
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emmanuel Jairaj Moses | -- | 1224 | 2022-04-21 06:06:43 | | | |
| 2 | Emmanuel Jairaj Moses | + 1 word(s) | 1225 | 2022-04-21 06:08:56 | | | | |
| 3 | Catherine Yang | -31 word(s) | 1194 | 2022-04-21 06:15:39 | | | | |
| 4 | Catherine Yang | + 31 word(s) | 1225 | 2022-04-22 10:31:09 | | | | |
| 5 | Catherine Yang | Meta information modification | 1225 | 2022-04-22 10:32:08 | | | | |
| 6 | Catherine Yang | Meta information modification | 1225 | 2022-04-22 10:35:58 | | | | |
| 7 | Catherine Yang | -31 word(s) | 1194 | 2022-04-22 10:38:50 | | |
Video Upload Options
Vascularization is another hallmark of cancer, whereby cancer cells promote the formation of blood vessels to deliver nutrients for fast-growing solid tumors. The most well-known process of vascularization is angiogenesis. In normal cells and tissues, the angiogenesis is a controlled process that is turned on or off depending on the needs of the cells; however, in cancerous cells and tumors, the angiogenesis process is continuous and there is a dysregulation of pro- and antiangiogenesis factors . This continuous activation of angiogenesis allows the cancer cells to form blood vessels to obtain sufficient nutrients for continuous growth and proliferation. There are other ways tumors can achieve vascularization, such as vascular co-option, intussusceptive microvascular growth and vasculogenic mimicry.
1. Vascularization Mechanisms in Cancer Cells
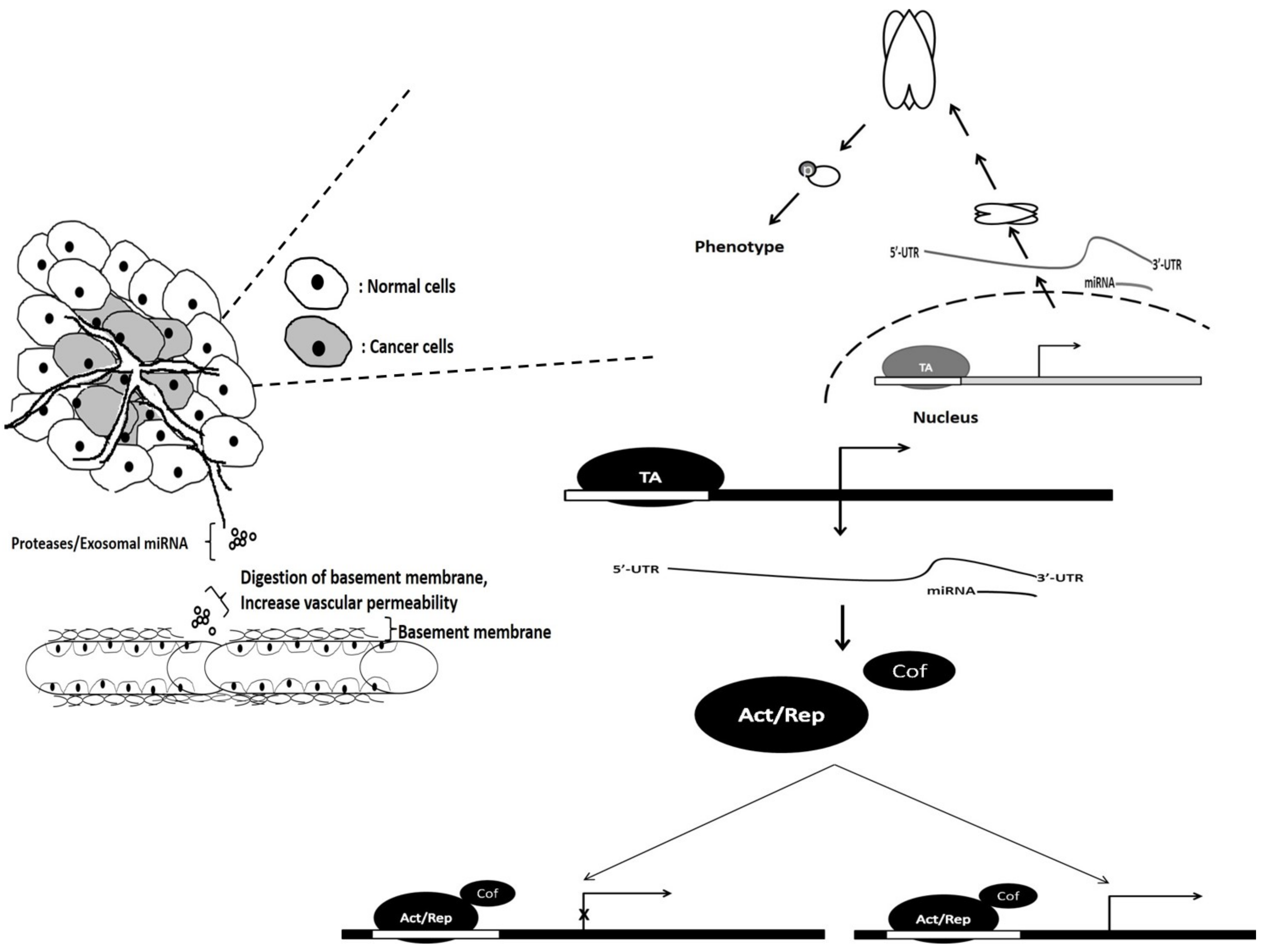
2. The Role of miRNAs in the Vascularization of Cancer Cells
| No | miRNA | Cancer | Target | Action | Reference |
|---|---|---|---|---|---|
| 1 | miR-124-3p | Glioblastoma | NRP-1, transcriptional | Overexpression leads to the attenuation of angiogenesis | [14] |
| 2 | miR-526b/miR-655 | Breast cancer | PTEN tumor suppressor, transcriptional | Overexpression improved angiogenesis suggesting roles as oncomiR via PTEN-regulated HIF1-α pathway | [15] |
| 3 | miR-9 | Nasopharyngeal Carcinoma | MDK, exosomal secretion | Suppression of miR-9 in patient suggest its role as oncomiR. Overexpression attenuated tubal formation HUVECs | [16] |
| 4 | miR-205 | Ovarian Cancer | PTEN tumor suppressor, exosomal secretion | Treatment of HUVECs with miR-205 exosome leads to an increase in tubal formation | [17] |
