Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Johanne Poudrier | -- | 1134 | 2022-04-18 04:28:25 | | | |
| 2 | Conner Chen | Meta information modification | 1134 | 2022-04-18 04:35:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Poudrier, J. Marginal Zone Precursor-Like in the Context of HIV. Encyclopedia. Available online: https://encyclopedia.pub/entry/21858 (accessed on 08 February 2026).
Poudrier J. Marginal Zone Precursor-Like in the Context of HIV. Encyclopedia. Available at: https://encyclopedia.pub/entry/21858. Accessed February 08, 2026.
Poudrier, Johanne. "Marginal Zone Precursor-Like in the Context of HIV" Encyclopedia, https://encyclopedia.pub/entry/21858 (accessed February 08, 2026).
Poudrier, J. (2022, April 18). Marginal Zone Precursor-Like in the Context of HIV. In Encyclopedia. https://encyclopedia.pub/entry/21858
Poudrier, Johanne. "Marginal Zone Precursor-Like in the Context of HIV." Encyclopedia. Web. 18 April, 2022.
Copy Citation
Marginal zone (MZ) B-cells are innate-like, and possess a polyreactive B-cell receptor (BCR) and several pattern recognition receptors (PRR) [1,2]. They are known to generate low-affinity first-line antibody responses against invading pathogens such as encapsulated bacteria.
MZp
1. Introduction
Marginal zone (MZ) B-cells are innate-like, and possess a polyreactive B-cell receptor (BCR) and several pattern recognition receptors (PRR) [1][2]. They are known to generate low-affinity first-line antibody responses against invading pathogens such as encapsulated bacteria [3].
2. MZp in the Context of HIV
Initial work with HIV-Tg mice showed an expanded marginal zone in the spleen of these animals, as well as B-cell hyperactivity and hyperglobulinemia with elevated anti-nuclear auto-antibodies [4]. Interestingly, the numerous extra-follicular IgM bright plasma-cells were found in the spleen of these HIV-Tg mice [4]. Notably, BAFF levels were found to be in excess in the serum of these animals [5]. Similar observations have been made with BAFF-Tg mice [6]. In agreement with findings with HIV-Tg mice, it can be shown that frequencies of MZp are increased in the blood of HIV-infected individuals from the Montreal primary HIV infection (PHI) cohort, as soon as in the acute phase, and despite HAART; they are concomitant with excessive BAFF levels which persist throughout, suggesting that deregulations of MZ population frequencies in the HIV context could involve excess BAFF [7][8]. As such, and as mentioned above, BAFF signals are important for the selection of the MZ B-cell pool [9]. The fact that BAFF has been shown to increase the expression of NOTCH2, whose signal is essential to MZ cell-fate decision, suggests that in excessive BAFF contexts, increased NOTCH2 may skew differentiation towards the MZ type, contributing to their increased frequencies [9][10].
Chemokines such as CCL20 and CCL25 were found in excess in the blood of HIV-infected individuals from the Montreal PHI cohort, and MZp from these individuals strongly migrated in response to these chemokines in vitro [11]. CCL20 and CCL25 are important chemokines that allow B-cell migration to peripheral sites such as the mucosal associated lymphoid tissues (MALT) [12][13]. This modulation in MZp migratory capacities could also help explain the increased frequencies of MZp in the blood, as these cells are being actively recruited to peripheral sites; where they are possibly solicited in an attempt to control HIV inflammation in places where the active battle against the virus is held. Notably, populations such as MZ accumulated in lymphoid organs of SIV-infected macaques [14]. Importantly, MZps from the blood of HIV-infected individuals from the Montreal PHI cohort express α4β7, shown to bind to gp120 and be important for mucosal migration (data not published). It is possible that some MZps be naturally recruited to the MALT, where they perform Breg- and antibody-producing activities [14]. As such, the fact that MZ populations are capable of CSR could suggest their being related to the recently reported α4β7 IgA-expressing Bregs, promoted by APRIL via TACI [15][16]. Any disturbance in the activities of such populations is likely to have a deleterious outcome.
Importantly, the recent work shows that the Breg potential of blood MZps from HIV-infected individuals of the Montreal PHI cohort is severely altered despite therapy, and suggests that BAFF may directly contribute to this altered profile. Given the association of excess BAFF with hyperglobulinemia and autoimmune manifestations, it is reasonable to think that in such circumstances, MZps are rather driven to antibody production, the desirability of which is questionable.
