The protein kinase C (PKC) family belongs to serine-threonine kinases and consists of several subtypes. Increasing evidence suggests that PKCs are critical players in carcinogenesis. Interest-ingly, PKCs exert both promotive and suppressive effects on tumor cell growth and metastasis, which have attracted immense attention. PKCs play a potential role as a target for therapeutic intervention in cancer from basic research and clinical trials.
1. Introduction
Protein kinase C (PKC) isoenzymes are a prototypical class of serine/threonine kinases that are conserved throughout evolution and widely expressed in almost all cell types. They consist of domain permutations in their N-terminal regulatory regions linked by variable sequences to a highly conserved C-terminal kinase domain. The biological role of PKC isoenzymes has been intensely studied in both various physiological and pathological conditions. In addition, PKC isoenzymes are implicated in multiple signal transduction pathways that turn environment cues into cellular actions. The PKC signaling pathway has critical roles in regulating gene expression, as well as cell proliferation, differentiation, migration, survival and death. Inactivation or dysregulation of PKC isoenzymes may cause different pathologies, such as allergy
[1], inflammatory diseases
[2], diabetes
[3] and autoimmune diseases
[4][5]. Recent scientific breakthroughs and technological advancements have improved our understanding of the functional roles of PKCs in cancer development and progression. Moreover, PKC enzymes can serve as promising targets for cancer treatment
[6].
2. PKC Inhibitors for Cancer Therapy
Since the PKC isoforms are involved in multiple signaling pathways, they are attractive targets for the treatment of various human diseases, including cancers. Different approaches have been proposed to identify the selective regulators of PKC isoforms. For instance, ATP-competitive molecule inhibitors that bind to the ATP site of PKC enzymes, phorbol esters and other derivative activators or inhibitors that bind to the C1 domain for diacylglycerol (DAG)-binding simulation, and peptides that block the interaction between the regulatory region and RACK of PKC enzymes (Table 1).
Table 1. Inhibitors of PKC isoforms.
| Structure |
Name |
Specificity |
Inhibition Mechanism |
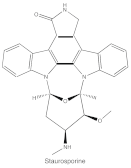 |
Staurosporine |
Pan-PKCs |
competitive interfere with the ATP binding site |
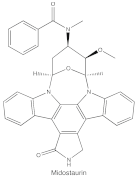 |
Midostaurin
(PKC412, CGP41251, N-benzoylstaurosporine) |
cPKCs, nPKCs |
competitive interfere with the ATP binding site |
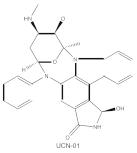 |
7-Hydroxystaurosporine (UCN-01) |
cPKCs, nPKCs |
competitive interfere with the ATP binding site |
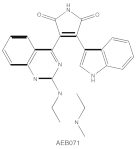 |
Sotrastaurin
(AEB071) |
PKCα, β, θ, δ |
competitive interfere with the ATP binding site |
 |
Enzastaurin
(LY317615) |
PKCβ |
competitive interfere with the ATP binding site |
 |
Bryostatin 1 |
PKCα, PKCε, and PKCη |
competitive interfere with the phorbol ester binding site |
2.1. ATP-Competitive Molecule Inhibitors
ATP-competitive molecule inhibitors are molecules that regulate high-affinity interaction with the ATP binding motif within the catalytic domain of PKC isoforms. Staurosporine is a natural and water-soluble pan-inhibitor of PKC. It has been demonstrated that staurosporine could selectively reverse chronic myeloid leukemia resistance to imatinib by inhibiting PKCα-dependent CDC23 inhibition and arresting the cell cycle in the G2/M phase
[7]. Midostaurin (PKC412, CGP41251, N-benzoylstaurosporine), a semi-synthetic derivative of staurosporine, shows stronger inhibition on cPKC than nPKC and is inactive towards PKCζ. Midostaurin exhibited a potent anti-tumor effect on rituximab-resistant Burkitt’s lymphoma (BL) cells by reducing the phosphorylation of PKC and promoting proapoptotic activity. Moreover, the combination of midostaurin and rituximab dramatically improved the survival of mice bearing the resistant BL cells compared to rituximab monotherapy
[8]. 7-Hydroxystaurosporine (UCN-01) has a similar preference for PKC as midostaurin and exhibits anticancer activity via dampening cell cycle progression from the G1 to S phase
[9]. Several clinical studies indicated that UCN-01 could be administered safely and well-tolerated
[10][11]. Sotrastaurin (also known as AEB071) is a broadly selective molecule that blocks the catalytic activity of PKC enzymes, such as PKCα, PKCβ, PKCθ and PKCδ. Targeted PKC inhibition with sotrastaurin exerted an antitumor effect on uveal melanoma cells harboring GNAQ mutations via suppressing PKC/ERK1/2 and PKC/NF-κB pathways
[12]. Enzastaurin (also known as LY317615) is an oral selective PKCβ inhibitor that exhibits a synergistic antitumor effect on non-small cell lung cancer (NSCLC) cell growth when combined with pemetrexed
[13]. However, the ATP binding motif shares high sequence homology and structural similarity among kinases, which makes it difficult for the inhibitors to achieve the required selectivity.
