
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucia Malaguarnera | -- | 2985 | 2022-04-27 10:52:39 | | | |
| 2 | Camila Xu | + 260 word(s) | 3245 | 2022-04-27 11:00:12 | | |
Video Upload Options
Skeletal muscle dysfunction is frequently associated with chronic obstructive pulmonary disease (COPD), which is characterized by a permanent airflow limitation, with a worsening respiratory disorder during disease evolution. COPD is a progressive lung disease, characterized by an irreversible airflow limitation. In COPD, the pathophysiological changes related to the chronic inflammatory state affect oxidant–antioxidant balance, which is one of the main mechanisms accompanying extra-pulmonary comorbidity such as muscle wasting. Muscle impairment is characterized by alterations on muscle fiber architecture, contractile protein integrity, and mitochondrial dysfunction. Vitamin D deficiency affects oxidative stress and mitochondrial function influencing disease course through an effect on muscle function in COPD patients.
1. Introduction
2. Mechanisms Mediating Muscular Wasting in COPD Patients
3. Vitamin D Metabolism and Biological Function in the Muscle
4. Vitamin D Deficiency and Muscle Weakness
5. Role of Vitamin D in Anti-Oxidative Mechanisms Implicated in Muscular Wasting in COPD Patients
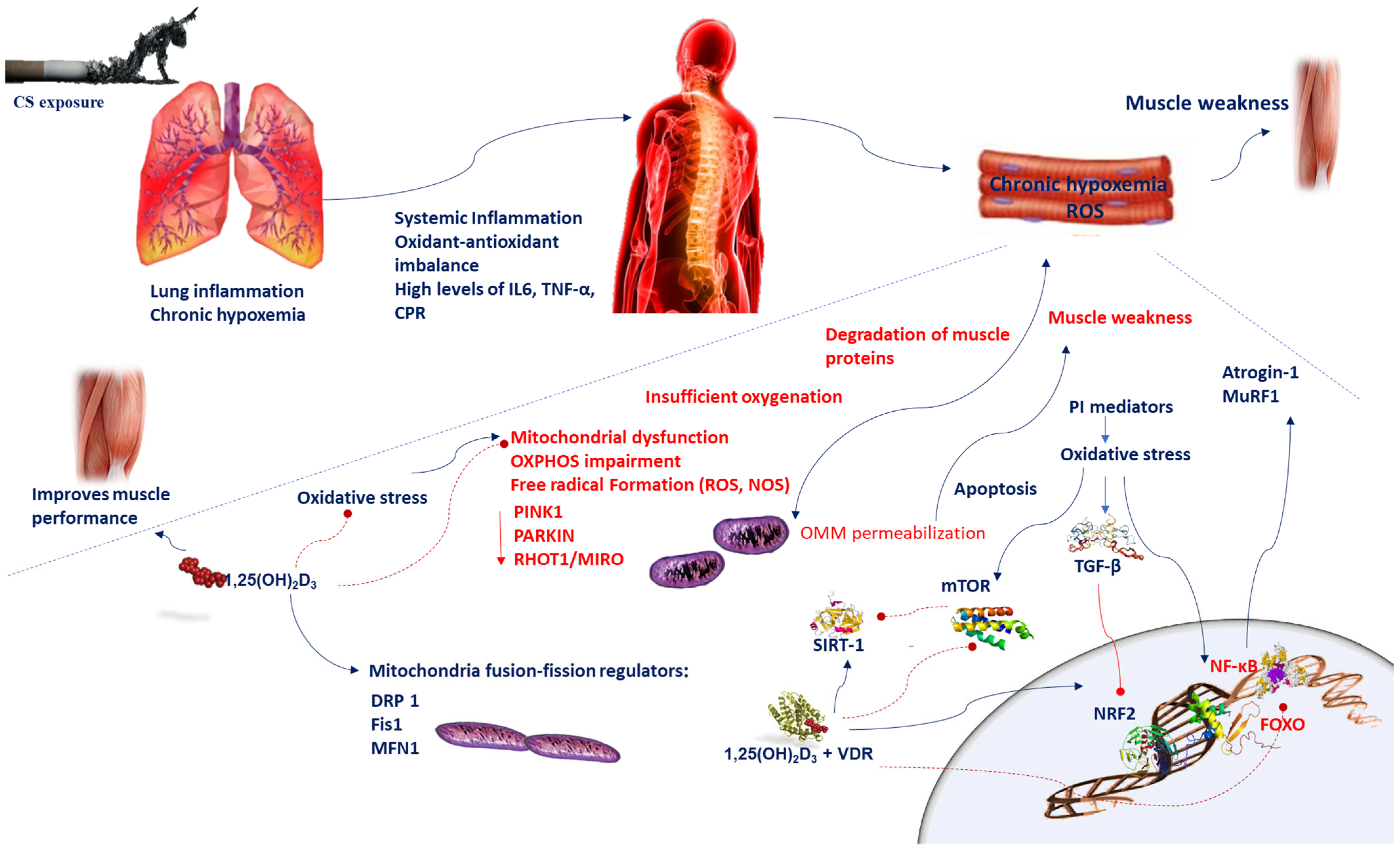 Figure 1. Chronic hypoxemia contributes to inflammation, which generates mitochondrial dysfunction, impairments in mitochondrial turnover, and oxidant–antioxidant imbalance. Reduced respiration causes insufficient oxygenation and mitochondrial dysfunction, which in turn leads to alteration of mitochondrial OXPHOS and increases ROS levels. A disproportionate