2. Vitamin D Signaling in Breast Cancer
Synthesis of vitamin D3 in the skin requires UV radiation, which can induce DNA damage, preferentially cyclobutane pyrimidine dimers and (6-4)-pyrimidine-pyrimidone photoproducts, which are found in many skin cancers, including melanoma (reviewed in
[22]). On the other hand, vitamin D3 protects the skin against UV-induced aging and DNA damage causing cancer. This raises the question about the general anticancer properties of vitamin D3.
Usually, the biological effects of vitamin D are classified into nongenomic and genomic (reviewed in
[23]). Genomic effects are mediated by VDR, RXR, and VDREs and result in long-term, sometimes delayed, biological consequences. The nongenomic pathway includes rapid cellular effects of 1,25(OH)2D, such as the protection of VDR-defective human fibroblasts against DNA damage induced by UV mediated by endoplasmic reticulum stress protein 57 (ERP57, MARRS)
[24]. Therefore, the anticancer effects of vitamin D3 may be underlined by its genomic and nongenomic actions (reviewed in
[25]).
The anticancer effect of vitamin D3 is not limited to UV-induced skin cancers. Colston et al. and Abe et al. were the first to show the anticancer properties of vitamin D in vitro
[26][27]. Subsequently, many in vivo and in vitro studies showed the protective action of vitamin D against various cancers and identified several genes important in cancer transformation as targets in the genomic action of vitamin D (reviewed in
[9]). In general, the antitumor effects of vitamin D are underlined by modulation of specific signaling pathways that are involved in tumor growth (
