Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, M.; , .; Kimchi, E.; Staveley-O'carroll, K.; Li, G. Gut Microbiota Interaction-Derived Metabolites and NAFLD. Encyclopedia. Available online: https://encyclopedia.pub/entry/22027 (accessed on 07 February 2026).
Yang M, , Kimchi E, Staveley-O'carroll K, Li G. Gut Microbiota Interaction-Derived Metabolites and NAFLD. Encyclopedia. Available at: https://encyclopedia.pub/entry/22027. Accessed February 07, 2026.
Yang, Ming, , Eric Kimchi, Kevin Staveley-O'carroll, Guangfu Li. "Gut Microbiota Interaction-Derived Metabolites and NAFLD" Encyclopedia, https://encyclopedia.pub/entry/22027 (accessed February 07, 2026).
Yang, M., , ., Kimchi, E., Staveley-O'carroll, K., & Li, G. (2022, April 20). Gut Microbiota Interaction-Derived Metabolites and NAFLD. In Encyclopedia. https://encyclopedia.pub/entry/22027
Yang, Ming, et al. "Gut Microbiota Interaction-Derived Metabolites and NAFLD." Encyclopedia. Web. 20 April, 2022.
Copy Citation
Gut microbiota-derived components and metabolites play pivotal roles in shaping intrahepatic immunity during the progression of nonalcoholic fatty liver disease (NAFLD) or nonalcoholic steatohepatitis (NASH). With the advance of techniques, such as single-cell RNA sequencing (scRNA-seq), each subtype of immune cells in the liver has been studied to explore their roles in the pathogenesis of NAFLD. In addition, new molecules involved in gut microbiota-mediated effects on NAFLD are found.
NAFLD
NASH
gut microbiota
metabolite
1. Gut Microbiota-Derived Metabolites in the Pathogenesis NAFLD and NASH
Lipopolysaccharides (LPS), a major component of Gram-negative bacterial cell membrane, plays a pivotal in the pathogenesis of mouse and human nonalcoholic fatty liver disease (NAFLD) via Toll-like receptor 4 (TLR4) signaling pathway [1]. In addition to gut microbial components, metabolites derived from gut microbiota also impact hepatic function, including amino acids, secondary bile acids, ethanol, lipids, and SCFAs. For example, a tryptophan-derived metabolite indole-3-propionic acid (IPA) by gut microbiota showed anti-NASH ability in rats by reducing gut LPS leakage, which can activate hepatic macrophages to produce proinflammatory cytokines (for example, tumor necrosis factor (TNF)-α and interleukin (IL)-1β) to cause liver inflammation and fibrosis [2][3]. An updated summary in the following context is to describe the function of metabolites in the development of NAFLD from recent findings.
1.1. Amino Acids
Plasma amino acids (AAs), such as glutamate and valine, are shown to increase in NAFLD patients with or without obesity compared to non-NAFLD controls [4]. Hoyles et al. reported that dysregulation of branched-chain amino acid and aromatic amino acid metabolism was positively associated with hepatic inflammation and steatosis in non-diabetic obese women, resulting from gut microbial dysbiosis with the richness of genes for dietary lipid metabolism and LPS biosynthesis [5]. This was also showed that phenylacetic acid (PAA), a microbiota-derived metabolite from aromatic amino acid phenylalanine, was positively associated with hepatic steatosis. Another one showed that limiting glycine source or inhibiting glycine biosynthetic genes such as alanine-glyoxylate aminotransferase 1 (AGXT1) accelerated diet-induced NASH and hyperlipidemia [6]. Treatment with a tripeptide DT-109 (Gly-Gly-L-Leu) ameliorated mouse NASH features induced by a high-fat, cholesterol, and fructose diet by enhancing liver mitochondrial fatty acid β-oxidation (FAO) and stimulating de novo glutathione synthesis [6]. Thus, modulating AA metabolites can potentially inhibit the progression of NAFLD.
