
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masashi Tanaka | -- | 1490 | 2022-04-25 12:34:14 | | | |
| 2 | Jason Zhu | -1 word(s) | 1489 | 2022-04-26 04:48:46 | | |
Video Upload Options
Layered metal nitride halides MNX (M = Ti, Zr, Hf; X = Cl, Br, I) have two polymorphs, including α- and β-forms, which have the FeOCl and SmSI structures, respectively. These compounds are band insulators and become metals and show superconductivity after electron doping by intercalating alkali metals between the layers. The superconductivity of β-form had been extensively characterized from decades ago, but it is not easy to consistently interpret all experimental results using conventional phonon-mediated Bardeen–Cooper–Schriefer mechanisms. The titanium compound TiNCl crystallizes only in the α-form structure. TiNCl also exhibits superconductivity as high as ~16 K after electron doping by intercalating metals and/or organic basis. It is important to compare the superconductivity of different M–N networks. However, α-form compounds are vulnerable to moisture, unlike β-form ones. The intercalation compounds are even more sensitive to humid air.
1. Introduction
2. Micro-PES of Electron-Doped TiNCl
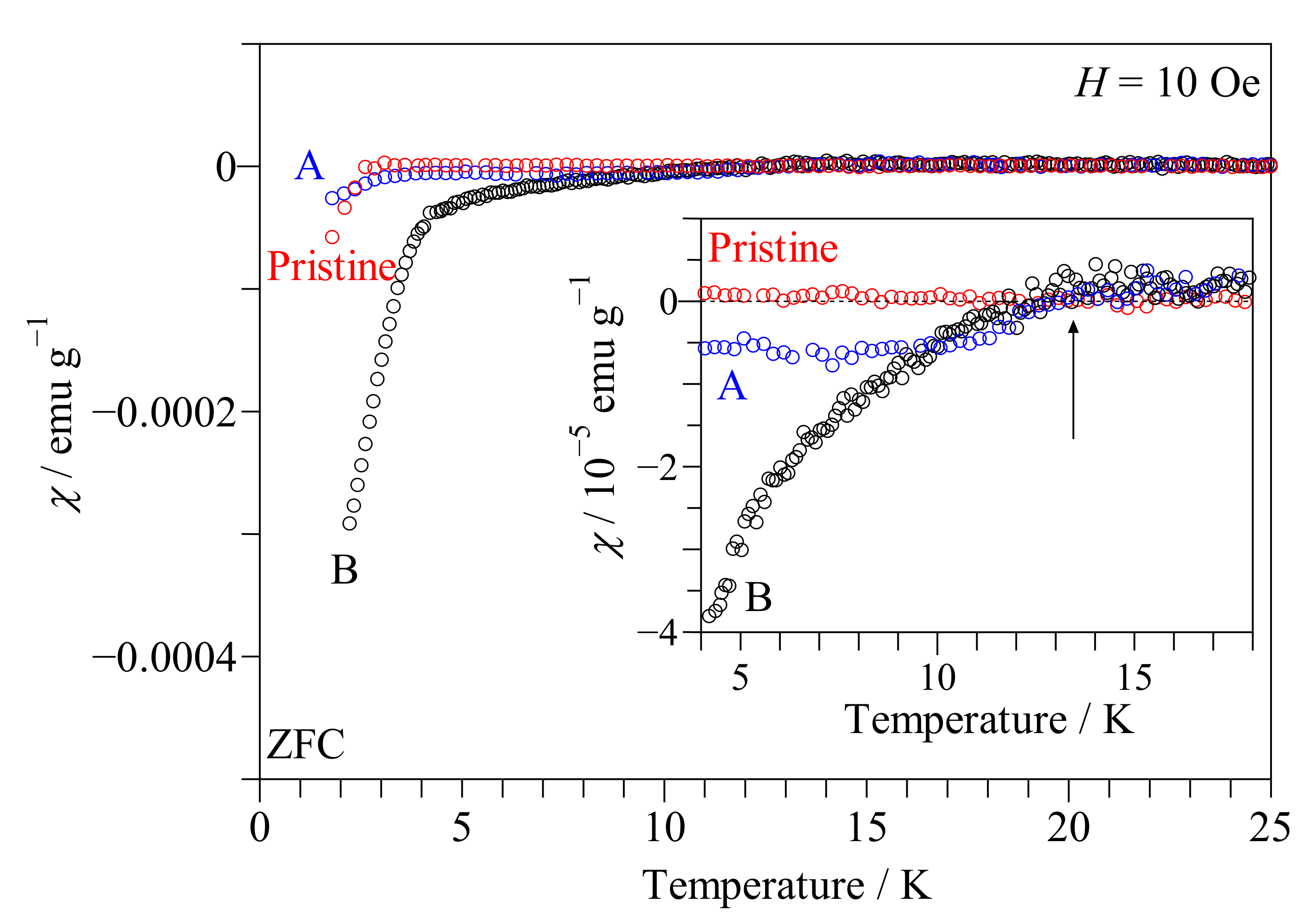
3. Metalization of TiNCl Induced by Soft X-ray Irradiation
References
- Yamanaka, S.; Yasunaga, T.; Yamaguchi, K.; Tagawa, M. Structure and superconductivity of the intercalation compounds of TiNCl with pyridine and alkali metals as intercalants. J. Mater. Chem. 2009, 19, 2573.