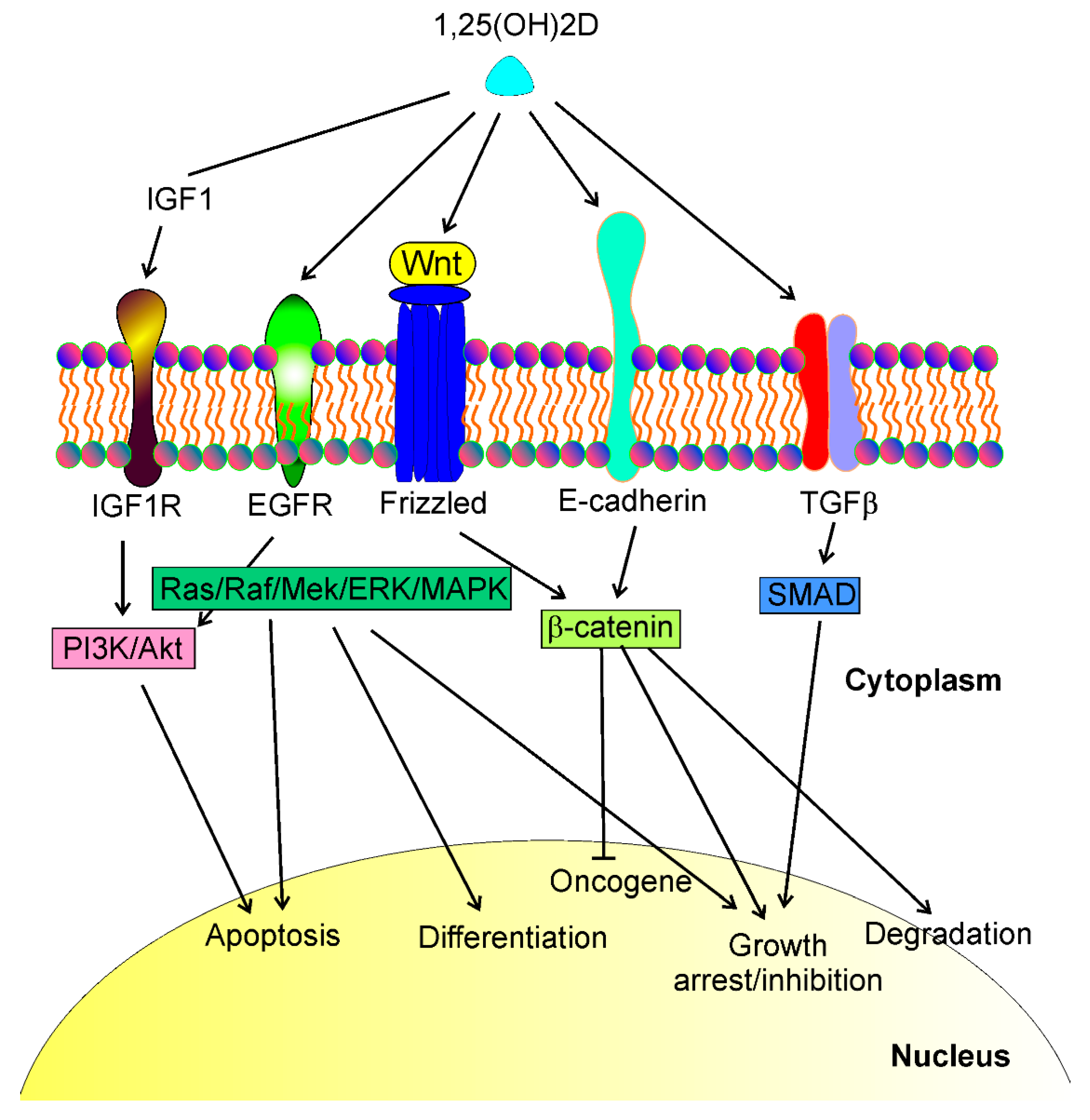
Figure 1). Tumor growth is determined by the fate of cancer cells that may differentiate, arrest the cell cycle, and undergo apoptosis and/or degradation.
Figure 1. 1,25(OH)2D, a biologically active metabolite of vitamin D, may interact with different transmembrane proteins and affect several signaling pathways that regulate essential phenomena in cancer cells such as apoptosis, differentiation, growth arrest, cell cycle, oncogene and tumor suppressor expression, degradation. These proteins include, but are not limited to, insulin growth factor 1 (IGF1) receptor (IGF1R), epidermal growth factor receptor (EGFR), Wnt/frizzled proteins, E-cadherin, and transforming growth factor-beta (TGFβ). Interactions of 1,25(OH)2D with these proteins lead to a cascade of downstream events resulting in the activation of several signaling pathways, including PI3K/Akt, Ras/Raf/Mek/ERK/MAPK, β-catenin, and SMAD (phosphatidylinositol 3-kinase/AKT serine/threonine kinase, KRAS proto-oncogene/Raf-1 proto-oncogene/mitogen-activated protein kinase kinase 1, SMAD family members, respectively). These signaling pathways may directly regulate the life and death of cancer cells or control the transcription of genes whose products contribute to cancer transformation.
Vitamin D3/VDR signaling is implicated in the morphogenesis of the postnatal mammary gland, negatively regulating its growth
[28]. Therefore, impaired vitamin D/VDR signaling may promote the abnormal development of the mammary gland, which may contribute to young age-onset breast cancer cases.