2.2. Phorbol Esters and Other Derivatives
Phorbol esters exhibit pharmacological properties via interacting with the C1 domain in PKC, similar to the lipid second messenger DAG, but in a competitive manner
[14]. It has been demonstrated that phorbol esters induce cancer cell apoptosis via PKC activation and downregulation of several proto-oncogenes (c-Myc, c-Fos and c-Jun)
[15]. Unlike DAG, phorbol esters are not readily metabolized and subsequently fasten PKC in an open and active conformation that is susceptible to ubiquitination, dephosphorylation and degradation. Thus, while phorbol esters induce acute and constitutive activation of PKC, chronic loss or downregulation of all conventional and novel PKC isoforms occurs
[16]. Although PKC is the major receptor of phorbol esters, phorbol esters also target other receptors and cause local inflammation. Bryostatin 1 is a macrocyclic lactone that antagonizes the pro-tumor effects of phorbol esters with exceptionally high affinity, leading to significant inhibition of PKCα, PKCε and PKCη, as well as peripheral blood monocytes in patients with advanced malignancies
[17]. DAG-lactones are a group of DAG analogs synthesized by a pharmacophore-guided approach, which afford higher affinity for PKC isoforms compared with DAG. HK434 and HK654, two designed compounds of DAG-lactones with highly potent DAG surrogates, are found to induce apoptosis in LNCaP prostate cancer cells by activating PKCα
[18]. It is worth noting that the structure of the C1 domain in PKC isoforms is highly conserved, making it arduous to fabricate isoform-selective compounds that target the C1 domain.
2.3. Lipid Analogs
Several lipid analogs have been developed, such as safingol, ilmofosine, perifosine and aurothiomalate, which target activation of PKC at the regulatory domain. Safingol, L-threo-dihydrosphingosine, displayed selective inhibition for PKCα and induced an endonuclease G-mediated cell apoptosis of oral squamous cell carcinoma cells in a caspase-independent manner
[19]. Safingol is also a putative inhibitor of sphingosine kinase 1 (SphK), which catalyzes sphingosine 1-phosphate (S1P) and mediates cancer cell growth. A phase I clinical trial showed that a combination of safingol with cisplatin was tolerable in patients with advanced solid tumors
[20]. Ilmofosine (BM41.440, 1-hexadecylthio-2-methoxymethyl-rac-glycero-3-phosphocholine), a thioether lysophospholipid derivative of lysophosphocholine, is a PKC inhibitor found to induce G2-arrest and Cdc2 kinase suppression via affecting the formation of Cdc2/cyclin B1 complexes
[21]. Ilmosofine exhibits concentration-dependent anticancer activities in various solid tumor models
[22][23]. However, no tumor regression occurred in patients with non-small cell bronchogenic carcinoma when using a schedule of continuous infusion for 5 days and a dose of 300 mg/m
2/day of ilmofosine
[24]. Perifosine (D-21226) is a synthetic alkyl phospholipid that demonstrates anticancer effects via inhibition of mitogen-activated protein kinases (MAPK), Akt and PKC
[25][26][27]. However, according to the trial results of the National Cancer Institute of Canada Clinical Trials Group, perifosine does not show evidence of anticancer activity in patients with previously untreated metastatic or locally advanced soft tissue sarcoma, when given orally on day 1 (900 mg cycle 1, 300 mg cycle 2+) and 2–21 (150 mg daily each cycle)
[27]. Aurothiomalate is an anti-rheumatoid gold agent that inhibits PKCλ/ι binding to the adaptor molecule Par6, which regulates Rac1 and induces anchorage-independent growth of NSCLC cells
[28]. It also demonstrates a pro-apoptotic effect on advanced prostate cancer and hepatocellular carcinoma
[29][30].
2.4. Others
Antisense oligonucleotides offer one approach to target genes that regulate cancer cell proliferation, apoptosis, and signaling pathways. Recent clinical trials have confirmed the anti-tumor activity of antisense oligonucleotides with safety and tolerability
[31]. Affinitak (ISIS-3521/LY900003) is an antisense oligonucleotide that specifically blocks PKCα
[32]. A phase II study demonstrated that ISIS-3521 is effective and safe in patients with relapsed low-grade non-Hodgkin’s lymphoma
[33]. However, there is no evidence of any clinical activity or target modulation of ISIS-3521 in patients with metastatic colorectal cancer
[34], and no objective responses are observed, even in combination with ISIS-5132 (an antisense inhibitor of Raf-1 kinase)
[35].
3. Conclusions
It is obvious that PKC isoforms are involved in cancer progression and represent important targets for cancer therapy. However, the biological functions of PKC isoforms are likely to vary across different tumor types. Studies of cancer-associated mutations revealed that the PKC family members are inactivated in cancer, representing a tumor-suppressive function
[36]. Based on this theory, strategies of cancer therapy should focus on restoring rather than suppressing PKC function. It also explains why numerous attempts to target PKC isoforms in cancer have yielded very limited success. Clinical trials demonstrated that the use of PKC inhibitors for cancer treatment not only failed but also worsened patient outcomes in some cases. Current efforts of small molecule compounds have been focused on the kinase domain of PKC enzymes. However, PKC enzymes shared a well-conserved kinase domain, which makes it a major challenge to obtain small molecule modulators with high specificity and sensitivity toward a specific PKC isoform. Despite this, PKC isoforms are promising anti-tumor targets and additional efforts are needed to improve the efficacy and selectivity of PKC isoform inhibitors.