Interestingly, MZ B-cells were shown to bind to glycoproteins of the HIV Env, such as gp120, via C-type lectins—such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN)—and the mannose receptor, or via their polyreactive BCR; and a fraction of IgG and IgA produced following gp120 stimulation in the presence of BAFF was shown to recognize gp120 [17]. Additionally, the stimulating effect of gp120 on MZ populations is enhanced in the presence of BAFF [17]. MZ B-cell populations can also recognise HIV Env proteins such as gp41 through TLR10 and CD21 via the complement system [18][19] (see Figure 1). However, the exact contribution of MZ and MZp to anti-Env Abs and/or to hyperglobulinemia and auto-antibodies needs further assessment.
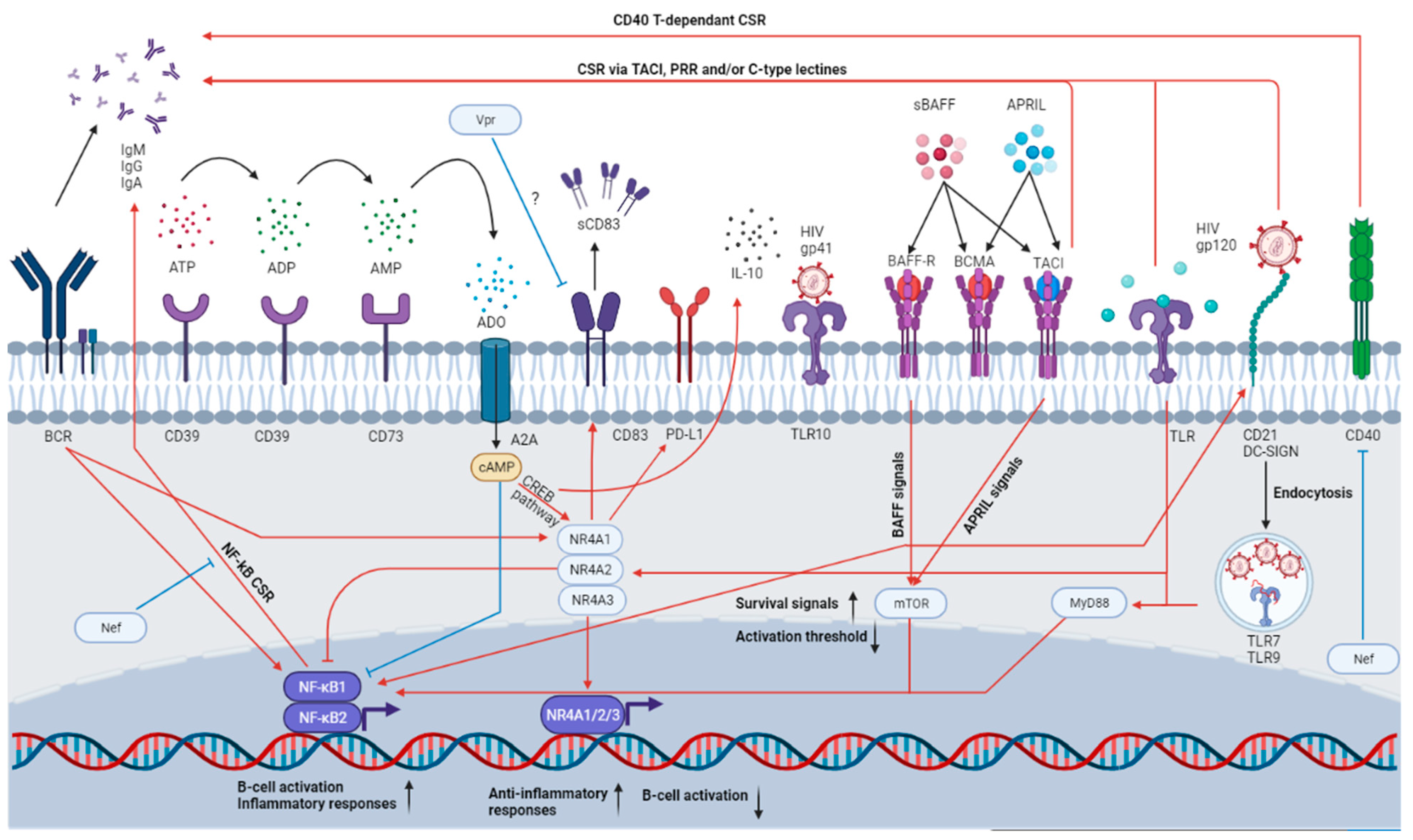
Figure 1. MZp immune functions and how they can be affected in the HIV context. MZps possess a strong Breg potential, as attested by the expression of several immunoregulatory molecules. Indeed, MZps express CD39 and CD73, which will convert the extracellular ATP into ADO. This molecule will then be uptaken by purinergic receptors such as A2A, which will induce cAMP accumulation in the cytosol and the activation of the CREB pathway. CREB will induce the expression of CREB-induced elements such as the NR4A molecules and IL-10, which will allow for the maintenance of a regulatory phenotype. The NR4As will then induce the expression of even more immunoregulatory molecules such as CD83 and PD-L1, while also impeding unwanted cell activation by the BCR or the TLR. However, this homeostasis is heavily altered in the HIV context due to the chronic inflammation, excess BAFF and viral proteins. For instance, MZ B-cells are able to class-switch following CD40 engagement and subsequent NF-kB pathway activation. However, in the HIV context, HIV Nef could impede this CSR. HIV gp120 could activate B-cells by cross-linking DC-SIGN an action that is enhanced by BAFF. Excess BAFF could induce TACI-dependent CSR by activating the mTOR pathway, which intersects with the TLR pathway (also engaged due to HIV-mediated recognition by TLR7, expressed by MZ B-cells), lowering the MZ activation threshold. HIV proteins such as Vpr could also directly affect MZp immunoregulatory protein expression such as CD83. Thus, in the HIV context, MZps could possibly lose their immunoregulatory functions, become easily activated and produce poor-affinity antibodies, with possible auto-reactivity.