| 5 | miR-6868-5p | Colorectal Cancer | FOXM1, transcriptional | Overexpression leads to the reduction in endothelial tubal formation | [18] |
| 6 | miR-143-3p | Gallbladder Carcinoma | ITGA6, transcriptional | Suppression was observed in bad overall survival patients. Overexpression leads to increased tubal formation | [19] |
| 7 | miR-130b | Prostate cancer | TNF-α, transcriptional | Inhibition leads to attenuation of VEGFA, a downstream target of TNF-α suppressing angiogenesis | [20] |
| 8 | mR-23a | Nasopharyngeal Carcinoma | TSGA10, exosomal secretion | Exosomal overexpression enhanced angiogenesis | [21] |
| 9 | miR-21 | Renal cell carcinoma | PCD4, proteomal | Inhibition of miR-21 attenuated MMP levels, besides inhibiting angiogenesis | [22] |
| 10 | miR-574-5p | Gastric Cancer Cells | PTPN3 proteomal | Binds to PTPN3, enhancing ERK/JNK activity and driving angiogenesis | [23] |
| 11 | miR-27a | Pancreatic Cancer | BTG2, Exosomal | miR-27a was highly expressed in cancer tissue. Exosomal mir-27a stimulates HMVEC tubal formation. | [24] |
| 12 | miR-155 | Gastric Carcinoma | C-MYB/, Exosomal | Stimulates VEGF expression, leading to enhanced angiogenesis observed on HUVEC | [25] |
| 13 | miR-183-5p | Colorectal Cancer | FOXO1, Exosomal | CRC-derived- exosome enhanced tubal formation of HMEC-1 cells | [26] |
| 14 | miR-619-5p | Non-Small Cell Lung Cancer | RCAN1.4, Exosomal | Mimic transfection and leads to the increase in HUVEC tube length and tube abundance | [27] |
| 15 | miR-3064-5p | Hepatocellular carcinoma | FOXA1, transcriptional | Overexpression improves overall survival of mice and reduces tumor size; angiogenic factor suppression observed | [28] |
| 16 | miR-141 | Pancreatic cancer | TM5SF1 transcriptional | Angiogenic factors were induced following inhibition of miR-141 | [29] |
| 17 | miR-195 | Squamous cell lung cancer | VEGF transcriptional | miRNA-195 attenuates tubal formation | [30] |
| 18 | miR-136 | Gall Bladder cancer | MAP2K4 transcriptional | Mimic treatment resulted in activation of angiopoiesis | [31] |
| 19 | miR-302 | Chronic Myeloid leukemia | VEGFA, secretome | Low expression was associated with bad OS. Treatment of K562 media on HUVECS attenuate capillary formation |
[32] |