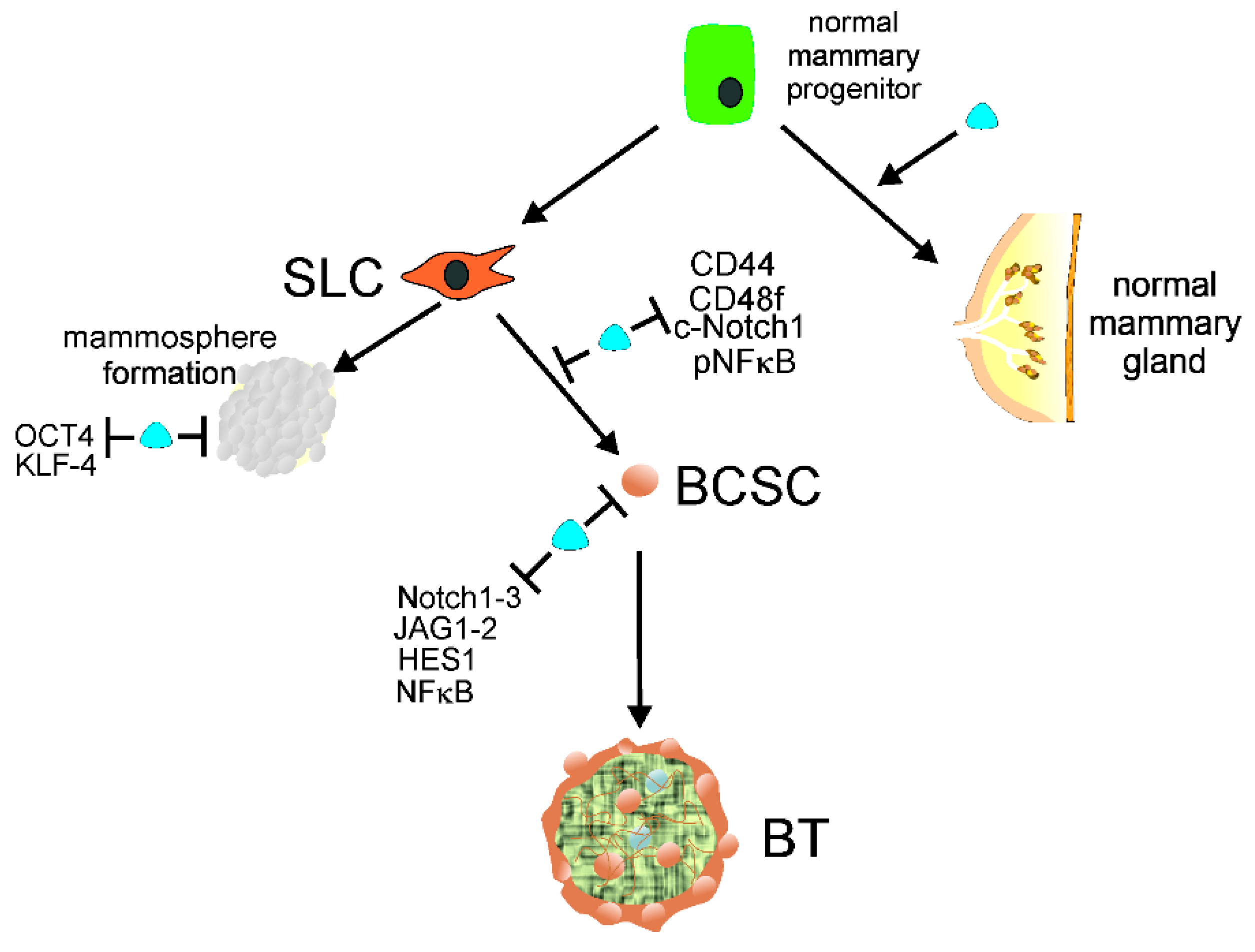
Malignant breast tumors are formed and maintained with the involvement of breast cancer stem cells (BCSCs)
[29]. Moreover, BCSCs are involved in progression to metastasis, relapse, and therapy resistance. 1,25(OH)2D and a gemini analog of vitamin D3 (BXL0124) were reported to inhibit mammosphere formation from mammary progenitors and downregulated the pluripotency markers octamer-binding transcription factor 4 (OCT4) and Kruper like factor 4 (KLF-4) in mammospheres
[30]. 1,25(OH)2D also repressed markers of stem cell-like phenotypes, including cluster of differentiation 44 (CD44), CD49f, cleaved Notch receptor 1 (c-Notch1), and phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells (pNFκB) in breast cancer. Subsequently, it was shown that 1,25(OH)2D and BXL0124 downregulated the pluripotency markers OCT4, CD44, and laminin subunit alpha 5 (LAMA5) in the mammospheres of a triple-negative breast cancer (TNBC) cell line
[31]. Furthermore, 1,25(OH)2D and its analog repressed other proteins important for the maintenance of BCSCs, including Notch1-3, jagged canonical Notch ligand 1 (JAG1-2), Hes family BHLH transcription factor 1 (HES1), and NFκB. Therefore, vitamin D3/VDR signaling may prevent acquiring a stem-like phenotype by normal mammary gland progenitors and somatic breast cancer cells (
Figure 2). Several pathways involved in cancer stemness are modulated by lncRNAs, and their targeting may be considered a new strategy to control tumor development, invasion, metastasis, and therapeutic resistance (e.g.,
[32][33][34]).