HIV proteins can directly affect MZ and MZp capacity and function. For instance, it has been shown than soluble Nef, possibly produced and released by the HIV reservoirs, penetrates B-cells and directly impedes CD40 signaling mediated through the NF-kB and STAT pathway, and thus, CSR [20]. Moreover, the HIV Viral protein R (Vpr) has been shown to downregulate CD83 expression in both macrophages and DCs [21][22]. Furthermore, as described above, HIV Env glycoproteins can directly activate MZ populations (Figure 1).
Lastly, consistent with the notion that they are highly solicited, MZps from the blood of HIV-infected individuals present an exhausted profile; this is depicted by the upregulation of the negative regulators CD22 and CD72, as well as the exhaustion markers CD85j and FCRL5. The expression of T-bet and CD11c were also upregulated by these MZps. Interestingly, T-bet and CD11c expression are related to extra-follicular B-cell responses and to a population identified as “age-associated B-cells”; these are also described in the contexts of chronic inflammation and autoimmunity (discussed below), and are dependent on IL-21R and TLR7 signalling [23]. Age-associated B-cells are thought to produce antibodies of poor affinity. Interestingly, MZps express both IL-21R and TLR7, which means that they have the potential to take part in the age-associated B-cell pool.
References
- Cerutti, A.; Cols, M.; Puga, I. Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat. Rev. Immunol. 2013, 13, 118–132.
- Palm, E.A.-K.; Kleinau, S. Marginal zone B cells: From housekeeping function to autoimmunity? J. Autoimmun. 2021, 119, 102627.
- Weill, J.-C.; Weller, S.; Reynaud, C.-A. Human Marginal Zone B Cells. Annu. Rev. Immunol. 2009, 27, 267–285.
- Poudrier, J.; Weng, X.; Kay, D.G.; Paré, G.; Calvo, E.L.; Hanna, Z.; Kosco-Vilbois, M.H.; Jolicoeur, P. The AIDS Disease of CD4C/HIV Transgenic Mice Shows Impaired Germinal Centers and Autoantibodies and Develops in the Absence of IFN-γ and IL-6. Immunity 2001, 15, 173–185.
- Hannah, Z.; Kay, D.; Poudrier, J.; Jolicoeur, P. Division of Experimental Medicine; McGill University: Montreal, QC, Canada, 2022; Unpublished work.
- Mackay, F.; Woodcock, S.A.; Lawton, P.; Ambrose, C.; Baetscher, M.; Schneider, P.; Tschopp, J.; Browning, J. Mice Transgenic for Baff Develop Lymphocytic Disorders along with Autoimmune Manifestations. J. Exp. Med. 1999, 190, 1697–1710.