| 20 | miR-148a miR-30 |
Glioblastoma | FIH1 | Regulates HIF1-α via binding directly to its inhibitor FIH1 and attenuating vascularization | [11] |
| 21 | miR-29b | Breast cancer | AKT3 | Overexpression resulted in the attenuation of vascularization by downregulating AKT3, which is crucial for VEGF activation | [33] |
| 22 | miR-140-5p | Breast cancer | VEGFA | Abrogates vascularization by binding and attenuating VEGFA | [34] |
| 23 | miR-1 | Gastric cancer | VEGFA | Inhibition of miR-1 leads to accumulation of VEGFA | [35] |
| 24 | miR-30d | Prostate cancer | MYPT1 | Downregulation resulted in the attenuation of angiogenesis, leading to reduction in endothelial capillary tube formation | [36] |
| 25 | miR-210 | Hepatocellular carcinoma | SMAD4, STAT6 | Promote angiogenesis by inhibiting SMAD4 and STAT6 | [13] |
References
- Doktorova, H.; Hrabeta, J.; Khalil, M.A.; Eckschlager, T. Hypoxia-induced chemoresistance in cancer cells: The role of not only HIF-1. Biomed. Pap. 2015, 159, 166–177.
- Lu, Y.; Qin, T.; Li, J.; Wang, L.; Zhang, Q.; Jiang, Z.; Mao, J. MicroRNA-140-5p inhibits invasion and angiogenesis through targeting VEGF-A in breast cancer. Cancer Gene Ther. 2017, 24, 386–392.
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 1–13.
- Batty, M.; Pugh, R.; Rathinam, I.; Simmonds, J.; Walker, E.; Forbes, A.; Anoopkumar-Dukie, S.; McDermott, C.M.; Spencer, B.; Christie, D.; et al. The Role of α1-Adrenoceptor Antagonists in the Treatment of Prostate and Other Cancers. Int. J. Mol. Sci. 2016, 17, 1339.
- Chang, Y.-C.; Chan, Y.-C.; Chang, W.-M.; Lin, Y.-F.; Yang, C.-J.; Su, C.-Y.; Huang, M.-S.; Wu, A.T.; Hsiao, M. Feedback regulation of ALDOA activates the HIF-1α/MMP9 axis to promote lung cancer progression. Cancer Lett. 2017, 403, 28–36.
- Li, Y.-Y.; Zheng, Y.-L. Hypoxia promotes invasion of retinoblastoma cells in vitro by upregulating HIF-1α/MMP9 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5361–5369.
- Oren, R.; Addadi, S.; Haziza, L.N.; Dafni, H.; Rotkopf, R.; Meir, G.; Fishman, A.; Neeman, M. Fibroblast recruitment as a tool for ovarian cancer detection and targeted therapy. Int. J. Cancer 2016, 139, 1788–1798.
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in In Vitro models of retinoblastoma. BMC Cancer 2017, 17, 434.