Figure 2. Normal mammary gland progenitors may develop into a normal mammary gland or acquire stem cell-like (SLC) properties to form mammospheres or/and transform into breast cancer stem cells (BCSCs). 1,25(OH)2D, a biologically active metabolite of vitamin D, along with its receptor, VDR, symbolized here as a blue tear, supports the normal development of mammary gland and prevents mammosphere formation by repressing the pluripotency markers OCT4 and KLF-4. Vitamin D may also prevent the conversion of SLC cells into BCSCs by downregulation of other pluripotency markers, including CD44, CD49f, c-Notch1, and pNFκB. Vitamin D may also repress proteins needed for the maintenance of BCSCs, such as Notch1-3, JAG1-2, HES1, and NFκB, preventing the initiation and formation of breast tumors (BTs). Abbreviations are defined in the main text.
The hormonal properties of vitamin D3, as part of the vitamin D3 endocrine system, may be responsible for the increased efficacy of tamoxifen, a selective inhibitor of estrogen receptor (ER) commonly applied in breast cancer therapy
[35]. Such an action of vitamin D seems to be especially important in the case of breast cancer in postmenopausal women who no longer maintain ovarian production of estrogen
[36]. Aromatase is a key enzyme in estrogen synthesis, and vitamin D was reported to inhibit it and repress the expression of estrogen receptor alpha (ERα) in breast tissue,
[37][38][39][40][41]. Therefore, vitamin D is particularly important in breast cancer ER-positive cases in postmenopausal women. Many lncRNAs were reported to play a role in the regulation of estrogen-dependent signaling (reviewed in
[42]).
The role of autophagy in cancer in general and in breast cancer in particular is emerging
[43][44][45]. Regulation of autophagy is considered a method to prevent breast cancer and break therapeutic resistance (reviewed in
[46]). It was shown that the vitamin D/VDR axis in breast cancer is regulated by many miRNAs
[47]. Upregulation of
miR-214 diminished VDR-mediated signaling in breast cancer cell lines with a concomitant positive correlation between VDR level and an inhibitor of the Hedgehog pathway
[48]. The
VDR gene has a sequence in its 3′-untranslated region recognized by
miR-125b whose overexpression in the MCF-7-line decreases VDR level
[49]. On the other hand, 1,25(OH)2D inhibited the expression of
miR-125b in MCF-7 cells
[50]. These results highlight the role of lncRNAs in mediating effects of vitamin D3 in breast cancer as one of the main mechanisms of biological action of lncRNAs is miRNAs sponging.
Other pathways and mechanisms may contribute to vitamin D3/VDR signaling in breast cancer (reviewed in
[10]). The results of research on breast cancer cell lines should be interpreted considering the high heterogeneity of breast cancer cases and different forms of VDR, as well as pleiotropy of biological action of vitamin D
[51]. Breast cancer cells, depending on origin, can be inherently vitamin D3 resistant or sensitive or acquire resistance during disease progression due to various effects, including methylation of the
VDR promoter
[52]. Heterogeneity of breast cancer also involves differences between specific breast compartments, which cause differential distribution of vitamin D3 in different regions of breast tumors due to different activity of enzymes involved in vitamin D3/VDR signaling.
In summary, several epidemiological studies show vitamin D3 deficiency in breast cancer patients. These association studies are supported by experimental research, but the mechanism underlying the potential beneficiary effect of vitamin D3 in breast cancer remains poorly understood. To apply vitamin D3 in breast cancer prevention and therapy effectively and safely, more controlled clinical trials and mechanistic laboratory studies are needed. 1,25(OH)2D may directly interact with several membrane-bound proteins to initiate a cascade of several signaling pathways that control cell growth and cell cycle arrest, cell migration, proliferation, and apoptosis important for cancer transformation.