
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ryan Sarkar | -- | 4568 | 2022-04-01 10:47:36 | | | |
| 2 | Bruce Ren | Meta information modification | 4568 | 2022-04-07 03:08:12 | | |
Video Upload Options
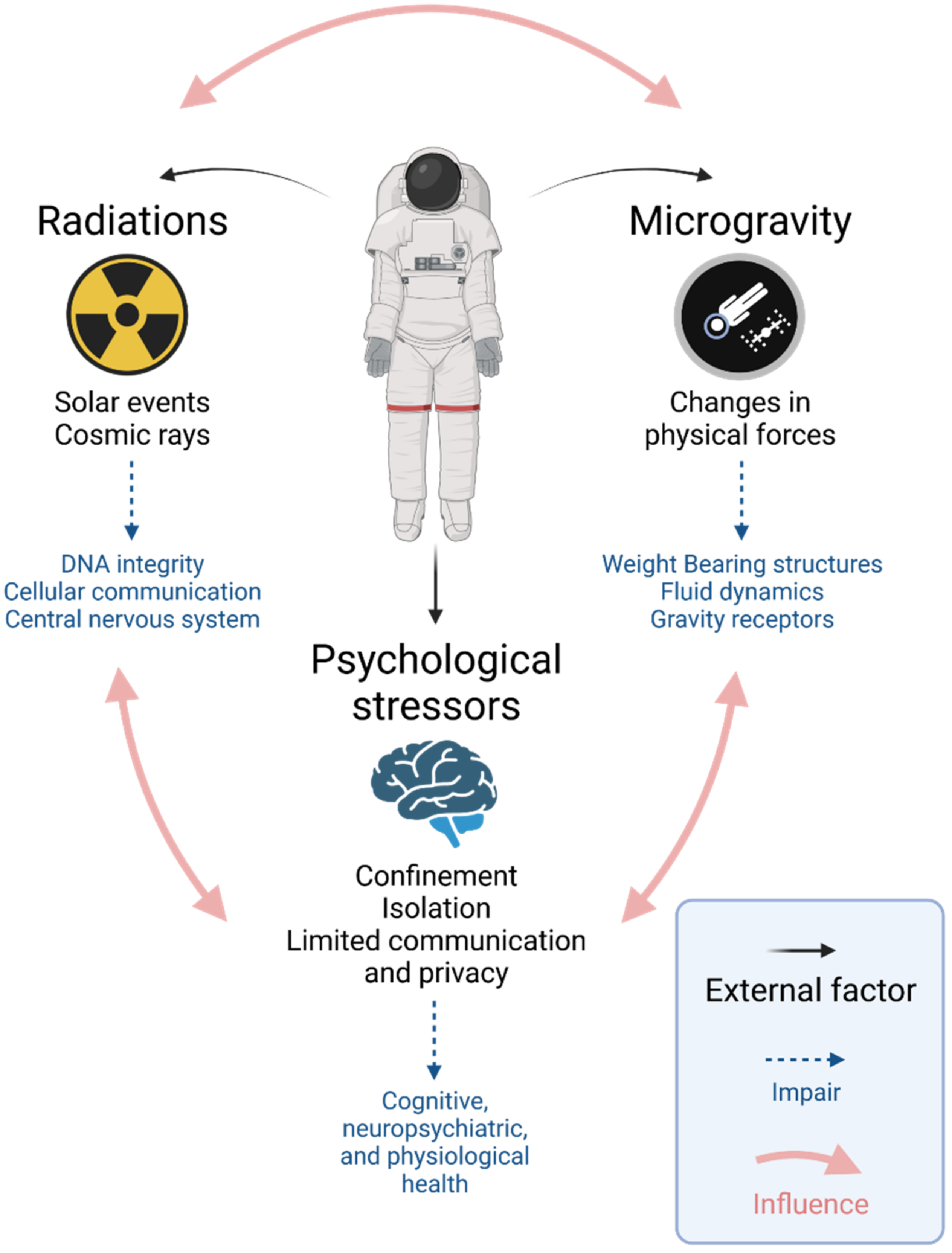
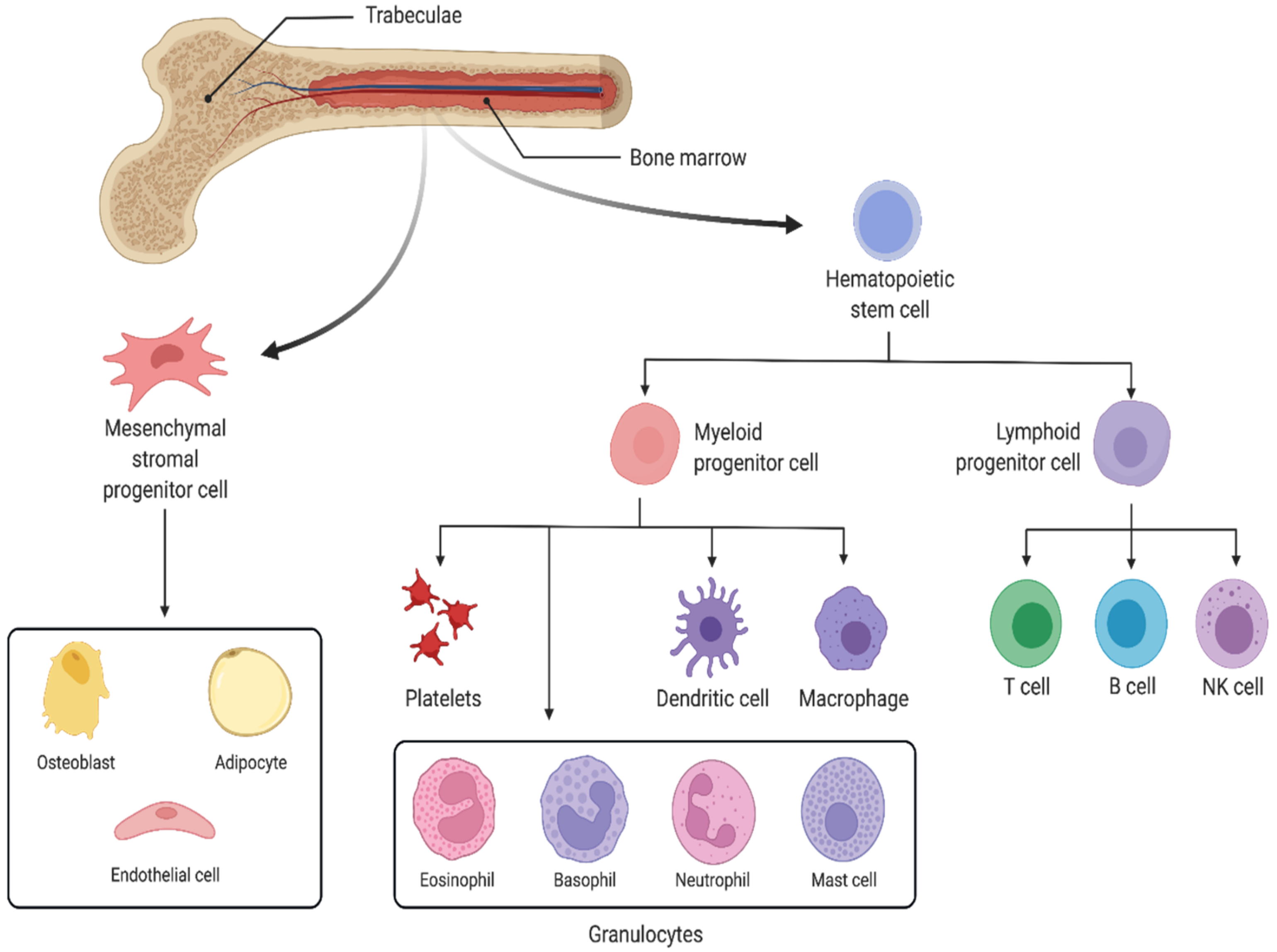
Spaceflight affects the body on every level. Reports on astronaut health identify bone marrow remodelling and dysfunction of the innate immune system as significant health risks of long-term habitation in space. Microgravity-induced alterations of the bone marrow induce physical changes to the bone marrow stem cell niche. Downstream effects on innate immunity are expected due to impaired hematopoiesis and myelopoiesis. To date, few studies have investigated these effects in real microgravity and the sparsely available literature often reports contrasting results. This emphasizes a need for the development of physiologically relevant in vitro models of the bone marrow stem cell niche, capable of delivering appropriate sample sizes for robust statistics.