stimulation of fission induces mitochondrial dysfunction. Reduction in the mitochondrial fusion factor OPA1 impairs the mitochondrial network and promotes apoptosis. Blockade of fission Fis1 or Drp1 inhibits mitochondrial fragmentation. Oxidative stress triggers the TGF-β signaling pathway, which induces inhibitory effect on Nrf2, which in turn inhibits endogenous antioxidants. Oxidative stress induces cellular senescence via FOXO transcription factors and decreases SIRT-1 expression and enzyme activity; ROS activate the PI3K-mTOR pathway. Vitamin D supplementation prevents the mitochondrial dysfunction and oxidative stress by setting MFN1/2, OPA1, and Drp1 expression. Oxidative stress activates NF-κB and FOXO pathways which influences muscle wasting in COPD patients. Vitamin D and VDR represses NF-κB and modulates the post-translational modification and function of FoxO proteins. The beneficial effects of SIRT-1 on mitochondrial function are regulated by vitamin D, which acts by increasing SIRT-1 formation. Abbreviations: CPR = C-reactive protein; Drp1 = dynamin-related protein 1; 1,25(OH)2D3 = 1,25-dihydroxyvitamin D3; Fis1 = fission protein 1; FOXO = forkhead box O; IL-6 = interleukin-6; MFN1/2 = mitofusin-1/2; mTOR = mammalian target of rapamycin; MuRF1 = muscle-specific RING finger protein 1;NOS = nitrogen species; Nrf2 = nuclear factor erythroid 2-related factor 2; NF-κB = nuclear factor kappa; OPA1 = optic atrophy protein 1; OXPHOS = oxidative phosphorylation; PI3K = phosphatidylinositol-3-kinase; ROS = reactive oxygen species; RHOT-1/MIRO = Ras homolog family member T-1; TGF-β = transforming growth factor-beta; TNF-α = tumor necrosis-alpha; sirtuin-1 = SIRT-1; VDR = vitamin D receptor.