1.2. Bile Acids
Bile acids (BAs) play important roles in NAFLD pathogenesis by modulating hepatic lipid and glucose metabolism, consisting of primary and secondary BAs [7]. Primary BAs such as chenodeoxycholic acid (CDCA) are produced in the liver, while gut microbiota can metabolize them to secondary BAs such as deoxycholic acid (DCA) [8]. BA receptors such as nuclear Farnesoid X receptor (FXR) and the Takeda G protein-coupled receptor 5 (TGR5) are important molecules that are involved in the modulation of energy metabolism and inflammation during metabolic disorders, including NAFLD [9]. For example, a high-fat diet (HFD)-induced development of NAFLD has been reported to be associated with a decrease in the ratio of non-12α-OH BAs (for example, HDCA/Hyodeoxycholic)/12α-OH BAs (for example, DCA) with downregulation of FXR and TGR5 and upregulation of cytochrome P450 family 7 subfamily A member 1 (CYP7A1) and TLR4 [10]. Modulating gut microbiota with an antibiotic cocktail can alleviate HFD-induced hepatic steatosis and inflammation in hamsters via upregulating cytochrome P450 family 7 subfamily B member 1 (CYP7B1) to increase hydrophilic BA synthesis [11].
1.3. Choline Metabolism
Choline can be metabolized by the gut microbiota to trimethylamine (TMA), which is absorbed in the liver and further converted to trimethylamine N-oxide (TMAO) by flavin-containing monooxygenase 3 (FMO3) [12]. In addition to choline, TMA precursors such as L-carnitine and betaine are rich in diets (for example, red meat and eggs), and overconsumption of these diets can increase TMAO in plasma to promote NAFLD through activation of oxidative stress, unfolded protein response, and change of bile acid metabolism [13]. A prospective one was showed that plasma levels of TMAO were positively associated with all-cause mortality in human NAFLD patients but not in non-NAFLD patients, which was independent of traditional risk factors, such as triglyceride glucose, and body mass index (BMI) [14]. TMA-producing bacteria consist of enzymes choline-TMA lyase (CutC), carnitine oxygenase (CntA), and betaine reductase (GrdH), such as Firmicutes [14][15][16][17]. In addition, several choline-deficient diets were applied to induced mouse NASH and liver fibrosis models [18].
1.4. Ethanol
Excessive consumption of alcohol causes alcohol fatty liver disease (AFLD). Endogenous ethanol produced by gut microbiota can impair mitochondrial function and promotes NAFLD development [19]. Gavage of ethanol-producing gut microbiota (for example, Klebsiella pneumoniae) to mice can increase ethanol production, increase liver injury, and impair mitochondrial function in mice, indicating a causative factor for NAFLD [19]. Fasting ethanol concentration in plasma has been shown to be positively associated with insulin resistance in children with NAFLD compared to controls [20]. Further in mice also showed that impaired activity of alcohol dehydrogenase (ADH) in the liver tissue is the major cause of ethanol concentration increase instead of an increase in endogenous ethanol synthesis [20]. Thus, ethanol either produced endogenously by gut microbiota or caused by impaired ADH in the liver can impact NAFLD progression.
1.5. Fiber
Dietary fibers (DF) consist of carbohydrate polymers resistant to digestive enzymes in the small intestine, which can be digested by bacteria in the large intestine [21]. DF can be divided into soluble and insoluble forms based on the solubility in water, and soluble fibers can be degraded into SCFAs [22]. Supplementation of oligofructose, a DF, is helpful to reduce body weight in obese adults [23]. Obese patients with consumption of higher insoluble fiber consumption (≥7.5 g/day) had improvement in the fatty liver index, hepatic steatosis index, and NAFLD liver fat score, while patients with fruit fiber consumption (≥8.8 g/day) showed significant improvements in gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) [24]. A clinical trial was also showed that consumption of a low-carbohydrate and high-fiber diet with education can effectively reduce the body weight and body fat of NAFLD patients and improve metabolic disorders [25]. One of the underlying mechanisms is to change gut permeability, as evidenced by the reduction in serum levels of zonulin in NAFLD patients with DF [26].
Fermentation of DF can impact the diversity of gut microbiota. For example, a meta-analysis revealed that DF intervention can increase the abundance of Bifidobacterium and Lactobacillus genera compared to placebo or low-fiber consumption, which is associated with a high concentration of butyrate in feces [27]. Consumption of brans such as oat and rye containing 50% DF can reduce body weight gain and ameliorate Western diet (WD)-induced liver inflammation via altering gut metabolism such as indole production [28].
1.6. Short-Chain Fatty Acids
SCFAs, consisting of acetate, propionate, and butyrate, are produced by gut microbiota from dietary fibers and starch. They play important roles in energy metabolism, tissue homeostasis, and immune regulation.