- Gómez-Escudero, J.; Clemente, C.; García-Weber, D.; Acín-Pérez, R.; Millán, J.; Enríquez, J.A.; Bentley, K.; Carmeliet, P.; Arroyo, A.G. PKM2 regulates endothelial cell junction dynamics and angiogenesis via ATP production. Sci. Rep. 2019, 9, 1–18.
- Roux, Q.; Gavard, J. Endothelial Cell-Cell Junctions in Tumor Angiogenesis. In Tumor Angiogenesis Key Target for Cancer Therapy; Springer: Cham, Switzerland, 2019; pp. 91–119.
- Wong, H.-K.A.; El Fatimy, R.; Onodera, C.; Wei, Z.; Yi, M.; Mohan, A.; Gowrisankaran, S.; Karmali, P.; Marcusson, E.; Wakimoto, H.; et al. The Cancer Genome Atlas Analysis Predicts MicroRNA for Targeting Cancer Growth and Vascularization in Glioblastoma. Mol. Ther. 2015, 23, 1234–1247.
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 1–14.
- Lin, X.-J.; Fang, J.-H.; Yang, X.-J.; Zhang, C.; Yuan, Y.; Zheng, L.; Zhuang, S.-M. Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo. Mol. Ther.-Nucleic Acids 2018, 11, 243–252.
- Zhang, G.; Chen, L.; Khan, A.A.; Li, B.; Gu, B.; Lin, F.; Su, X.; Yan, J. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int. J. Cancer 2018, 143, 635–644.
- Hunter, S.; Nault, B.; Ugwuagbo, K.; Maiti, S.; Majumder, M. Mir526b and Mir655 Promote Tumour Associated Angiogenesis and Lymphangiogenesis in Breast Cancer. Cancers 2019, 11, 938.
- Lu, J.; Liu, Q.-H.; Wang, F.; Tan, J.-J.; Deng, Y.-Q.; Peng, X.-H.; Liu, X.; Zhang, B.; Xu, X.; Li, X.-P. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 1–12.
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220.
- Wang, Y.; Wu, M.; Lei, Z.; Huang, M.; Li, Z.; Wang, L.; Cao, Q.; Han, D.; Chang, Y.; Chen, Y.; et al. Dysregulation of miR-6868-5p/FOXM1 circuit contributes to colorectal cancer angiogenesis. J. Exp. Clin. Cancer Res. 2018, 37, 292.
- Jin, Y.-P.; Hu, Y.-P.; Wu, X.-S.; Wu, Y.-S.; Ye, Y.-Y.; Li, H.-F.; Liu, Y.-C.; Jiang, L.; Liu, F.-T.; Zhang, Y.-J.; et al. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018, 9, 1–15.
- Mu, H.Q.; He, Y.H.; Wang, S.B.; Yang, S.; Wang, Y.J.; Nan, C.J.; Bao, Y.F.; Xie, Q.P.; Chen, Y.H. MiR-130b/TNF-α/NF-κB/VEGFA loop inhibits prostate cancer angiogenesis. Clin. Transl. Oncol. 2020, 22, 111–121.
- Bao, L.; You, B.; Shi, S.; Shan, Y.; Zhang, Q.; Yue, H.; Zhang, J.; Zhang, W.; Shi, Y.; Liu, Y.; et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018, 37, 2873–2889.
- Fan, B.; Jin, Y.; Zhang, H.; Zhao, R.; Sun, M.; Sun, M.; Yuan, X.; Wang, W.; Wang, X.; Chen, Z.; et al. MicroRNA-21 contributes to renal cell carcinoma cell invasiveness and angiogenesis via the PDCD4/c-Jun (AP-1) signalling pathway. Int. J. Oncol. 2020, 56, 178–192.
- Zhang, S.; Zhang, R.; Xu, R.; Shang, J.; He, H.; Yang, Q. MicroRNA-574-5p in gastric cancer cells promotes angiogenesis by targeting protein tyrosine phosphatase non-receptor type 3 (PTPN3). Gene 2020, 733, 144383.