Figure 1. Chronic hypoxemia contributes to inflammation, which generates mitochondrial dysfunction, impairments in mitochondrial turnover, and oxidant–antioxidant imbalance. Reduced respiration causes insufficient oxygenation and mitochondrial dysfunction, which in turn leads to alteration of mitochondrial OXPHOS and increases ROS levels. A disproportionate stimulation of fission induces mitochondrial dysfunction. Reduction in the mitochondrial fusion factor OPA1 impairs the mitochondrial network and promotes apoptosis. Blockade of fission Fis1 or Drp1 inhibits mitochondrial fragmentation. Oxidative stress triggers the TGF-β signaling pathway, which induces inhibitory effect on Nrf2, which in turn inhibits endogenous antioxidants. Oxidative stress induces cellular senescence via FOXO transcription factors and decreases SIRT-1 expression and enzyme activity; ROS activate the PI3K-mTOR pathway. Vitamin D supplementation prevents the mitochondrial dysfunction and oxidative stress by setting MFN1/2, OPA1, and Drp1 expression. Oxidative stress activates NF-κB and FOXO pathways which influences muscle wasting in COPD patients. Vitamin D and VDR represses NF-κB and modulates the post-translational modification and function of FoxO proteins. The beneficial effects of SIRT-1 on mitochondrial function are regulated by vitamin D, which acts by increasing SIRT-1 formation. Abbreviations: CPR = C-reactive protein; Drp1 = dynamin-related protein 1; 1,25(OH)2D3 = 1,25-dihydroxyvitamin D3; Fis1 = fission protein 1; FOXO = forkhead box O; IL-6 = interleukin-6; MFN1/2 = mitofusin-1/2; mTOR = mammalian target of rapamycin; MuRF1 = muscle-specific RING finger protein 1;NOS = nitrogen species; Nrf2 = nuclear factor erythroid 2-related factor 2; NF-κB = nuclear factor kappa; OPA1 = optic atrophy protein 1; OXPHOS = oxidative phosphorylation; PI3K = phosphatidylinositol-3-kinase; ROS = reactive oxygen species; RHOT-1/MIRO = Ras homolog family member T-1; TGF-β = transforming growth factor-beta; TNF-α = tumor necrosis-alpha; sirtuin-1 = SIRT-1; VDR = vitamin D receptor.| Experimental Data | Model | Sample Size | Tissue | Approach | Reference |
|---|---|---|---|---|---|
| Evaluation of oxygen consumption, biogenesis, dynamics, and nuclear genes encoding variations. | Human | // | Muscle biopsies. Primary human skeletal muscle cells. |
Supplementation of 1α,25-Dihydroxyvitamin D3 (10-8 M) for 48 h. VDR expression in human muscle cells and skeletal muscle homogenates. Effect of 1α,25(OH)2D mitochondrial oxygen consumption and in expression of: mitochondrial proteins that alter mitochondrial fusion; proteins associated with mitochondrial fission; phosphorylated pyruvate dehydrogenase and pyruvate dehydrogenase kinase 4; genes encoding mitochondrial proteins; and genes encoding cellular signaling and growth-regulatory pathways in adult human skeletal muscle cells. Knockdown of VDR with silencing RNA in skeletal muscle cells to detect the effects of 1α,25(OH)2D3 on OCR. |
[45] |
| Assessment of oxidative and nitrosative stress parameters. | Rat | // | Fasting blood samples analysis. C2C12 cell culture. | Supplementation of 1,25(OH)2D3 (1 nM and 10 nM) for 24 h. Effect of VDD in muscle oxidative stress in a rat model. Pre/post treatment of C2C12 muscle cells with vitamin D offers protection against oxidative stress induced muscle proteolysis. VDD increase in activities of the glutathione-dependent enzymes and decrease in SOD and catalase enzymes in the rat muscle. Pre/post treatment of C2C12 muscle cells with vitamin D correct total protein degradation, 20S proteasomal enzyme activity, muscle atrophy gene markers and expression of proteasome subunit genes induced by oxidative stress. |
[51] |