1. Hematopoiesis, Innate Immunity, and Spaceflight Conditions
2. Response of the Innate Immune System to Spaceflight Conditions
2.1. Response of Innate Immune Cells to Microgravity and Ionizing Radiation
2.1.1. Hematopoietic Stem Cells
2.1.2. Peripheral Blood Mononuclear Cells
2.1.3. Monocytes and Macrophages
2.1.4. Osteocytes
2.1.5. Natural Killer Cells
2.1.6. Granulocytes
2.2. Response to Microgravity of the Innate Immune System in Animal Models
References
- Institute of Medicine (IOM). Safe Passage: Astronaut Care for Exploration Missions; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-07585-5.
- Axpe, E.; Chan, D.; Abegaz, M.F.; Schreurs, A.S.; Alwood, J.S.; Globus, R.K.; Appel, E.A. A human mission to Mars: Predicting the bone mineral density loss of astronauts. PLoS ONE 2020, 15, e0226434.
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118.
- Chancellor, J.C.; Scott, G.B.I.; Sutton, J.P. Space radiation: The number one risk to astronaut health beyond low earth orbit. Life 2014, 4, 491–510.
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437.
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859.
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049.
- Domaratskaya, E.I.; Michurina, T.V.; Bueverova, E.I.; Bragina, E.V.; Nikonova, T.A.; Starostin, V.I.; Khrushchov, N.G. Studies on clonogenic hemopoietic cells of vertebrate in space: Problems and perspectives. Adv. Space Res. 2002, 30, 771–776.
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942.
- Blaber, E.; Marçal, H.; Burns, B.P. Bioastronautics: The Influence of Microgravity on Astronaut Health. Astrobiology 2010, 10, 463–473.
- Crucian, B.; Stowe, R.; Mehta, S.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Sams, C. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 2013, 33, 456–465.
- Verhaar, A.P.; Hoekstra, E.; Tjon, A.S.W.; Utomo, W.K.; Deuring, J.J.; Bakker, E.R.M.; Muncan, V.; Peppelenbosch, M.P. Dichotomal effect of space flight-associated microgravity on stress-activated protein kinases in innate immunity. Sci. Rep. 2014, 4, 5468.
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013.
- Sibonga, J.D. Spaceflight-induced bone loss: Is there an Osteoporosis Risk? Curr. Osteoporos. Rep. 2013, 11, 92–98.
- Sibonga, J.D.; Spector, E.R.; Johnston, S.L.; Tarver, W.J.; Reeves, J.M. Evaluating bone loss in ISS astronauts. Aerosp. Med. Hum. Perform. 2015, 86, A38–A44.
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404.
- Mitroulis, I.; Kalafati, L.; Hajishengallis, G.; Chavakis, T. Myelopoiesis in the Context of Innate Immunity. J. Innate Immun. 2018, 10, 365–372.
- Schultze, J.L.; Mass, E.; Schlitzer, A. Emerging Principles in Myelopoiesis at Homeostasis and during Infection and Inflammation. Immunity 2019, 50, 288–301.
