for improving the anti-corrosion properties of thin Al2O3 films deposited using atomic layer deposition on steel. Thin Solid Film. 2013, 534, 384–393.
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Salmi, E.; Ritala, M.; Marcus, P. The role of surface preparation in corrosion protection of copper with nanometer-thick ALD alumina coatings. Appl. Surf. Sci. 2016, 387, 1054–1061.
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523.
- Díaz, B.; Härkönen, E.; Światowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Ritala, M. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175.
- Shen, G.X.; Chen, Y.C.; Lin, L.; Lin, C.J.; Scantlebury, D. Study on a hydrophobic nano-TiO2 coating and its properties for corrosion protection of metals. Electrochim. Acta 2005, 50, 5083–5089.
- Lorencik, S.; Yu, Q.L.; Brouwers, H.J.H. Photocatalytic coating for indoor air purification: Synergetic effect of photocatalyst dosage and silica modification. Chem. Eng. J. 2016, 306, 942–952.
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959.
- Giolando, D.M. Transparent self-cleaning coating applicable to solar energy consisting of nano-crystals of titanium dioxide in fluorine doped tin dioxide. Sol. Energy 2016, 124, 76–81.
- Deyab, M.A.; Keera, S.T. Effect of nano-TiO2 particles size on the corrosion resistance of alkyd coating. Mater. Chem. Phys. 2014, 146, 406–411.
- Ammar, A.U.; Shahid, M.; Ahmed, M.K.; Khan, M.; Khalid, A.; Khan, Z.A. Electrochemical Study of Polymer and Ceramic-Based Nanocomposite Coatings for Corrosion Protection of Cast Iron Pipeline. Materials 2018, 11, 332.
- De Falco, G.; de Filippis, G.; Scudieri, C.; Vitale, L.; Commodo, M.; Minutolo, P.; D’Anna, A.; Ciambelli, P. Nano-TiO2 Coating Layers with Improved Anticorrosive Properties by Aerosol Flame Synthesis and Thermophoretic Deposition on Aluminium Surfaces. Materials 2021, 14, 2918.
- Fairbrother, F. The Chemistry of Niobium and Tantalum; Elsevier: Amsterdam, The Netherlands, 1967.
- Rahmati, B.; Sarhan AA, D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480.
- Díaz, B.; Światowska, J.; Maurice, V.; Pisarek, M.; Seyeux, A.; Zanna, S.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Chromium and tantalum oxide nanocoatings prepared by filtered cathodic arc deposition for corrosion protection of carbon steel. Surf. Coat. Technol. 2012, 206, 3903–3910.
- Xu, H.; Jiang, Y.; Fan, X.; Wang, Y.; Liu, G. Growth and dielectric properties of Ta2O5 single crystal by the floating zone method. Cryst. Res. Technol. 2012, 47, 903–908.
- Habibi, M.H.; Mokhtari, R. Novel sulfur-doped niobium pentoxide nanoparticles: Fabrication, characterization, visible light sensitization and redox charge transfer study. J. Sol-Gel Sci. Technol. 2011, 59, 352–357.
- Chaluvaraju, B.V.; Ganiger, S.K.; Murugendrappa, M.V. Synthesis, Characterization and DC Conductivity Studies of Polypyrrole/Tantalum Pentoxide Composites. Int. J. Latest Technol. Eng. 2014, 3, 33–36.
- Hu, W.; Xu, J.; Lu, X.; Hu, D.; Tao, H.; Munroe, P.; Xie, Z.-H. Corrosion and wear behaviours of a reactive-sputter-deposited Ta2O5 nanoceramic coating. Appl. Surf. Sci. 2016, 368, 177–190.
- Díaz, B.; Światowska, J.; Maurice, V.; Seyeux, A.; Härkönen, E.; Ritala, M.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Tantalum oxide nanocoatings prepared by atomic layer and filtered cathodic arc deposition for corrosion protection of steel: Comparative surface and electrochemical analysis. Electrochim. Acta 2013, 90, 232–245.
- Ma, J.J.; Xu, J.; Jiang, S.; Munroe, P.; Xie, Z.-H. Effects of pH value and temperature on the corrosion behavior of a Ta2N nanoceramic coating in simulated polymer electrolyte membrane fuel cell environment. Ceram. Int. 2016, 42, 16833–16851.
- Cheng, J.; Xu, J.; Liu, L.L.; Jiang, S. Electrochemical Corrosion Behavior of Ta2N Nanoceramic Coating in Simulated Body Fluid. Materials 2016, 9, 772.
- Hirpara, J.; Chawla, V.; Chandra, R. Anticorrosive Behavior Enhancement of Stainless Steel 304 through Tantalum-Based Coatings: Role of Coating Morphology. J. Mater. Eng. Perform. 2021, 30, 1895–1905.
More
Information
Subjects:
Materials Science, Ceramics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
29 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