| Serum and lung tissue analysis. | Human | 180 COPD patients | Human lung tissues and serum samples of COPD. | Level of pulmonary VDR-positive nuclei between COPD patients and control subjects Correlations of pulmonary function with pulmonary DJ-1, Nrf-2 and VDR in COPD patients. |
[57] |
| Antioxidant and antiaging effects of 1,25Dihydroxyvitamin D by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. | Mouse | 120 | Skin, lung, liver, kidney, and spleen. | Two different supplementation: thrice weekly of 2.2 IU vitamin D/g or 1,25(OH)2D3 (1 μg/kg) until death. Effects of a high-calcium/phosphate diet, of 1,25(OH)2D3, and of antioxidant supplementation on lifespan, body weight, skin morphology; on oxidative stress, DNA damage, protein expression of oncogenes and tumor suppressive genes; and on cell proliferation and senescence in 1α(OH)ase−/− mice. |
[58] |
| Improvement in parameters of mitochondrial function in vitamin-D-deficient individuals after vitamin D supplementation. | Human | 12 subjects with severe vitamin D deficiency | Serum samples | Effect of cholecalciferol therapy (20 000 IU supplementation on alternate days for 10–12 weeks) in muscle mitochondrial maximal oxidative phosphorylation after exercise in symptomatic, vitamin-D-deficient individuals. | [60] |
| FOXO1 activation in the skeletal muscle of global VDR-null mice. | Mouse | VDR−/− mice administered a diet enriched with calcium and phosphorus; SMVDR−/− mice generated by crossing VDRloxp/loxp mice with mice with muscle-specific Cre recombinase expression under the control of the myosin light chain 1f (MLC 1f) genomic locus; C2C12 muscle cells. | Treatment of C2C12 muscle cells with 1,25-dihydroxyvitamin D (100 nM for 48 h) to detect FOXO1 expression, nuclear translocation, and activity. Evaluation of FOXO1 activation in knockdown VDR mice. | [61] | |
| Effect of vitamin D supplementation on oxidative stress. | Mouse | Eight mice for each experimental group. | Adipocyte cell culture model | Supplementation of cholecalciferol (67 IU VD/kg daily for last 8 weeks) to detect the effects of 1,25(OH)2D3 supplementation in NOX4, Nrf2 SIRT-1 expression, ROS production, NF-κB and AMPK phosphorylation. | [62] |
References
- Barnes, P.J.; Burney, P.G.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076.
- Rabe, K.F.; Halpin, D.M.G.; Han, M.K.; Miravitlles, M.; Singh, D.; Grönke, L.; Voß, F.; Martinez, F.J. Composite endpoints in COPD: Clinically important deterioration in the UPLIFT trial. Respir. Res. 2020, 21, 177.
- Dumitru, L.; Iliescu, A.; Dinu, H.; Badea, R.; Savulescu, S.; Huidu, S.; Berteanu, M. Disability in COPD and Chronic Heart Failure Is the Skeletal Muscle the Final Common Pathway? Maedica (Bucur) 2013, 8, 206–213.
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62.
- Natanek, S.A.; Gosker, H.R.; Slot, I.G.; Marsh, G.S.; Hopkinson, N.S.; Man, W.D.; Tal-Singer, R.; Moxham, J.; Kemp, P.R.; Schols, A.M.; et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (Copd); implications for stratified medicine? Muscle Nerve 2013, 48, 488–497.
- Jaitovich, A.; Barreiro, E. Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. What We Know and Can Do for Our Patients. Am. J. Respir. Crit. Care Med. 2018, 198, 175–186.
- Khan, D.M.; Ullah, A.; Randhawa, F.A.; Iqtadar, S.; Butt, N.F.; Waheed, K. Role of Vitamin D in reducing number of acute exacerbations in Chronic Obstructive Pulmonary Disease (COPD) patients. Pak. J. Med. Sci. 2017, 33, 610–614.
- Li, X.; He, J.; Yu, M.; Sun, J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020, 9, 286–297.
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345.
- Redzic, M.; Lewis, R.M.; Thomas, D.T. Relationship between 25-hydoxyvitamin D, muscle strength, and incidence of injury in healthy adults: A systematic review. Nutr. Res. 2013, 33, 251–258.