- Chou, D.B.; Frismantas, V.; Milton, Y.; David, R.; Pop-Damkov, P.; Ferguson, D.; MacDonald, A.; Vargel Bölükbaşı, Ö.; Joyce, C.E.; Moreira Teixeira, L.S.; et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 2020, 4, 394–406.
- Pecaut, M.J.; Mao, X.W.; Bellinger, D.L.; Jonscher, K.R.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Mohney, R.P.; Gridley, D.S. Is spaceflight-induced immune dysfunction linked to systemic changes in metabolism? PLoS ONE 2017, 12, e0174174.
- Hammond, T.G.; Allen, P.L.; Birdsall, H.H. Effects of space flight on mouse liver versus kidney: Gene pathway analyses. Int. J. Mol. Sci. 2018, 19, 4106.
- Taylor, G.R.; Konstantinova, I.; Sonnenfeld, G.; Jennings, R. Changes in the Immune System During and After Spaceflight. Adv. Space Biol. Med. 1997, 6, 1–32.
- Kaur, I.; Simons, E.R.; Castro, V.A.; Mark Ott, C.; Pierson, D.L. Changes in neutrophil functions in astronauts. Brain. Behav. Immun. 2004, 18, 443–450.
- Kaur, I.; Simons, E.R.; Castro, V.A.; Ott, C.M.; Pierson, D.L. Changes in monocyte functions of astronauts. Brain. Behav. Immun. 2005, 19, 547–554.
- Bigley, A.B.; Agha, N.H.; Baker, F.L.; Spielmann, G.; Kunz, H.E.; Mylabathula, P.L.; Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 2019, 126, 842–853.
- Taylor, G.R.; Neale, L.S.; Dardano, J.R. Immunological analyses of U.S. space shuttle crewmembers. Aviat. Space Environ. Med. 1986, 57, 213–217.
- Plett, P.A.; Frankovitz, S.M.; Abonour, R.; Orschell-Traycoff, C.M. Proliferation of human hematopoietic bone marrow cells in simulated microgravity. In Vitro Cell. Dev. Biol. 2001, 37, 73–78.
- Plett, P.A.; Abonour, R.; Frankovitz, S.M.; Orschell, C.M. Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp. Hematol. 2004, 32, 773–781.
- Wang, P.; Tian, H.; Zhang, J.; Qian, J.; Li, L.; Shi, L.; Zhao, Y. Spaceflight/microgravity inhibits the proliferation of hematopoietic stem cells by decreasing Kit-Ras/cAMP-CREB pathway networks as evidenced by RNA-Seq assays. FASEB J. 2019, 33, 5903–5913.
- Puca, A.; Russo, G.; Giordano, A. Properties of mechano-transduction via simulated microgravity and its effects on intracellular trafficking of VEGFR’s. Oncotarget 2012, 3, 426–434.
- Davis, T.A.; Wiesmann, W.; Kidwell, W.; Cannon, T.; Kerns, L.; Serke, C.; Delaplaine, T.; Pranger, A.; Lee, K.P. Effect of spaceflight on human stem cell hematopoiesis: Suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 1996, 60, 69–76.
- Adamo, L.; Naveiras, O.; Wenzel, P.L.; McKinney-Freeman, S.; Mack, P.J.; Gracia-Sancho, J.; Suchy-Dicey, A.; Yoshimoto, M.; Lensch, M.W.; Yoder, M.C.; et al. Biomechanical forces promote embryonic haematopoiesis. Nature 2009, 459, 1131–1135.
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 2019, 10, 327.
- Choi, J.S.; Harley, B.A.C. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 2017, 3, e1600455.
- Kent, D.; Copley, M.; Benz, C.; Dykstra, B.; Bowie, M.; Eaves, C. Regulation of Hematopoietic Stem Cells by the Steel Factor/KIT Signaling Pathway. Clin. Cancer Res. 2008, 14, 1926–1930.