- Shang, D.; Xie, C.; Hu, J.; Tan, J.; Yuan, Y.; Liu, Z.; Yang, Z. Pancreatic cancer cell–derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J. Cell. Mol. Med. 2020, 24, 588–604.
- Deng, T.; Zhang, H.; Yang, H.; Wang, H.; Bai, M.; Sun, W.; Wang, X.; Si, Y.; Ning, T.; Zhang, L.; et al. Exosome miR-155 Derived from Gastric Carcinoma Promotes Angiogenesis by Targeting the c-MYB/VEGF Axis of Endothelial Cells. Mol. Ther.-Nucleic Acids 2020, 19, 1449–1459.
- Shang, A.; Wang, X.; Gu, C.; Liu, W.; Sun, J.; Zeng, B.; Chen, C.; Ji, P.; Wu, J.; Quan, W.; et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging 2020, 12, 8352–8371.
- Kim, D.H.; Park, S.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Sung, K.J.; Sung, Y.H.; Choi, C.-M.; Yun, M.; Yi, Y.-S.; et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020, 475, 2–13.
- Zhang, P.; Ha, M.; Li, L.; Huang, X.; Liu, C. MicroRNA-3064-5p sponged by MALAT1 suppresses angiogenesis in human hepatocellular carcinoma by targeting the FOXA1/CD24/Src pathway. FASEB J. 2020, 34, 66–81.
- Xu, D.; Yang, F.; Wu, K.; Xu, X.; Zeng, K.; An, Y.; Xu, F.; Xun, J.; Lv, X.; Zhang, X.; et al. Lost miR-141 and upregulated TM4SF1 expressions associate with poor prognosis of pancreatic cancer: Regulation of EMT and angiogenesis by miR-141 and TM4SF1 via AKT. Cancer Biol. Ther. 2020, 21, 354–363.
- Liu, H.; Chen, Y.; Li, Y.; Li, C.; Qin, T.; Bai, M.; Zhang, Z.; Jia, R.; Su, Y.; Wang, C. miR-195 suppresses metastasis and angiogenesis of squamous cell lung cancer by inhibiting the expression of VEGF. Mol. Med. Rep. 2019, 20, 2625–2632.
- Niu, J.; Li, Z.; Li, F. Overexpressed microRNA-136 works as a cancer suppressor in gallbladder cancer through suppression of JNK signaling pathway via inhibition of MAP2K4. Am. J. Physiol. Liver Physiol. 2019, 317, G670–G681.
- Cao, J.; Li, L.; Han, X.; Cheng, H.; Chen, W.; Qi, K.; Chen, C.; Wu, Q.; Niu, M.; Zeng, L.; et al. miR-302 cluster inhibits angiogenesis and growth of K562 leukemia cells by targeting VEGFA. OncoTargets Ther. 2019, 12, 433–441.
- Li, Y.; Cai, B.; Shen, L.; Dong, Y.; Lu, Q.; Sun, S.; Liu, S.; Ma, S.; Ma, P.X.; Chen, J. MiRNA-29b suppresses tumor growth through simultaneously inhibiting angiogenesis and tumorigenesis by targeting Akt3. Cancer Lett. 2017, 397, 111–119.
- Lu, Y.; Yu, S.-S.; Zong, M.; Fan, S.-S.; Lu, T.-B.; Gong, R.-H.; Sun, L.-S.; Fan, L.-Y. Glucose-6-Phosphate Isomerase (G6PI) Mediates Hypoxia-Induced Angiogenesis in Rheumatoid Arthritis. Sci. Rep. 2017, 7, 40274.
- Xie, M.; Dart, D.A.; Guo, T.; Xing, X.-F.; Cheng, X.-J.; Du, H.; Jiang, W.G.; Wen, X.-Z.; Ji, J.-F. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer 2017, 21, 41–54.
- Lin, Z.-Y.; Chen, G.; Zhang, Y.-Q.; He, H.-C.; Liang, Y.-X.; Ye, J.-H.; Liang, Y.-K.; Mo, R.-J.; Lu, J.-M.; Zhuo, Y.-J.; et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol. Cancer 2017, 16, 1–14.