1.6.1. Acetate
Oral administration of branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, significantly increased the abundance of gut Ruminococcus flavefaciens and portal acetic acid concentration, resulting in a reduction in hepatic fat accumulation [29]. In addition, a molecular mechanism one was showed that BCAA treatment inhibited the expression of lipogenesis-related enzymes such as fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC). It has been reported that both butyrate and propionate show predominantly anti-obesity effects, whereas acetate has more potential to promote obesity and lipogenesis in the liver and adipose tissue [30].
1.6.2. Propionate
A randomized controlled trial one was showed that dietary supplementation with inulin that is mainly metabolized into acetate in the colon increased intrahepatocellular lipid. In contrast, dietary supplementation of inulin-propionate ester, which is designed to deliver propionate to the colon and to attenuate the acetate-mediated increase in intrahepatocellular lipid [31].
1.6.3. Butyrate
Supplementation with grape polyphenols reduced Western diet (WD)-induced adiposity and hepatic steatosis in mice by increasing the abundance of Akkermansia muciniphila and butyrate and sugar expenditure in the distal intestine [32].
Overall, the dietary metabolites or metabolites derived from gut microbiota impact the progression of NAFLD and NASH (Figure 1).
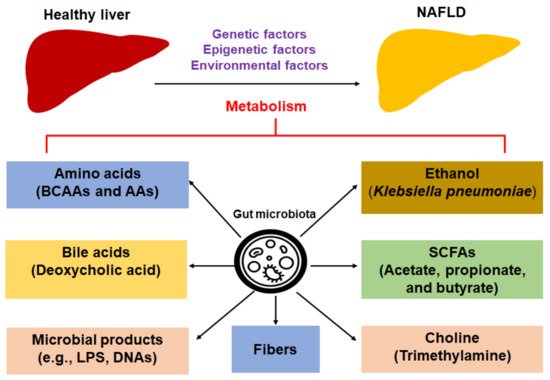
Figure 1. Dietary metabolites or metabolites derived from gut microbiota impact the progression of NAFLD. Abbreviations: AAA, aromatic amino acid; BCAA: branched-chain amino acid; LPS, lipopolysaccharide; NAFLD, nonalcoholic fatty liver disease; SCFAs, short-chain fatty acids.
2. Intrahepatic Immunity in NAFLD and NASH in Diet-Induced Murine Models and Human Patients
The intrahepatic immune response plays an essential role in the progression of NAFLD/NASH. Gut microbiota-derived metabolites and components circulating in the portal vein system can enter the liver to modulate intrahepatic immunity to impact NAFLD. This process is involved in a complicated communication among different liver non-parenchymal cells, including macrophages, monocytes, T cells, B cells, neutrophils, and HSCs [33]. Herein, it was update some recent findings in this field to explore new molecules or cell subtypes in the pathogenesis of NALFD. Animal models of steatosis, NAFLD, and NASH have been summarized in recent publications [34], which are briefly mentioned with the discussion of immune activation.
2.1. Macrophages/Monocytes
The composition of liver macrophages was altered in mice fed a high-fat high-sucrose diet (60% fat and 10% sucrose), with a decrease in liver resident macrophage Kupffer cells (KCs) and an increase in monocyte-derived macrophages (MdMs) detected by single-cell RNA sequencing (scRNA-seq) [35]. A subset of MdMs shows the phenotype of lipid-associated macrophages (LAMs) characterized by the expression of triggering receptor expressed on myeloid cells 2 (Trem2), cluster of differentiation (CD)63, CD9, and glycoprotein nonmetastatic melanoma protein B (Gpmnb) [35]. In addition, Cc chemokine receptor (CCR)2 expression is critically important for the recruitment of this population. Gut microbiota-derived tryptophan metabolites tryptamine and indole-3-acetate (I3A) can attenuate the expression of TNF-α, IL-1β, and MCP-1 on macrophages exposed to palmitate and LPS [36]. Those cytokines expressed by macrophages can promote NAFLD progression.