- Yin, Q.; Ylvisaker, E.R.; Pickett, W.E. Spin and charge fluctuations in α-structure layered nitride superconductors. Phys. Rev. B 2011, 83, 014509.
- Felser, C.; Seshadri, R. Electronic structures and instabilities of ZrNCl and HfNCl: Implications for superconductivity in the doped compounds. J. Mater. Chem. 1999, 9, 459–464.
- Kuroki, K. Spin-fluctuation-mediated d+id’ pairing mechanism in doped β-MNCl (M=Hf, Zr) superconductors. Phys. Rev. B 2010, 81, 104502.
- Kusakabe, K. Pair-hopping mechanism of superconductivity activated by the nano-space layered structure. J. Phys. Chem. Solids 2012, 73, 1546–1549.
- Kasahara, Y.; Kuroki, K.; Yamanaka, S.; Taguchi, Y. Unconventional superconductivity in electron-doped layered metal nitride halides MNX (M = Ti, Zr, Hf; X = Cl, Br, I). Physica C 2015, 514, 354–367.
- Yin, Z.P.; Kutepov, A.; Kotliar, G. Correlation-Enhanced Electron-Phonon Coupling: Applications of GW and Screened Hybrid Functional to Bismuthates, Chloronitrides, and Other High-Tc Superconductors. Phys. Rev. X 2013, 3, 021011.
- Sugimoto, A.; Sakai, Y.; Ekino, T.; Zhang, S.; Tanaka, M.; Yamanaka, S.; Gabovich, A.M. Scanning Tunnelling Microscopy and Spectroscopy of the Layered Nitride Superconductor α-NaxTiNCl. Phys. Procedia 2016, 81, 73–76.
- Sugimoto, A.; Shohara, K.; Ekino, T.; Zheng, Z.; Yamanaka, S. Nanoscale electronic structure of the layered nitride superconductors α-KxTiNCl and β-HfNCl y observed by scanning tunneling microscopy and spectroscopy. Phys. Rev. B 2012, 85, 144517.
- Yokoya, T.; Ishiwata, Y.; Shin, S.; Shamoto, S.; Iizawa, K.; Kajitani, T.; Hase, I.; Takahashi, T. Changes of electronic structure across the insulator-to-metal transition of quasi-two-dimensional Na-intercalated β-HfNCl studied by photoemission and X-ray absorption. Phys. Rev. B 2001, 64, 153107.
- Takeuchi, T.; Tsuda, S.; Yokoya, T.; Tsukamoto, T.; Shin, S.; Hirai, A.; Shamoto, S.; Kajitani, T. Soft X-ray emission and high-resolution photoemission study of quasi-two-dimensional superconductor NaxHfNCl. Physica C 2003, 392–396, 127–129.
- Yokoya, T.; Takeuchi, T.; Tsuda, S.; Kiss, T.; Higuchi, T.; Shin, S.; Iizawa, K.; Shamoto, S.; Kajitani, T.; Takahashi, T. Valence-band photoemission study of β-ZrNCl and the quasi-two-dimensional superconductor NaxZrNCl. Phys. Rev. B 2004, 70, 193103.
- Ino, A.; Yamazaki, K.; Yamasaki, T.; Higashiguchi, M.; Shimada, K.; Namatame, H.; Taniguchi, M.; Oguchi, T.; Chen, X.; Yamanaka, S. Angle-resolved-photoemission study of layer-structured nitride β-HfNCl. J. Electron Spectros. Relat. Phenom. 2005, 144–147, 667–669.
- Kataoka, N.; Terashima, K.; Tanaka, M.; Hosoda, W.; Taniguchi, T.; Wakita, T.; Muraoka, Y.; Yokoya, T. µ-PES Studies on TiNCl and Quasi-two-dimensional Superconductor Na-intercalated TiNCl. J. Phys. Soc. Jpn. 2019, 88, 104709.
- Tanaka, M.; Kataoka, N.; Matsumoto, R.; Inumaru, K.; Takano, Y.; Yokoya, T. Synthetic Route of Layered Titanium Nitride Chloride TiNCl Using Sodium Amide. ACS Omega 2022, 7, 6375–6380.
- Zhu, L.; Ohashi, M.; Yamanaka, S. Zirconium nitride derived from layer-structured β-ZrNCl by deintercalation of chlorine layers. Chem. Mater. 2002, 14, 4517–4521.
- Saeki, Y.; Matsuzaki, R.; Yajima, A.; Akiyama, M. Reaction Process of Titanium Tetrachloride with Ammonia in the Vapor Phase and Properties of the Titanium Nitride Formed. Bull. Chem. Soc. Jpn. 1982, 55, 3193–3196.
- Sosnov, E.A.; Malkov, A.A.; Malygin, A.A. Chemical transformations at the silica surface upon sequential interactions with titanium tetrachloride and ammonia vapors. Russ. J. Gen. Chem. 2015, 85, 2533–2540.
- Hegde, R.I.; Fiordalice, R.W.; Tobin, P.J. TiNCl formation during low-temperature, low-pressure chemical vapor deposition of TiN. Appl. Phys. Lett. 1993, 62, 2326–2328.
- Kataoka, N.; Tanaka, M.; Hosoda, W.; Taniguchi, T.; Fujimori, S.; Wakita, T.; Muraoka, Y.; Yokoya, T. Soft X-ray irradiation induced metallization of layered TiNCl. J. Phys. Condens. Matter 2021, 33, 035501.