- Fontaine, J.; Chagnon-Choquet, J.; Valcke, H.S.; Poudrier, J.; Roger, M.; the Montreal Primary HIV Infection and Long-Term Non-Progressor Study Groups. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 2011, 117, 145–155.
- Chagnon-Choquet, J.; Fontaine, J.; Poudrier, J.; Roger, M.; for the Montreal Primary HIV Infection and Slow Progressor Study Groups. IL-10 and Lymphotoxin-α Expression Profiles within Marginal Zone-Like B-Cell Populations Are Associated with Control of HIV-1 Disease Progression. PLOS ONE 2014, 9, e101949.
- Pillai, S.; Cariappa, A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 2009, 9, 767–777.
- Jia, W.; Poe, J.C.; Su, H.; Anand, S.; Matsushima, G.K.; Rathmell, J.C.; Maillard, I.; Radojcic, V.; Imai, K.; Reyes, N.J.; et al. BAFF promotes heightened BCR responsiveness and manifestations of chronic GVHD after allogeneic stem cell transplantation. Blood 2021, 137, 2544–2557.
- Gauvin, J.; Chagnon-Choquet, J.; Poudrier, J.; Roger, M.; Cohorts, M.P.H.I.A.S.P. Fluctuations in Blood Marginal Zone B-Cell Frequencies May Reflect Migratory Patterns Associated with HIV-1 Disease Progression Status. PLoS ONE 2016, 11, e0155868.
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426.
- Svensson, M. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest 2002, 110, 1113–1121.
- Zouali, M.; Richard, Y. Marginal Zone B-Cells, a Gatekeeper of Innate Immunity. Front. Immunol. 2011, 2, 63.
- Fehres, C.M.; Van Uden, N.O.; Yeremenko, N.G.; Fernandez, L.; Salinas, G.F.; Van Duivenvoorde, L.M.; Huard, B.; Morel, J.; Spits, H.; Hahne, M.; et al. APRIL Induces a Novel Subset of IgA+ Regulatory B Cells That Suppress Inflammation via Expression of IL-10 and PD-L1. Front. Immunol. 2019, 10.
- Sintes, J.; Gentile, M.; Zhang, S.; Garcia-Carmona, Y.; Magri, G.; Cassis, L.; Segura-Garzón, D.; Ciociola, A.; Grasset, E.K.; Bascones, S.; et al. mTOR intersects antibody-inducing signals from TACI in marginal zone B cells. Nat. Commun. 2017, 8, 1462.
- He, B.; Qiao, X.; Klasse, P.J.; Chiu, A.; Chadburn, A.; Knowles, D.M.; Moore, J.P.; Cerutti, A. HIV-1 Envelope Triggers Polyclonal Ig Class Switch Recombination through a CD40-Independent Mechanism Involving BAFF and C-Type Lectin Receptors. J. Immunol. 2006, 176, 3931–3941.
- Henrick, B.M.; Yao, X.-D.; Zahoor, M.; Abimiku, A.; Osawe, S.; Rosenthal, K.L. TLR10 Senses HIV-1 Proteins and Significantly Enhances HIV-1 Infection. Front. Immunol. 2019, 10, 482.
- Moir, S.; Malaspina, A.; Li, Y.; Chun, T.-W.; Lowe, T.; Adelsberger, J.; Baseler, M.; Ehler, L.A.; Liu, S.Jr.; et al. B Cells of HIV-1–Infected Patients Bind Virions through Cd21–Complement Interactions and Transmit Infectious Virus to Activated T Cells. J. Exp. Med. 2000, 192, 637–646.
- Qiao, X. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in by-stander B cells. Nat. Immunol. 2006, 7, 302–310.
- Majumder, B. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: Im-plications for viral immune escape. J. Virol. 2005, 79, 7990–8003.
- Muthumani, K.; Hwang, D.S.; Choo, A.Y.; Mayilvahanan, S.; Dayes, N.S.; Thieu, K.P.; Weiner, D.B. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int. Immunol. 2004, 17, 103–116.
- Jenks, S.A.; Cashman, K.S.; Woodruff, M.C.; Lee, F.E.; Sanz, I. Extrafollicular responses in humans andSLE. Immunol. Rev. 2019, 288, 136–148.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
483
Revisions:
2 times
(View History)
Update Date:
18 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