2.2. NK Cells
The number of natural killer (NK) cells was increased in a methionine- and choline-deficient diet (MCD)-induced mouse NASH liver via C-X-C motif chemokine ligand (CXCL)10/chemokine receptor (CXCR)3 signaling [37]. These intrahepatic NK cells expressed low levels of protein Ki67, indicating a reduced proliferation ability. In addition, depletion of NK cells induced hepatic infiltration of MdMs with M2-like phenotype, advancing liver inflammation and fibrosis [37]. Another one showed that CD56brightNK cells decreased in intrahepatic lymphocytes in NAFLD patients, while CD56dimNK cells increased compared to that in healthy controls, indicating the complex roles of each subtype of NK cells in NAFLD [38]. However, another one showed that there was only a minor change in NK cell activation and inhibitory markers from NASH patients, except natural killer group 2 member D (NKG2D) [39]. Natural cytotoxicity triggering receptor 1 (NKp46)+ NK cells can inhibit the progression of NASH and liver fibrosis via suppressing the expression of profibrogenic genes as well as M2 polarization (anti-inflammatory phenotype) of liver macrophages [40]. Therefore, the role of NK cells is dependent on their subtypes.
2.3. NKT Cells
Activation of invariant natural killer T (iNKT) cell subsets was shown in choline-deficient L-amino acid-defined HFD (CDAHFD)-induced murine NASH, accompanying the accumulation of plasmacytoid dendritic cells (pDCs) [41]. In addition, the frequency of iNKT cells was increased in peripheral blood mononuclear cells (PBMCs) from NASH patients compared to that in healthy controls. The axis of CXCR6/CXCL16 plays an essential role in the recruitment of NKT cells in fatty liver, liver fibrosis, and liver cancer [42][43]. Gut microbiota such as Clostridium spp. induced secondary bile species (sBAs) activated liver sinusoidal endothelial cells (LSECs) to produce the chemokine CXCL16 to attract accumulation of hepatic CXCR6+NKT cells [44]. CD1d-deficient mice lacking NKT cells on a high-fat high carbohydrate (HFHC) showed reduced body weight and hepatic triglyceride content, mRNA expression of α-smooth muscle actin (α-SMA), collagen type 1 alpha 1 (Col1α1) and alpha 2 (Col1α2), and infiltration of macrophages, with improved NAFLD activity scores [45]. Overall, NKT cells are normally increased in the liver, accompanying the development of NAFLD and NASH.
2.4. Neutrophils
Neutrophils are one of the first response cells that are recruited to the injury site to participate in the inflammatory response and tissue repair. Neutrophil depletion treated with antibody 1A8 (200 μg/mouse per week for four times) can reduce body weight gain and attenuate liver lipid accumulation with activation of lipid β-oxidation in HFD-fed mice compared to mice treated with isotype control [46]. Neutrophil depletion was also associated with a reduction in expression of inflammatory cytokines, such as TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1/CCL2) [46].
2.5. CD4 T Cells
Different subtypes of CD4+ T cells play different roles in NAFLD pathogenesis. Fatty acid composition (e.g., the ratio of C16:1n7/C16:0) can modulate the frequency of CD4+ T cell profiles in PBMCs of NAFLD patients, with an increase in CD25+CD45+CD4+ T cells and a decrease in PD1+CD4+ T cells [47].
Obesity increased the accumulation of inflammatory hepatic CXCR3+ T helper 17 (Th17) cells and concomitant expression of IL-17a, interferon (IFN)-γ, and TNF-α, resulting in NAFLD progression [48]. Cellular metabolism impacts the inflammatory phenotype of hepatic Th17 cells, especially by pyruvate kinase M2 (PKM2)-mediated glycolytic pathway [48]. The ratio of Th17 and regulatory T (Treg) cells is critically important in the pathogenesis of NAFLD and liver inflammation. Feeding an HFD increased the frequency of liver Th17 cells; meanwhile, it caused a decrease in Tregs in mice compared to ND feeding mice, resulting in an increased Th17/Treg ratio, progression of NAFLD, and liver inflammation [49]. IL-17+CD4+ T cells were significantly increased in the liver during NAFL to NASH progression [50]. The increase in Th17 cells in NASH patients was positively correlated with an increased blood concentration of LPS [51].
Hepatic infiltration of Tregs was increased in CD62L-deficient mice, which was associated with less hepatic lipid accumulation, reduced liver fibrosis, and improved insulin resistance [52]. However, adoptive transfer of Tregs from healthy wild-type mice to mice fed a high-fat, high-fructose diet (HFHFD) promoted hepatic steatosis due to infiltration of Tregs in subcutaneous adipose tissue and/or a decrease in Th1 cells [53].