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154.
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human osteogenic differentiation in Space: Proteomic and epigenetic clues to better understand osteoporosis. Sci. Rep. 2019, 9, 8343.
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627.
- Moreno-Villanueva, M.; Feiveson, A.H.; Krieger, S.; Brinda, A.M.K.; Von Scheven, G.; Bürkle, A.; Crucian, B.; Wu, H. Synergistic effects of weightlessness, isoproterenol, and radiation on DNA damage response and cytokine production in immune cells. Int. J. Mol. Sci. 2018, 19, 13689.
- Ludtka, C.; Moore, E.; Allen, J.B. The effects of simulated microgravity on macrophage phenotype. Biomedicines 2021, 9, 1205.
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2021, 18, 1489–1502.
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Seebacher, C.; Uhl, R.; Neelam, S.; Zhang, Y.; Levine, H.; Ullrich, O. Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages. Int. J. Mol. Sci. 2019, 20, 2402.
- Ludtka, C.; Silberman, J.; Moore, E.; Allen, J.B. Macrophages in microgravity: The impact of space on immune cells. NPJ Microgravity 2021, 7, 13.
- Pergola, C.; Schubert, K.; Pace, S.; Ziereisen, J.; Nikels, F.; Scherer, O.; Hüttel, S.; Zahler, S.; Vollmar, A.M.; Weinigel, C.; et al. Modulation of actin dynamics as potential macrophage subtype-targeting anti-tumour strategy. Sci. Rep. 2017, 7, 41434.
- Adrian, A.; Schoppmann, K.; Sromicki, J.; Brungs, S.; von der Wiesche, M.; Hock, B.; Kolanus, W.; Hemmersbach, R.; Ullrich, O. The oxidative burst reaction in mammalian cells depends on gravity. Cell Commun. Signal. 2013, 11, 98.
- Brungs, S.; Kolanus, W.; Hemmersbach, R. Syk phosphorylation–a gravisensitive step in macrophage signalling. Cell Commun. Signal. 2015, 13, 9.
- Thiel, C.S.; de Zélicourt, D.; Tauber, S.; Adrian, A.; Franz, M.; Simmet, D.M.; Schoppmann, K.; Hauschild, S.; Krammer, S.; Christen, M.; et al. Rapid adaptation to microgravity in mammalian macrophage cells. Sci. Rep. 2017, 7, 43.
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599.
- Maier, J.A.M. Impact of simulated microgravity on cell cycle control and cytokine release by U937 cells. Int. J. Immunopathol. Pharmacol. 2006, 19, 279–286.
- Paulsen, K.; Tauber, S.; Goelz, N.; Simmet, D.M.; Engeli, S.; Birlem, M.; Dumrese, C.; Karer, A.; Hunziker, S.; Biskup, J.; et al. Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity—Results of an in vitro experiment on board of the Shenzhou-8 space mission. Acta Astronaut. 2014, 94, 277–292.
- Paulsen, K.; Tauber, S.; Dumrese, C.; Bradacs, G.; Simmet, D.M.; Gölz, N.; Hauschild, S.; Raig, C.; Engeli, S.; Gutewort, A.; et al. Regulation of ICAM-1 in Cells of the Monocyte/Macrophage System in Microgravity. Biomed Res. Int. 2015, 2015, 538786.
- Moser, D.; Sun, S.J.; Li, N.; Biere, K.; Hoerl, M.; Matzel, S.; Feuerecker, M.; Buchheim, J.I.; Strewe, C.; Thiel, C.S.; et al. Cells’ Flow and Immune Cell Priming under alternating g-forces in Parabolic Flight. Sci. Rep. 2019, 9, 11276.
- Pajevic, P.D.; Spatz, J.M.; Garr, J.; Adamson, C.; Misener, L. Osteocyte biology and space flight. Curr. Biotechnol. 2013, 2, 179.