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498.
- Hornikx, M.; Van Remoortel, H.; Lehouck, A.; Mathieu, C.; Maes, K.; Gayan-Ramirez, G.; Decramer, M.; Troosters, T.; Janssens, W. Vitamin D supplementation during rehabilitation in COPD: A secondary analysis of a randomized trial. Respir. Res. 2012, 13, 84.
- Rezk, N.A.S.A.; Aly, N.Y.A.; Hewidy, A.A.H. Effect of vitamin D replacement in chronic obstructive pulmonary disease patients with vitamin D deficiency. Egypt J. Chest Dis. Tuberc. 2015, 64, 353–357.
- Heulens, N.; Korf, H.; Janssens, W. Innate immune modulation in chronic obstructive pulmonary disease: Moving closer toward vitamin D therapy. J. Pharmacol. Exp. Ther. 2015, 353, 360–368.
- Casabona, A.; Valle, M.S.; Laudani, L.; Crimi, C.; Russo, C.; Malaguarnera, L.; Crimi, N.; Cioni, M. Is the Power Spectrum of Electromyography Signal a Feasible Tool to Estimate Muscle Fiber Composition in Patients with COPD? J. Clin. Med. 2021, 10, 3815.
- Sharanya, A.; Ciano, M.; Withana, S.; Kemp, P.R.; Polkey, M.I.; Sathyapala, S.A. Sex differences in COPD-related quadriceps muscle dysfunction and fibre abnormalities. Chron. Respir. Dis. 2019, 16, 1479973119843650.
- Beauchamp, M.K.; Sibley, K.M.; Lakhani, B.; Romano, J.; Mathur, S.; Goldstein, R.S.; Brooks, D. Impairments in systems underlying control of balance in COPD. Chest 2012, 141, 1496–1503.
- Nantsupawat, N.; Lane, P.; Siangpraipunt, O.; Gadwala, S.; Nugent, K. Gait Characteristics in Patients with Chronic Obstructive Pulmonary Disease. J. Prim. Care Community Health. 2015, 6, 222–226.
- Valle, M.S.; Casabona, A.; Di Fazio, E.; Crimi, C.; Russo, C.; Malaguarnera, L.; Crimi, N.; Cioni, M. Impact of chronic obstructive pulmonary disease on passive viscoelastic components of the musculoarticular system. Sci. Rep. 2021, 11, 18077.
- De Boer, M.D.; Selby, A.; Atherton, P.; Smith, K.; Seynnes, O.R.; Maganaris, C.N.; Maffulli, N.; Movin, T.; Narici, M.V.; Rennie, M.J. The temporal responses of protein synthesis, gene expression and cell signaling in human quadriceps muscle and patellar tendon to disuse. J. Physiol. 2007, 585, 241–251.
- Swallow, E.B.; Reyes, D.; Hopkinson, N.S.; Man, W.D.; Porcher, R.; Cetti, E.J.; Moore, A.J.; Moxham, J.; Polkey, M.I. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007, 62, 115–120.
- Lakhdar, R.; Rabinovich, R.A. Can muscle protein metabolism be specifically targeted by nutritional support and exercise training in chronic obstructive pulmonary disease? J. Thorac. Dis. 2018, 10 (Suppl. S12), S1377–S1389.
- Nishiki, K.; Nojiri, M.; Kato, R.; Shinomiya, S.; Oikawa, T.; Ishizaki, T.; Toga, H.; Mizuno, S. Serum Creatinine/Cystatin C Ratio Associated with Cross-Sectional Area of Erector Spinae Muscles and Pulmonary Function in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2021, ume 16, 3513–3524.
- Owens, D.J.; Sharples, A.P.; Polydorou, I.; Alwan, N.; Donovan, T.; Tang, J.; Fraser, W.D.; Cooper, R.G.; Morton, J.P.; Stewart, C.; et al. A systems-based investigation into vitamin D and skeletal muscle repair, regeneration, and hypertrophy. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1019–E1031.