2.6. CD8 T Cells
Liver CD8+ T cells were increased in obese patients with NASH, which was associated with the expression of α-SMA, a marker of HSC activation [54]. Depletion of liver CD8+ T cells reduced hepatic macrophages and α-SMA expression in obesity or hyperlipidemia-induced NASH mice, but not in lean mice [54]. RNA-seq data showed that perforin deficiency increased proinflammatory cytokine expression in hepatic CD8+ T cells in mice with NASH [55]. Perforin-deficient mice fed with a methionine- and choline-deficient diet (MCD) displayed an increase in CD8+ T cell accumulation and activation with the expression of proinflammatory cytokines, but not CD4+ T cells and NK cells. Ex vivo ones revealed that microbiota-derived extracts in NAFLD-HCC patients compared to that can induce an immunosuppressive phenotype in human PBMCs, characterized by a suppression of CD8+ T cells and expansion of Tregs [56]. NAFLD promotes CD8+ T cell activation and suppresses its cytotoxicity to tumor cells by inducing immune tolerance.
2.7. B Cells
Fecal microbiota transplantation (FMT) of gut microbiota from human NAFLD patients into recipient mice can accelerate NASH progression via inducing accumulation and activation of liver B cells [57]. ScRNA-seq data showed that intrahepatic B cells in NASH mice display proinflammatory phenotype with activation of myeloid differentiation primary response protein 88 (MyD88) signaling pathway [57]. Furthermore, depletion of B cells suppressed NASH progression, whereas adoptive transfer of B cells from NASH liver can induce NASH, indicating the pathogenic role of B cells in NASH.
Activation of HSCs, the major cells that contribute to liver fibrosis, is mediated by the activation of intrahepatic immunity during NASH. For example, proinflammatory cytokines such as TNF-α, transforming growth factor (TGF)-β1, and IL-1β expressed by intrahepatic macrophages can activate HSCs to promote the progression of liver fibrosis and NASH [58]. In contrast, a recent one showed that tissue-resident memory CD8+ T cells can trigger apoptosis of activated HSCs via Fas (TNF receptor superfamily, member 6)/FasL-mediated signaling [59]. Therefore, the immune activation, hepatocyte injury, and activation of HSCs are cross-talked with each other during NAFLD development and progression (Figure 2).
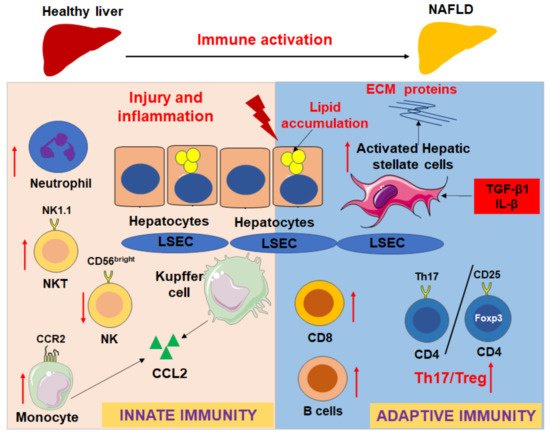
Figure 2. Innate and adaptive immune responses in the progression of NAFLD and liver fibrosis. Red arrows show that the immune cells will be recruited into the fatty liver during NAFLD development, such as CCR2+ monocytes/macrophages and neutrophils; the ratio of Th17/Tregs increases, NKT cell, CD8 T cells, and B cells are activated and increased in different extend according to different models; however, CD56brightNK cells are decreased. The immune activation and hepatocyte injury will impact the activation of hepatic stellate cells (HSCs) to express extracellular matrix (ECM) proteins via upregulation of profibrotic and proinflammatory cytokines, such as TGF-β1 and IL-β.
References
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin Producers Overgrowing in Human Gut Microbiota as the Causative Agents for Nonalcoholic Fatty Liver Disease. mBio 2020, 11.
- Zhao, Z.H.; Xin, F.Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14.
- Sehgal, R.; Ilha, M.; Vaittinen, M.; Kaminska, D.; Männistö, V.; Kärjä, V.; Tuomainen, M.; Hanhineva, K.; Romeo, S.; Pajukanta, P.; et al. Indole-3-Propionic Acid, a Gut-Derived Tryptophan Metabolite, Associates with Hepatic Fibrosis. Nutrients 2021, 13, 3509.
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158.
- Hoyles, L.; Fernández-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080.
- Rom, O.; Liu, Y.; Liu, Z.; Zhao, Y.; Wu, J.; Ghrayeb, A.; Villacorta, L.; Fan, Y.; Chang, L.; Wang, L.; et al. Glycine-based treatment ameliorates NAFLD by modulating fatty acid oxidation, glutathione synthesis, and the gut microbiome. Sci. Transl. Med. 2020, 12.