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Transl. 2017, 10, 86–93.
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369.
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk Between Bone and Immune System. Front. Endocrinol. 2019, 10, 236.
- Divieti Pajevic, P.; Krause, D.S. Osteocyte regulation of bone and blood. Bone 2019, 119, 13–18.
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front. Immunol. 2020, 11, 58.
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1.
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16.
- Cao, D.; Song, J.; Ling, S.; Niu, S.; Lu, L.; Cui, Z.; Li, Y.; Hao, S.; Zhong, G.; Qi, Z.; et al. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 2019, 33, 6904–6918.
- Buravkova, L.B.; Rykova, M.P.; Grigorieva, V.; Antropova, E.N. Cell interactions in microgravity: Cytotoxic effects of natural killer cells in vitro. J. Gravit. Physiol. 2004, 11, P177–P180.
- Buravkova, L.; Romanov, Y.; Rykova, M.; Grigorieva, O.; Merzlikina, N. Cell-to-cell interactions in changed gravity: Ground-based and flight experiments. Acta Astronaut. 2005, 57, 67–74.
- Buravkova, L.B.; Grigor’eva, O.V.; Rykova, M.P.; Grigor’ev, A.I. Cytotoxic activity of natural killer cells in vitro under microgravity. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect. 2008, 421, 275–277.
- Li, Q.; Mei, Q.; Huyan, T.; Xie, L.; Che, S.; Yang, H.; Zhang, M.; Huang, Q. Effects of simulated microgravity on primary human NK cells. Astrobiology 2013, 13, 703–714.
- Mylabathula, P.L.; Li, L.; Bigley, A.B.; Markofski, M.M.; Crucian, B.E.; Mehta, S.K.; Pierson, D.L.; Laughlin, M.S.; Rezvani, K.; Simpson, R.J. Simulated microgravity disarms human NK-cells and inhibits anti-tumor cytotoxicity in vitro. Acta Astronaut. 2020, 174, 32–40.
- Shao, D.; Ye, L.; Zhu, B.; Li, Q.; Yang, H.; Shi, J.; Huang, Q.; Zhao, W. Mechanisms of the Effect of Simulated Microgravity on the Cytotoxicity of NK Cells Following the DNA Methylation of NKG2D and the Expression of DAP10. Microgravity Sci. Technol. 2021, 33, 6.
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80.
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68.
- Paul, A.M.; Mhatre, S.D.; Cekanaviciute, E.; Schreurs, A.S.; Tahimic, C.G.T.; Globus, R.K.; Anand, S.; Crucian, B.E.; Bhattacharya, S. Neutrophil-to-Lymphocyte Ratio: A Biomarker to Monitor the Immune Status of Astronauts. Front. Immunol. 2020, 11, 564950.
- Meloni, M.A.; Galleri, G.; Camboni, M.G.; Pippia, P.; Cogoli, A.; Cogoli-Greuter, M. Modeled microgravity affects motility and cytoskeletal structures. J. Gravit. Physiol. 2004, 11, P197–P198.
- Blaber, E.A.; Dvorochkin, N.; Torres, M.L.; Yousuf, R.; Burns, B.P.; Globus, R.K.; Almeida, E.A.C. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014, 13, 181–201.
- Bonnefoy, J.; Ghislin, S.; Beyrend, J.; Coste, F.; Calcagno, G.; Lartaud, I.; Gauquelin-Koch, G.; Poussier, S.; Frippiat, J.P. Gravitational experimental platform for animal models, a new platform at ESA’s terrestrial facilities to study the effects of micro-and hypergravity on aquatic and rodent animal models. Int. J. Mol. Sci. 2021, 22, 62961.
- Morey, E.R. Spaceflight and Bone Turnover: Correlation with a New Rat Model of Weightlessness. Bioscience 1979, 29, 168–172.
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377.