- Valle, M.S.; Russo, C.; Casabona, A.; Crimi, N.; Crimi, C.; Colaianni, V.; Cioni, M.; Malaguarnera, L. Anti-inflammatory role of vitamin D in muscle dysfunctions of patients with COPD: A comprehensive review. Minerva Med. 2022.
- Pojednic, R.M.; Ceglia, L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc. Sport Sci. Rev. 2014, 42, 76–81.
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80 (Suppl. S6), 1689S–1696S.
- Ginde, A.A.; Wolfe, P.; Jr Camargo, C.A.; Schwartz, R.S. Defining vitamin D status by secondary hyperparathyroidism in the U.S. population. J. Endocrinol. Investig. 2012, 35, 42–48.
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective role of vitamin D against oxidative stress in diabetic retinopathy. Diabetes Metab. Res. Rev. 2021, 37, e3447.
- Olsson, K.; Saini, A.; Strömberg, A.; Alam, S.; Lilja, M.; Rullman, E.; Gustafsson, T. Evidence for Vitamin D Receptor Expression and Direct Effects of 1α,25(OH)2D3 in Human Skeletal Muscle Precursor Cells. Endocrinology 2016, 157, 98–111.
- Bellido, T.; Boland, R. Effects of 1,25-dihydroxy-vitamin D3 on phosphate accumulation by myoblasts. Horm. Metab. Res. 1991, 23, 113–116, Erratum in Horm. Metab. Res. 1991, 23, 356.
- Garcia, L.A.; King, K.K.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 2011, 152, 2976–2986.
- Pfeifer, M.; Begerow, B.; Minne, H.W. Vitamin D and muscle function. Osteoporos Int. 2002, 13, 187–194.
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78.
- Shuler, F.D.; Wingate, M.K.; Moore, G.H.; Giangarra CGiangarra, C. Sports health benefits of vitamin d. Sports Health 2012, 4, 496–501.
- Ranathunga, R.M.T.K.; Hill, T.R.; Mathers, J.C.; Francis, R.M.; Prentice, A.; Schoenmakers, I.; Aspray, T.J. Vitamin D in Older People Study group. No effect of monthly supplementation with 12000 IU, 24000 IU or 48000 IU vitamin D3 for one year on muscle function: The vitamin D in older people study. J. Steroid. Biochem Mol. Biol. 2019, 190, 256–262.
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 5, 1253–1275.
- Tomlinson, P.B.; Joseph, C.; Angioi, M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J. Sci. Med. Sport. 2015, 18, 575–580.
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633.
- Sakai, S.; Suzuki, M.; Tashiro, Y.; Tanaka, K.; Takeda, S.; Aizawa, K.; Hirata, M.; Yogo, K.; Endo, K. Vitamin D receptor signaling enhances locomotive ability in mice. J. Bone Miner. Res. 2015, 30, 128–136.
- Van Schoor, N.M.; de Jongh, R.T.; Daniels, J.M.; Heymans, M.W.; Deeg, D.J.; Lips, P. Peak expiratory flow rate shows a gender-specific association with vitamin D deficiency. J. Clin. Endocrinol. Metab. 2012, 97, 2164–2171.
- Girgis, C.M.; Cha, K.M.; Houweling, P.J.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.J.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610.
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003, 144, 5138–5144.
- Romme, E.A.; Rutten, E.P.; Smeenk, F.W.; Spruit, M.A.; Menheere, P.P.; Wouters, E.F. Vitamin D status is associated with bone mineral density and functional exercise capacity in patients with chronic obstructive pulmonary disease. Ann. Med. 2013, 45, 91–96.
- Ryan, Z.C.; Craig, T.A.; Folmes, C.D.; Wang, X.; Lanza, I.R.; Schaible, N.S.; Salisbury, J.L.; Nair, K.S.; Terzic, A.; Sieck, G.C.; et al. 1α,25-Dihydroxyvitamin D3 Regulates Mitochondrial Oxygen Consumption and Dynamics in Human Skeletal Muscle Cells. J. Biol. Chem. 2016, 291, 514–528.