- Jiao, N.; Baker, S.S.; Chapa-Rodriguez, A.; Liu, W.; Nugent, C.A.; Tsompana, M.; Mastrandrea, L.; Buck, M.J.; Baker, R.D.; Genco, R.J.; et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67, 1881–1891.
- Qi, X.; Yang, M.; Stenberg, J.; Dey, R.; Fogwe, L.; Alam, M.S.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Gut microbiota mediated molecular events and therapy in liver diseases. World J. Gastroenterol. 2020, 26, 7603–7618.
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.
- Yang, C.; Wan, M.; Xu, D.; Pan, D.; Xia, H.; Yang, L.; Sun, G. Flaxseed Powder Attenuates Non-Alcoholic Steatohepatitis via Modulation of Gut Microbiota and Bile Acid Metabolism through Gut-Liver Axis. Int. J. Mol. Sci. 2021, 22, 858.
- Sun, L.; Pang, Y.; Wang, X.; Wu, Q.; Liu, H.; Liu, B.; Liu, G.; Ye, M.; Kong, W.; Jiang, C. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm. Sin. B 2019, 9, 702–710.
- Fennema, D.; Phillips, I.R.; Shephard, E.A. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab. Dispos. 2016, 44, 1839–1850.
- Li, X.; Hong, J.; Wang, Y.; Pei, M.; Wang, L.; Gong, Z. Trimethylamine-N-Oxide Pathway: A Potential Target for the Treatment of MAFLD. Front. Mol. Biosci. 2021, 8, 733507.
- Flores-Guerrero, J.L.; Post, A.; van Dijk, P.R.; Connelly, M.A.; Garcia, E.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Circulating trimethylamine-N-oxide is associated with all-cause mortality in subjects with nonalcoholic fatty liver disease. Liver Int. 2021, 41, 2371–2382.
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front. Microbiol. 2019, 10, 2966.
- León-Mimila, P.; Villamil-Ramírez, H.; Li, X.S.; Shih, D.M.; Hui, S.T.; Ocampo-Medina, E.; López-Contreras, B.; Morán-Ramos, S.; Olivares-Arevalo, M.; Grandini-Rosales, P.; et al. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 2021, 47, 101183.
- Tan, X.; Liu, Y.; Long, J.; Chen, S.; Liao, G.; Wu, S.; Li, C.; Wang, L.; Ling, W.; Zhu, H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019, 63, e1900257.
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.F.; Aithal, G.P.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767.
- Chen, X.; Zhang, Z.; Li, H.; Zhao, J.; Wei, X.; Lin, W.; Zhao, X.; Jiang, A.; Yuan, J. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020, 35, 2009–2019.
- Engstler, A.J.; Aumiller, T.; Degen, C.; Dürr, M.; Weiss, E.; Maier, I.B.; Schattenberg, J.M.; Jin, C.J.; Sellmann, C.; Bergheim, I. Insulin resistance alters hepatic ethanol metabolism: Studies in mice and children with non-alcoholic fatty liver disease. Gut 2016, 65, 1564–1571.
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut Fermentation of Dietary Fibres: Physico-Chemistry of Plant Cell Walls and Implications for Health. Int. J. Mol. Sci. 2017, 18, 2203.
- Prasad, K.N.; Bondy, S.C. Dietary Fibers and Their Fermented Short-Chain Fatty Acids in Prevention of Human Diseases. Mech. Ageing Dev. 2018.
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759.
- Cantero, I.; Abete, I.; Monreal, J.I.; Martinez, J.A.; Zulet, M.A. Fruit Fiber Consumption Specifically Improves Liver Health Status in Obese Subjects under Energy Restriction. Nutrients 2017, 9, 667.
- Chen, J.; Huang, Y.; Xie, H.; Bai, H.; Lin, G.; Dong, Y.; Shi, D.; Wang, J.; Zhang, Q.; Zhang, Y.; et al. Impact of a low-carbohydrate and high-fiber diet on nonalcoholic fatty liver disease. Asia Pac. J. Clin. Nutr. 2020, 29, 483–490.
- Krawczyk, M.; Maciejewska, D.; Ryterska, K.; Czerwińka-Rogowska, M.; Jamioł-Milc, D.; Skonieczna-Żydecka, K.; Milkiewicz, P.; Raszeja-Wyszomirska, J.; Stachowska, E. Gut Permeability Might be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients 2018, 10, 1793.
