
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandra Bernardo | + 2157 word(s) | 2157 | 2022-03-08 08:56:20 | | | |
| 2 | Fani Sousa | + 3 word(s) | 2160 | 2022-03-21 20:41:16 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 2160 | 2022-03-22 04:46:29 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 2160 | 2022-03-22 04:47:23 | | |
Video Upload Options
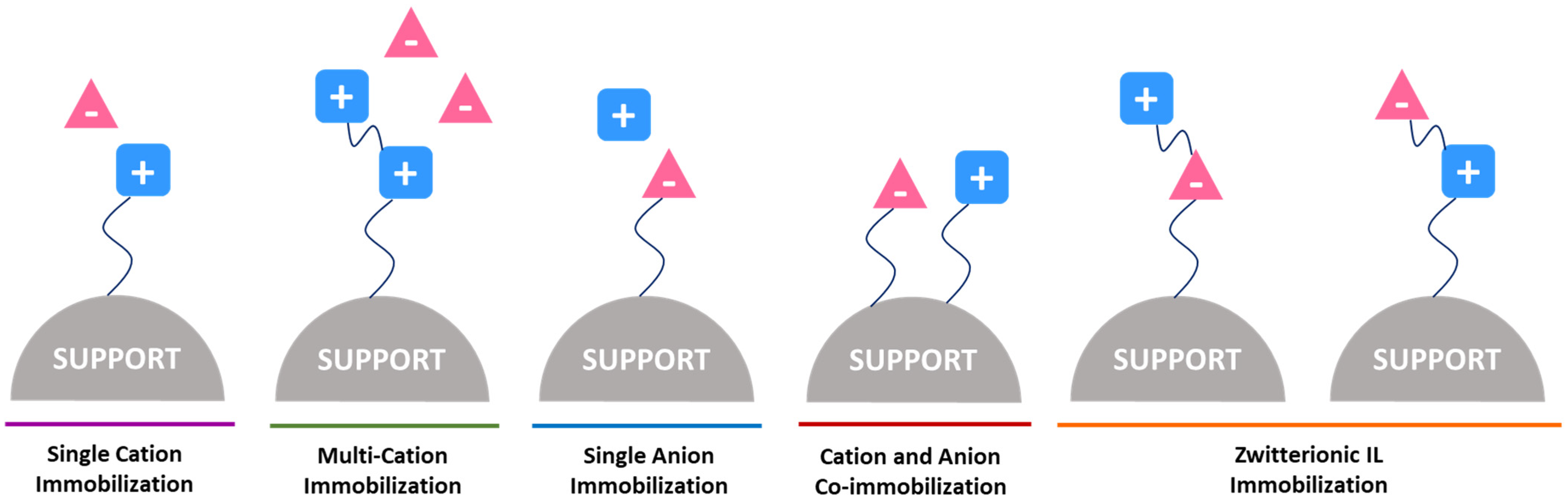
Ionic liquids (ILs) have been investigated as novel ligands in chromatographic matrices, denominated as supported ionic liquids (SILs). ILs are organic salts with a wide structural diversity, which can display a multi-modal behavior because they present positive/negative charged groups, and can be tailored by the introduction of several functional groups and alkyl moieties of different lengths. SILs maintain the valuable features of ILs with the addition of being supported, thus avoiding the use of large amounts of ILs. Despite the fact that the liquid state of ILs is being lost when immobilized, their capability to establish a plethora of interactions is kept, allowing them to be used in hydrophilic, hydrophobic, affinity, multi-modal and ion-exchange chromatography. Due to their advantages, IL-modified materials have been recently synthetized and proven to be an important new type of stationary phases in liquid chromatography.
1. Introduction
2. Ionic Liquids
2.1. Supported Ionic Liquids (SILs) in Analytical Methods
2.2. ILs as Ligands of Chromatographic Supports
References
- Nikolin, B.; Imamović, B.; Medanhodzić-Vuk, S.; Sober, M. High perfomance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 2004, 4, 5–9.