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345.
- Jackson, A.S.; Shrikrishna, D.; Kelly, J.L.; Kemp, S.V.; Hart, N.; Moxham, J.; Polkey, M.I.; Kemp, P.; Hopkinson, N.S. Vitamin D and skeletal muscle strength and endurance in COPD. Eur. Respir. J. 2013, 41, 309–316.
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839.
- Modesti, L.; Danese, A.; Angela Maria Vitto, V.; Ramaccini, D.; Aguiari, G.; Gafà, R.; Lanza, G.; Giorgi, C.; Pinton, P. Mitochondrial Ca2+Signaling in Health, Disease and Therapy. Cells 2021, 10, 1317.
- Gomes, L.C.; Scorrano, L. Mitochondrial morphology in mitophagy and macroautophagy. Biochim. Biophys. Acta 2013, 1833, 205–212.
- Bhat, M.; Ismail, A. Vitamin D treatment protects against and reverses oxidative stress induced muscle proteolysis. J. Steroid. Biochem. Mol. Biol. 2015, 152, 171–179.
- Van der Meijden, K.; Bravenboer, N.; Dirks, N.F.; Heijboer, A.C.; den Heijer, M.; de Wit, G.M.; Offringa, C.; Lips, P.; Jaspers, R.T. Effects of 1,25(OH)2 D3 and 25(OH)D3 on C2C12 Myoblast Proliferation, Differentiation, and Myotube Hypertrophy. J. Cell. Physiol. 2016, 231, 2517–2528.
- Seldeen, K.L.; Berman, R.N.; Pang, M.; Lasky, G.; Weiss, C.; MacDonald, B.A.; Thiyagarajan, R.; Redae, Y.; Troen, B.R.; Vitamin, D. Insufficiency Reduces Grip Strength, Grip Endurance and Increases Frailty in Aged C57Bl/6J Mice. Nutrients 2020, 12, 3005.
- Chen, Y.; Li, Q.; Liu, Y.; Shu, L.; Wang, N.; Wu, Y.; Sun, X.; Wang, L. Attenuation of hyperoxia-induced lung injury in neonatal rats by 1α,25-Dihydroxyvitamin D3. Exp. Lung Res. 2015, 41, 344–352.
- Ke, C.Y.; Yang, F.L.; Wu, W.T.; Chung, C.H.; Lee, R.P.; Yang, W.T.; Subeq, Y.M.; Liao, K.W. Vitamin D3 Reduces Tissue Damage and Oxidative Stress Caused by Exhaustive Exercise. Int. J. Med. Sci. 2016, 13, 147–153.
- Srikuea, R.; Hirunsai, M.; Charoenphandhu, N. Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Sci. Rep. 2020, 10, 8239.
- Xiang, Y.; Fu, L.; Xiang, H.X.; Zheng, L.; Tan, Z.X.; Wang, L.X.; Cao, W.; Xu, D.X.; Zhao, H. Correlations among Pulmonary DJ-1, VDR and Nrf-2 in patients with Chronic Obstructive Pulmonary Disease: A Case-control Study. Int. J. Med. Sci. 2021, 18, 2449–2456.
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019, 18, e12951.
- Mathyssen, C.; Aelbrecht, C.; Serré, J.; Everaerts, S.; Maes, K.; Gayan-Ramirez, G.; Vanaudenaerde, B.; Janssens, W. Local expression profiles of vitamin D-related genes in airways of COPD patients. Respir. Res. 2020, 21, 137.
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513.
- Chen, S.; Villalta, S.A.; Agrawal, D.K. FOXO1 Mediates Vitamin D Deficiency-Induced Insulin Resistance in Skeletal Muscle. J. Bone Miner. Res. 2016, 31, 585–595.
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34.