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983.
- Kundi, Z.M.; Lee, J.C.; Pihlajamäki, J.; Chan, C.B.; Leung, K.S.; So, S.S.Y.; Nordlund, E.; Kolehmainen, M.; El-Nezami, H. Dietary Fiber from Oat and Rye Brans Ameliorate Western Diet-Induced Body Weight Gain and Hepatic Inflammation by the Modulation of Short-Chain Fatty Acids, Bile Acids, and Tryptophan Metabolism. Mol. Nutr. Food Res. 2021, 65, e1900580.
- Iwao, M.; Gotoh, K.; Arakawa, M.; Endo, M.; Honda, K.; Seike, M.; Murakami, K.; Shibata, H. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci. Rep. 2020, 10, 18768.
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119.
- Chambers, E.S.; Byrne, C.S.; Rugyendo, A.; Morrison, D.J.; Preston, T.; Tedford, C.; Bell, J.D.; Thomas, L.; Akbar, A.N.; Riddell, N.E.; et al. The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 372–376.
- Mezhibovsky, E.; Knowles, K.A.; He, Q.; Sui, K.; Tveter, K.M.; Duran, R.M.; Roopchand, D.E. Grape Polyphenols Attenuate Diet-Induced Obesity and Hepatic Steatosis in Mice in Association With Reduced Butyrate and Increased Markers of Intestinal Carbohydrate Oxidation. Front. Nutr. 2021, 8, 675267.
- Nati, M.; Haddad, D.; Birkenfeld, A.L.; Koch, C.A.; Chavakis, T.; Chatzigeorgiou, A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev. Endocr. Metab. Disord. 2016, 17, 29–39.
- Febbraio, M.A.; Reibe, S.; Shalapour, S.; Ooi, G.J.; Watt, M.J.; Karin, M. Preclinical Models for Studying NASH-Driven HCC: How Useful Are They? Cell Metab. 2019, 29, 18–26.
- Daemen, S.; Gainullina, A.; Kalugotla, G.; He, L.; Chan, M.M.; Beals, J.W.; Liss, K.H.; Klein, S.; Feldstein, A.E.; Finck, B.N.; et al. Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH. Cell Rep. 2021, 34, 108626.
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111.
- Fan, Y.; Zhang, W.; Wei, H.; Sun, R.; Tian, Z.; Chen, Y. Hepatic NK cells attenuate fibrosis progression of non-alcoholic steatohepatitis in dependent of CXCL10-mediated recruitment. Liver Int. 2020, 40, 598–608.
- Diedrich, T.; Kummer, S.; Galante, A.; Drolz, A.; Schlicker, V.; Lohse, A.W.; Kluwe, J.; Eberhard, J.M.; Schulze Zur Wiesch, J. Characterization of the immune cell landscape of patients with NAFLD. PLoS One 2020, 15, e0230307.
- Stiglund, N.; Strand, K.; Cornillet, M.; Stål, P.; Thorell, A.; Zimmer, C.L.; Näslund, E.; Karlgren, S.; Nilsson, H.; Mellgren, G.; et al. Retained NK Cell Phenotype and Functionality in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019, 10, 1255.
- Tosello-Trampont, A.C.; Krueger, P.; Narayanan, S.; Landes, S.G.; Leitinger, N.; Hahn, Y.S. NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 2016, 63, 799–812.
- Maricic, I.; Marrero, I.; Eguchi, A.; Nakamura, R.; Johnson, C.D.; Dasgupta, S.; Hernandez, C.D.; Nguyen, P.S.; Swafford, A.D.; Knight, R.; et al. Differential Activation of Hepatic Invariant NKT Cell Subsets Plays a Key Role in Progression of Nonalcoholic Steatohepatitis. J. Immunol. 2018, 201, 3017–3035.
- Zhu, H.; Zhang, Q.; Chen, G. CXCR6 deficiency ameliorates ischemia-reperfusion injury by reducing the recruitment and cytokine production of hepatic NKT cells in a mouse model of non-alcoholic fatty liver disease. Int. Immunopharmacol. 2019, 72, 224–234.
- Wehr, A.; Baeck, C.; Heymann, F.; Niemietz, P.M.; Hammerich, L.; Martin, C.; Zimmermann, H.W.; Pack, O.; Gassler, N.; Hittatiya, K.; et al. Chemokine receptor CXCR6-dependent hepatic NK T Cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 2013, 190, 5226–5236.