- Rosing, H.; Man, W.Y.; Doyle, E.; Bult, A.; Beijnen, J.H. Bioanalytical liquid chromatographic method validation. a review of current practices and procedures. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 329–354.
- Cramer, S.M.; Jayaraman, G. Preparative chromatography in biotechnology. Curr. Opin. Biotechnol. 1993, 4, 217–225.
- Jungbauer, A. Preparative chromatography of biomolecules. J. Chromatogr. A 1993, 639, 3–16.
- Guiochon, G. Preparative liquid chromatography. J. Chromatogr. A 2002, 965, 129–161.
- Ward, W.W.; Swiatek, G. Protein Purification. Curr. Anal. Chem. 2009, 5, 85–105.
- Valente, J.F.A.; Queiroz, J.A.; Sousa, F. Dilemma on plasmid DNA purification: Binding capacity vs selectivity. J. Chromatogr. A 2021, 1637, 461848.
- Martins, R.; Queiroz, J.A.; Sousa, F. Ribonucleic acid purification. J. Chromatogr. A 2014, 1355, 1–14.
- Lowe, C.R.; Lowe, A.R.; Gupta, G. New developments in affinity chromatography with potential application in the production of biopharmaceuticals. J. Biochem. Biophys. Methods 2001, 49, 561–574.
- Łącki, K.M.; Riske, F.J. Affinity Chromatography: An Enabling Technology for Large-Scale Bioprocessing. Biotechnol. J. 2020, 15, e1800397.
- Owczarek, B.; Gerszberg, A.; Hnatuszko-Konka, K. A Brief Reminder of Systems of Production and Chromatography-Based Recovery of Recombinant Protein Biopharmaceuticals. Biomed. Res. Int. 2019, 2019, 4216060.
- Sanchez-Garcia, L.; Martín, L.; Mangues, R.; Ferrer-Miralles, N.; Vázquez, E.; Villaverde, A. Recombinant pharmaceuticals from microbial cells: A 2015 update. Microb. Cell Factories 2016, 15, 1–7.
- Ho, J.R.; Chien, J. Trends in translational medicine and drug targeting and delivery: New insights on an old concept—targeted drug delivery with antibody–drug conjugates for cancers. J. Pharm. Sci. 2014, 103, 71–77.
- Arakawa, T.; Santarelli, X. Binding and Elution Properties of Mixed-Mode Chromatography and Its Applications for Purification. Curr. Protein Pept. Sci. 2019, 20, 3.
- Ren, X.; Zhang, K.; Gao, D.; Fu, Q.; Zeng, J.; Zhou, D.; Wang, L.; Xia, Z. Mixed-mode liquid chromatography with a stationary phase co-functionalized with ionic liquid embedded C18 and an aryl sulfonate group. J. Chromatogr. A 2018, 1564, 137–144.
- Shi, X.; Qiao, L.; Xu, G. Recent development of ionic liquid stationary phases for liquid chromatography. J. Chromatogr. A 2015, 1420, 1–15.
- Kuchenbuch, A.; Giernoth, R. Ionic liquids beyond simple solvents: Glimpses at the state of the art in organic chemistry. ChemistryOpen 2015, 4, 677.
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038.
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706.
- Shukla, S.K.; Pandey, S.; Pandey, S. Applications of ionic liquids in biphasic separation: Aqueous biphasic systems and liquid–liquid equilibria. J. Chromatogr. A 2018, 1559, 44–61.
- Ventura, S.P.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052.
- Freire, M.G.; Claudio, A.F.M.; Araujo, J.M.; Coutinho, J.A.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995.
- Sintra, T.E.; Nasirpour, M.; Siopa, F.; Rosatella, A.A.; Gonçalves, F.; Coutinho, J.A.; Afonso, C.A.; Ventura, S.P. Ecotoxicological evaluation of magnetic ionic liquids. Ecotoxicol. Environ. Saf. 2017, 143, 315–321.
- Taha, M.; Quental, M.V.; e Silva, F.A.; Capela, E.V.; Freire, M.G.; Ventura, S.P.; Coutinho, J.A. Good’s buffer ionic liquids as relevant phase-forming components of self-buffered aqueous biphasic systems. J. Chem. Technol. Biotechnol. 2017, 92, 2287–2299.
- Zhang, M.; Liang, X.; Jiang, S.; Qiu, H. Preparation and applications of surface-confined ionic-liquid stationary phases for liquid chromatography. Trac Trends Anal. Chem. 2014, 53, 60–72.
- Chen, W.; Zhang, Y.; Zhu, L.; Lan, J.; Xie, R.; You, J. A Concept of Supported Amino Acid Ionic Liquids and Their Application in Metal Scavenging and Heterogeneous Catalysis. J. Am. Chem. Soc. 2007, 129, 13879–13886.
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531.
- Nawała, J.; Dawidziuk, B.; Dziedzic, D.; Gordon, D.; Popiel, S. Applications of ionic liquids in analytical chemistry with a particular emphasis on their use in solid-phase microextraction. Trac Trends Anal. Chem. 2018, 105, 18–36.
- Pletnev, I.; Smirnova, S.; Shvedene, N. New directions in using ionic liquids in analytical chemistry. 1: Liquid–liquid extraction. J. Anal. Chem. 2019, 74, 625–658.
- Pino, V.; Afonso, A.M. Surface-bonded ionic liquid stationary phases in high-performance liquid chromatography—A review. Anal. Chim. Acta 2012, 714, 20–37.
- Vidal, L.; Riekkola, M.-L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41.
- Jandera, P.; Janás, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32.
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950.
- Han, D.; Row, K.H. Recent Applications of Ionic Liquids in Separation Technology. Molecules 2010, 15, 2405–2426.
- Qiu, H.; Wang, L.; Liu, X.; Jiang, S. Preparation and characterization of silica confined ionic liquids as chromatographic stationary phases through surface radical chain-transfer reaction. Analyst 2009, 134, 460–465.
- Bi, W.; Zhou, J.; Row, K.H. Separation of xylose and glucose on different silica-confined ionic liquid stationary phases. Anal. Chim. Acta 2010, 677, 162–168.
- Pietruszka, N.; Galaev, I.Y.; Kumar, A.; Brzozowski, Z.K.; Mattiasson, B. New Polymers Forming Aqueous Two-Phase Polymer Systems. Biotechnol. Prog. 2000, 16, 408–415.
- Qiao, L.; Wang, S.; Li, H.; Shan, Y.; Dou, A.; Shi, X.; Xu, G. A novel surface-confined glucaminium-based ionic liquid stationary phase for hydrophilic interaction/anion-exchange mixed-mode chromatography. J. Chromatogr. A 2014, 1360, 240–247.
- Neves, M.; Pereira, P.; Pedro, A.; Martins, J.; Trindade, T.; Queiroz, J.; Freire, M.; Sousa, F. Improved ionic-liquid-functionalized macroporous supports able to purify nucleic acids in one step. Mater. Today Bio 2020, 8, 100086.
- Qiu, H.; Jiang, S.; Takafuji, M.; Ihara, H. Polyanionic and polyzwitterionic azobenzene ionic liquid-functionalized silica materials and their chromatographic applications. Chem. Commun. 2013, 49, 2454–2456.
- Qiu, H.; Zhang, M.; Chen, J.; Gu, T.; Takafuji, M.; Ihara, H. Anionic and cationic copolymerized ionic liquid-grafted silica as a multifunctional stationary phase for reversed-phase chromatography. Anal. Methods 2014, 6, 469–475.
- Qiao, L.; Dou, A.; Shi, X.; Li, H.; Shan, Y.; Lu, X.; Xu, G. Development and evaluation of new imidazolium-based zwitterionic stationary phases for hydrophilic interaction chromatography. J. Chromatogr. A 2013, 1286, 137–145.