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360.
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.M.; Shivakumar, P.; Kohli, R. Hepatic Natural Killer T-cell and CD8+ T-cell Signatures in Mice with Nonalcoholic Steatohepatitis. Hepatol. Commun. 2017, 1, 299–310.
- Ou, R.; Liu, J.; Lv, M.; Wang, J.; Wang, J.; Zhu, L.; Zhao, L.; Xu, Y. Neutrophil depletion improves diet-induced non-alcoholic fatty liver disease in mice. Endocrine 2017, 57, 72–82.
- Seike, T.; Mizukoshi, E.; Yamada, K.; Okada, H.; Kitahara, M.; Yamashita, T.; Arai, K.; Terashima, T.; Iida, N.; Fushimi, K.; et al. Fatty acid-driven modifications in T-cell profiles in non-alcoholic fatty liver disease patients. J. Gastroenterol. 2020, 55, 701–711.
- Moreno-Fernandez, M.E.; Giles, D.A.; Oates, J.R.; Chan, C.C.; Damen, M.; Doll, J.R.; Stankiewicz, T.E.; Chen, X.; Chetal, K.; Karns, R.; et al. PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell Metab. 2021, 33, 1187–1204.
- He, B.; Wu, L.; Xie, W.; Shao, Y.; Jiang, J.; Zhao, Z.; Yan, M.; Chen, Z.; Cui, D. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunol. 2017, 18, 33.
- Rau, M.; Schilling, A.K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105.
- Wang, X.; Ji, D.; Zhu, B.; Jiang, S.; Han, L.; Wang, Y.; Mai, H.; Xu, S.; Jiang, H.; Wang, G.; et al. Contribution of endotoxin to Th17 bias in patients with non-alcoholic steatohepatitis. Microb. Pathog. 2020, 142, 104009.
- Drescher, H.K.; Schippers, A.; Rosenhain, S.; Gremse, F.; Bongiovanni, L.; Bruin, A.; Eswaran, S.; Gallage, S.U.; Pfister, D.; Szydlowska, M.; et al. L-Selectin/CD62L is a Key Driver of Non-Alcoholic Steatohepatitis in Mice and Men. Cells 2020, 9, 1106.
- Van Herck, M.A.; Vonghia, L.; Kwanten, W.J.; Vanwolleghem, T.; Ebo, D.G.; Michielsen, P.P.; De Man, J.G.; Gama, L.; De Winter, B.Y.; Francque, S.M. Adoptive Cell Transfer of Regulatory T Cells Exacerbates Hepatic Steatosis in High-Fat High-Fructose Diet-Fed Mice. Front. Immunol. 2020, 11, 1711.
- Breuer, D.A.; Pacheco, M.C.; Washington, M.K.; Montgomery, S.A.; Hasty, A.H.; Kennedy, A.J. CD8(+) T cells regulate liver injury in obesity-related nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G211–G224.
- Wang, T.; Sun, G.; Wang, Y.; Li, S.; Zhao, X.; Zhang, C.; Jin, H.; Tian, D.; Liu, K.; Shi, W.; et al. The immunoregulatory effects of CD8 T-cell-derived perforin on diet-induced nonalcoholic steatohepatitis. Faseb J. 2019, 33, 8490–8503.
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187.
- Barrow, F.; Khan, S.; Fredrickson, G.; Wang, H.; Dietsche, K.; Parthiban, P.; Robert, S.; Kaiser, T.; Winer, S.; Herman, A.; et al. Microbiota-Driven Activation of Intrahepatic B Cells Aggravates NASH Through Innate and Adaptive Signaling. Hepatology 2021, 74, 704–722.
- Yang, M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Astaxanthin Prevents Diet-Induced NASH Progression by Shaping Intrahepatic Immunity. Int. J. Mol. Sci. 2021, 22, 1037.
- Koda, Y.; Teratani, T.; Chu, P.S.; Hagihara, Y.; Mikami, Y.; Harada, Y.; Tsujikawa, H.; Miyamoto, K.; Suzuki, T.; Taniki, N.; et al. CD8(+) tissue-resident memory T cells promote liver fibrosis resolution by inducing apoptosis of hepatic stellate cells. Nat. Commun. 2021, 12, 4474.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
983
Revisions:
2 times
(View History)
Update Date:
21 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




